Summary
Elucidating fine architectures and functions of cellular and synaptic connections requires development of new flexible methods. Here, we created a concept called the “battle of transgenes,” based on which we generated strategies using genetically engineered battles of multiple recombinases. The strategies enabled split-tunable allocation of multiple transgenes. We demonstrated the versatility of these strategies and technologies in inducing strong and multi-sparse allocations of multiple transgenes. Furthermore, the combination of our transgenic strategy and expansion microscopy enabled three-dimensional high-resolution imaging of whole synaptic structures in the hippocampus with simultaneous visualizations of endogenous synaptic proteins. These strategies and technologies based on the battle of genes may accelerate the analysis of whole synaptic and cellular connections in diverse life science fields.
Subject Areas: Molecular Biology, Molecular Neuroscience, Neuroanatomy, Techniques in Neuroscience
Graphical Abstract
Highlights
-
•
Generation of BATTLE-recombinase systems for allocation of multiple transgenes
-
•
Split-tunable allocation in BATTLE-1 and multi-sparse allocation in BATTLE-2
-
•
Clear and strong labeling of dendrites and axons using BATTLE-2
-
•
3D high-resolution imaging of whole synapses in hippocampus in BATTLE-1EX
Molecular Biology; Molecular Neuroscience; Neuroanatomy; Techniques in Neuroscience
Introduction
Genes work cooperatively, competitively, and sometimes selfishly in the cells of the body (Dawkins, 1976). Cellular and synaptic connections are essential for almost all mammalian functions (Gumbiner, 1996, Südhof and Malenka, 2008). Genetic labeling of small groups of cells and synaptic structures is crucial to understanding the basal and functional mechanisms of the brain and body (Arenkiel and Ehlers, 2009). Blended expressions of multicolored fluorescent proteins are widely used for labeling neurons in neuroscience studies (Livet et al., 2007, Weissman and Pan, 2015, Sakaguchi et al., 2018), despite limitations in flexibility and tunability. To date, considerable efforts have been made to visualize and dissect whole synaptic structures using light microscopy (Nimchinsky et al., 2002, Rochefort and Konnerth, 2012), but most of these studies only succeeded in labeling half of the synaptic structures (Nimchinsky et al., 2002, Rochefort and Konnerth, 2012, Chen et al., 2015, Tillberg et al., 2016, Asano et al., 2018) such as spines or presynaptic terminals alone.
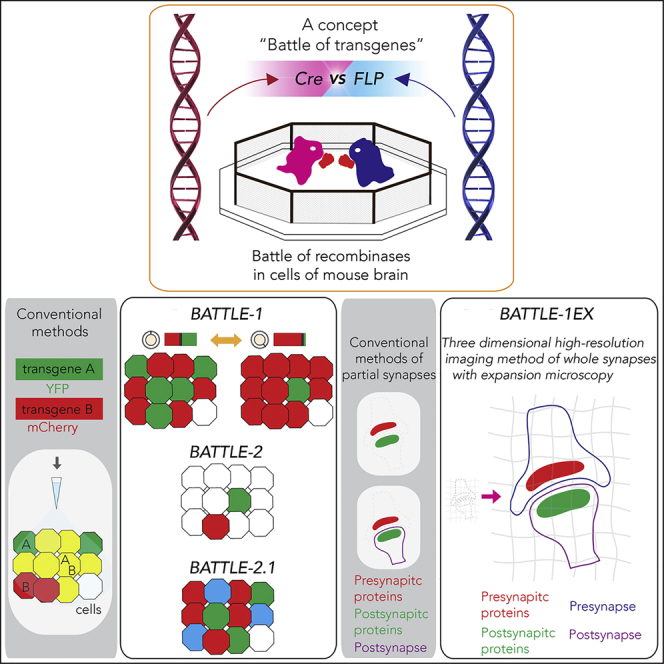
In this study, we created a concept called the “battle of the transgenes.” Based on this concept, we designed three strategies, named BATTLE-1, 2, and 2.1 enabling splittable, tunable, intense, multi-sparse, triple splittable allocation of transgenes. BATTLE-1 used two genetically engineered recombinase proteins and induced recombinase fights for the mutually exclusive allocation of transgenes. BATTLE-2 used two genetically engineered recombinase proteins and a genetically engineered shadow recombinase protein to induce intense, tunable, and multiple sparse allocation of transgenes. BATTLE-2.1 used three genetically engineered recombinase proteins to induce triple splittable allocation of transgenes.
We combined BATTLE-1 with a method called expansion microscopy (ExM) (Chen et al., 2015, Tillberg et al., 2016, Asano et al., 2018), a high-resolution method that allows three-dimensional (3D) high-resolution imaging of whole, synaptic structures of hippocampal neural circuits. We called this BATTLE-1EX.
Results
Battle of Recombinases and Genetically Engineered Strategies, BATTLE-1
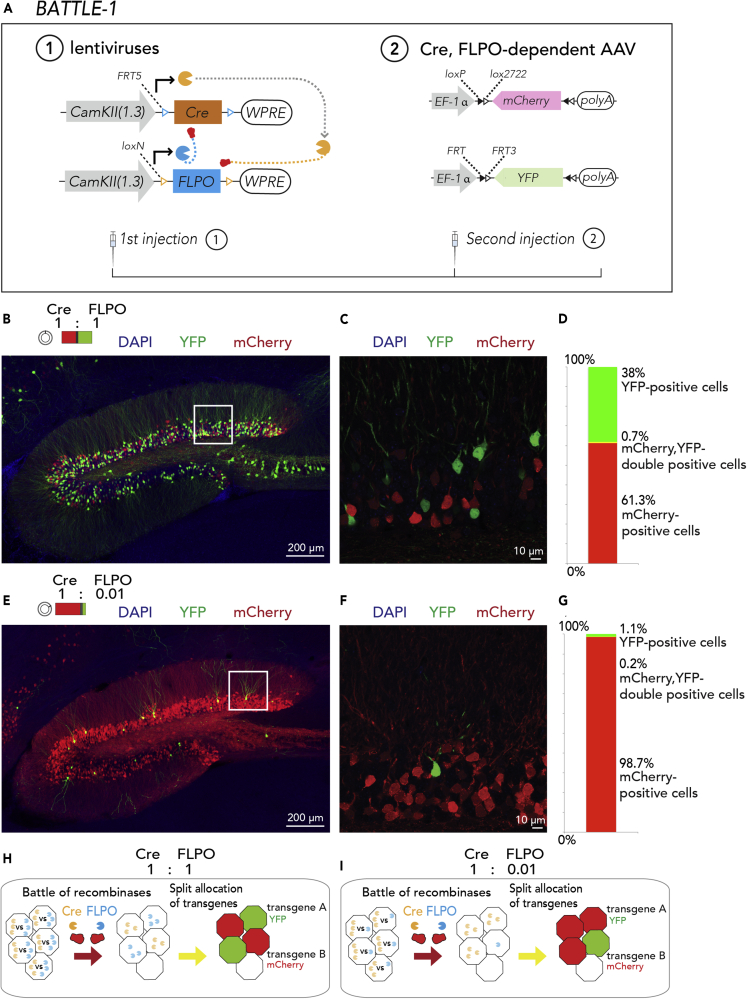
To establish the concept of the battle of transgenes, we generated genetically battle-oriented recombinase proteins. Cyclization recombination (Cre) and flippase O (FLPO) recombinase proteins can originally excise a transgene flanked by two similar recombinase recognition sites (Nagy, 2000) (such as loxP and flippase recognition target [FRT]). We designed multiple battle-oriented recombinases by aligning a Cre transgene flanked by two FRT mutant sites (Schlake and Bode, 1994) (FRT5) and an FLPO transgene flanked by two loci of X-over P1 (loxP) mutant sites (Livet et al., 2007) (loxN, Figure 1A). In this configuration, Cre is oriented to excise the FLPO transgene, which is then oriented to excise the Cre transgene.
Figure 1.
Battle of Recombinases
BATTLE-1: split-tunable allocation of transgenes by recombinase “fights.”
(A) In the BATTLE-1 technology, Cre and FLPO are expressed under the control of CamKIIa promoter. The Cre transgene flanked by two FRT5 is designed to be a target of the FLPO recombinase. The FLPO transgene flanked by two loxN is designed to be a target of the Cre recombinase. Cre- and FLPO-dependent AAVs expressing mCherry and YFP, respectively, were used.
(B) Representative maximum intensity projection image of injected hippocampus. When the Cre to FLPO ratio was 1:1, the splitting allocation of YFP and mCherry was observed in hippocampal neurons.
(C) Magnified confocal image of the boxed area in (B).
(D) Quantification of percentage of cells expressing fluorescent proteins in the dentate gyrus.
(E) Representative maximum intensity projection image of injected hippocampus. When the ratio of Cre to FLPO was 1:0.01, most of the cells expressed mCherry, whereas few cells expressed YFP in the hippocampus.
(F) Magnified confocal image of the boxed area in (E).
(G) Quantification of percentage of cells expressing fluorescent proteins in the dentate gyrus.
(H) Illustration shows the battle of recombinases and BATTLE-1 system at a Cre to FLPO ratio of 1:1. After the first injection of mixed viruses, Cre and FLPO started recombinase battles in each infected neuron. Then, only the winning recombinase survived to induce its specific mutually exclusive allocation of transgenes (mCherry or YFP).
(I) Illustration shows the battle of recombinases and BATTLE-1 system at Cre to FLPO ratio of 1:0.01. In this situation, Cre wins over FLPO in many cells. Then mCherry is allocated in many cells, whereas YFP is allocated in a few cells.
To achieve the battle of transgenes in the mouse brain, we simultaneously injected lentiviruses encoding Cre flanked with FRT5 and ones encoding FLPO flanked with loxN at the same ratio (Cre:FLPO = 1:1) into the mouse hippocampus. Three weeks later, we injected Cre- and FLPO-dependent adeno-associated viruses (AAVs) expressing mCherry and yellow fluorescent protein (YFP), respectively, into the same region. YFP and mCherry were co-expressed with CamKIIa proteins in hippocampal neurons under the control of the CamKIIa (1.3 kb) promoter, indicating their specific expression in excitatory neurons (Benson et al., 1992) (CamKIIa+:100% of 148 mCherry-positive neurons, 100% of 101 YFP-positive neurons, and 100% of one mCherry-YFP double-positive neuron in the dentate gyrus, N = 3 mice, Figure S1).
Mixed viral infections mostly induce co-allocation of multiple transgenes in many cases (Weissman and Pan, 2015, Sakaguchi et al., 2018, Gomez-Nicola et al., 2014, Chan et al., 2017). We observed that YFP and mCherry were separately allocated in a majority of the fluorescence-positive neurons in the dentate gyrus (Figures 1B–1D, mCherry+:YFP+:mCherry-YFP double+ = 61.3% ± 5.8%:38% ± 5.8%:0.7% ± 0.2%, mean ± SEM; n = 827 granule neurons, N = 5 mice) and in the CA2-3 region of the hippocampus (mCherry+:YFP+:mCherry-YFP double+ = 78% ± 2.7%:21.4% ± 2.7%:0.6% ± 0.4%, mean ± SEM; n = 292 pyramidal neurons in CA2-3, N = 5 mice). Only a few neurons showed co-allocation of the transgenes (Figure 1D). Furthermore, we performed experiments with different dosages and observed similar splitting allocations of YFP and mCherry in dentate gyrus: 500 nL dosage, mCherry+:YFP+:mCherry-YFP double+ = 55.9% ± 1.5%:43.8% ± 1.6%:0.3% ± 0.1%, mean ± SEM, n = 1,111 granule neurons, N = 3 mice; 1,500 nL dosage, mCherry+:YFP+:mCherry-YFP double+ = 57.4% ± 1.4%:41.9% ± 1.6%:0.7% ± 0.3%, mean ± SEM, n = 1,458 granule neurons, N = 3 mice (Figure S2). These data suggest that the battle of multiple recombinases occurred and induced splitting allocation of YFP and mCherry. We called this strategy, based on the battle of multiple recombinases, BATTLE-1.
Then, we similarly injected BATTLE-1 lentiviruses at different ratios (Cre: FLPO = 1: 0.01, Figure 1E). Under this condition, we observed biased and splitting allocation of transgenes in the dentate gyrus (Figures 1E–1G, mCherry+:YFP+:mCherry-YFP double+ = 98.7% ± 0.2%:1.1% ± 0.2%:0.2% ± 0.1%, mean ± SEM, n = 1,153 granule neurons, N = 3 mice) and the CA2-3 region (mCherry+:YFP+:mCherry-YFP double+ = 96.2% ± 0.8%:3.6% ± 0.9%:0.1% ± 0.1%, mean ± SEM, n = 442 pyramidal neurons in the CA2-3 region, N = 4 mice). These data suggest that the dominant battle of recombinases induced biased allocation of transgenes in the hippocampus. Therefore, we generated the battle of transgenes in the mouse hippocampus (Figures 1H and 1I). For this purpose, we generated the BATTLE-1 technology enabling the splitting and tunable allocation of multiple transgenes in the mouse hippocampus.
BATTLE-2 for Splittable, Tunable, Intense, and Multi-sparse Allocation of Multiple Transgenes
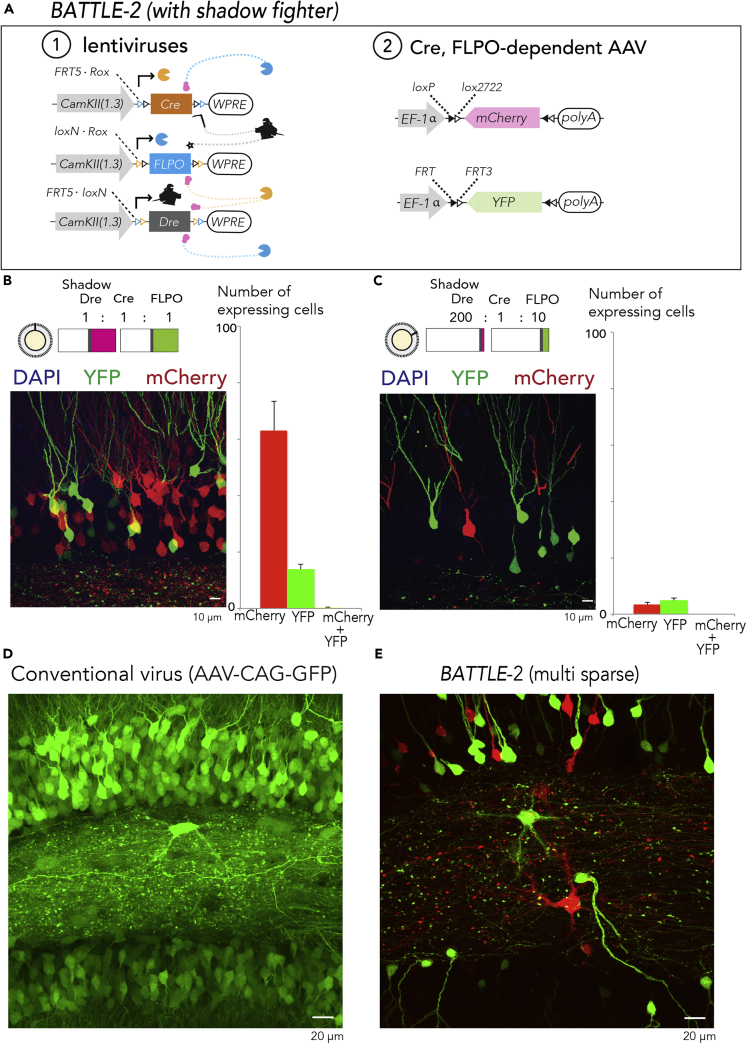
Next, we created the BATTLE-2 technology based on genetically engineered “fights” between triple recombinases consisting of Cre transgene flanked by two FRT5-rox sites, FLPO transgene flanked by two loxN-rox sites, and Dre recombinase (Anastassiadis et al., 2009) transgene flanked by two FRT5-loxN sites (Figure 2A). We used Cre- and FLPO-dependent AAVs expressing mCherry and YFP, respectively, in BATTLE-2. Under this configuration, the Dre recombinase, referred to as “shadow fighter,” did not induce the expression of specific transgenes. To test the BATTLE-2 technology, we simultaneously injected lentiviruses encoding Cre flanked with FRT5-rox, one encoding FLPO flanked with loxN-rox sites, and the one encoding a Dre transgene flanked by two FRT5-loxN sites at the same ratio (Cre:FLPO:Dre = 1:1:1, Figure 2A).
Figure 2.
BATTLE-2 (with Shadow Fighter): Split-Tunable and Multi-sparse Allocation of Transgenes Using the Battle of the Recombinases
(A) In BATTLE-2 technology, Cre, FLPO, and Dre are expressed under the control of CamKIIa promoter. Then, Cre excises FLPO and Dre transgenes, whereas FLPO excises Cre and Dre transgenes. In addition, Dre excises FLPO and Cre transgenes. Using AAVs, Cre induces mCherry expression and FLPO induces YFP expression. However, Dre as a shadow fighter did not induce expression of transgenes.
(B) (Left) A representative maximum intensity projection image of dentate gyrus infected with BATTLE-2 system when the ratio of Dre:Cre:FLPO was 1:1:1. (Right) Quantification of cell number of neurons expressing fluorescent protein in the dentate gyrus. Data are represented as mean ± SEM.
(C) (Left) Representative maximum intensity projection image of dentate gyrus infected with BATTLE-2 system when the ratio of Dre:Cre:FLPO was 200:1:10. (Right) Quantification of cell number of neurons expressing fluorescent protein in the dentate gyrus. Data are represented as mean ± SEM.
(D) Representative maximum intensity projection image of dentate gyrus infected with conventional virus AAV-CAG-GFP. Note that the morphologies of mossy cells are unclear.
(E) Representative maximum intensity projection image of dentate gyrus infected with BATTLE-2 (double sparse). Note that the morphologies of multiple mossy cells are clear using BATTLE-2 double sparse expression.
Three weeks later, we injected Cre-dependent AAV expressing mCherry and FLPO-dependent one expressing YFP in the same region. YFP and mCherry were co-expressed with CamKIIa proteins in hippocampal neurons under the control of the CamKIIa (1.3 kb) promoter, indicating their specific expression in excitatory neurons (Figure S3). We observed splitting allocation of mCherry and YFP in the dentate gyrus (Figure 2B, mCherry+:63.1 ± 10.2, YFP+:13.9 ± 1.8, and mCherry-YFP double+:0.3 ± 0.2, mean ± SEM, number of expressing cells/400 μm2, n = 618 granule neurons in the dentate gyrus, N = 3 mice) and CA2-3 (mCherry+:39.8 ± 8.5, YFP+:5.1 ± 1.4, and mCherry-YFP double+:0.6 ± 0.4, mean ± SEM, number of expressing cells/400 μm2, n = 364 pyramidal neurons in CA2-3, N = 3 mice). Furthermore, we observed similar splitting allocation of mCherry and YFP in dentate gyrus with different dosages of mixed lentiviruses: 500 nL dosage, mCherry+:YFP+:mCherry-YFP double+ = 73.3% ± 4.1%:26.3% ± 4.1%:0.5% ± 0.3%, mean ± SEM, n = 569 granule neurons, N = 4 mice; 1,000 nL dosage, mCherry+:YFP+:mCherry-YFP double+ = 81.1% ± 1.7%:18.9% ± 1.7%:0.2% ± 0.2%, mean ± SEM, n = 618 granule neurons, N = 3 mice; 1,500 nL dosage, mCherry+:YFP+:mCherry-YFP double+ = 80.8% ± 2.5%:18.5% ± 2.4%:0.7 ± 0.3%, mean ± SEM, n = 674 granule neurons, N = 3 mice (Figure S4).
To achieve multiple sparse allocation of transgenes, we injected a lentivirus encoding Cre flanked with FRT5-rox, one encoding FLPO flanked with loxP-rox sites, and another encoding Dre transgene flanked by two FRT5-loxP sites (Cre:FLPO:Dre = 1:10:200), following Cre- and FLPO-dependent AAV injection. BATTLE-2 induced intense and double sparse allocation of mCherry and YFP in granule neurons of the dentate gyrus (Figure 2C, mCherry+:3.4 ± 0.9, YFP+:4.9 ± 0.8, and mCherry-YFP double+: 0, mean ± SEM, number of expressing cells/400 μm2, n = 66 granule neurons in dentate gyrus, N = 4 mice) and pyramidal neurons in the CA2-3 (mCherry+:1.1 ± 0.4, YFP+:1.9 ± 0.3, and mCherry-YFP double+:0, mean ± SEM, number of expressing cells/400 μm2, n = 14 pyramidal neurons in CA2-3, N = 6 mice).
In contrast to the excessively dense green protein fluorescence (GFP) expression with unclear cell morphologies induced by conventional viruses (Figure 2D), dendritic and axonal morphologies were strongly, sparsely, and clearly labeled using the BATTLE-2 technology (Figure 2E and Video S1).
A representative maximum intensity projection image of the hippocampus infected by BATTLE-2. Blue, green, and red show DAPI, YFP, and mCherry, respectively. Note that BATTLE-2 technology multi-sparsely, clearly, and brightly labeled the somata, dendrites, and axons of granule neurons.
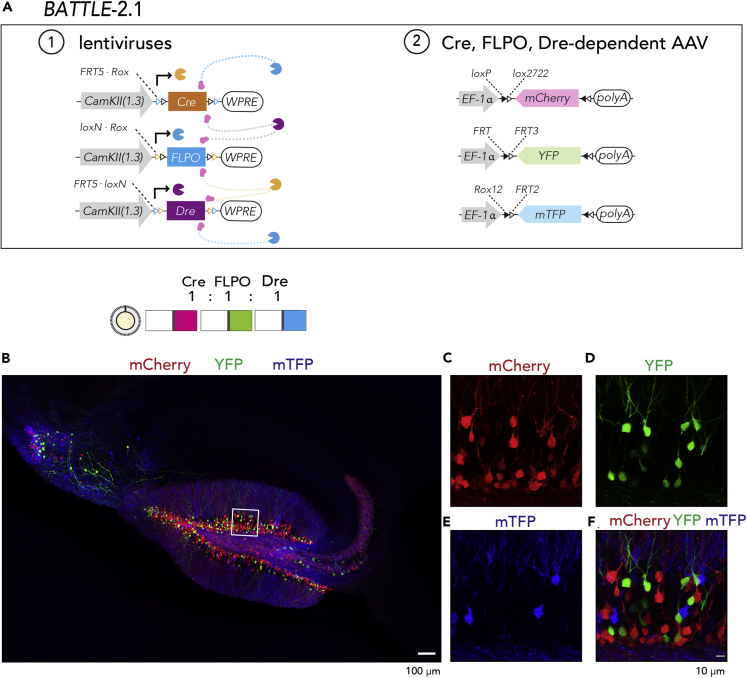
Furthermore, we created Dre-dependent AAV expressing mTFP (Chuang et al., 2015, Ai et al., 2006). To confirm Dre-specific recombination of Dre-dependent AAV, crosstalk activities between Cre, FLPO, and Dre were examined. We did not observe crosstalk activities in our experimental conditions (Figure S5).
Using BATTLE-2 with this AAV, we observed triple splitting allocations of mTFP, YFP, and mCherry in granule neurons of the dentate gyrus and pyramidal neurons in the CA2-3 and called this BATTLE-2.1: 500 nL dosage, mCherry+:mTFP+: YFP+:mTFP-YFP double+:mTFP-mCherry double+:mCherry-YFP double+:triple+ = 62.3% ± 2.4%:18.5% ± 1.8%:18.5% ± 1.7%:0%:0.4% ± 0.2%:0.2% ± 0.2%:0%, mean ± SEM, n = 740 granule neurons, N = 4 mice; 1,000 nL dosage, mCherry+:mTFP+: YFP+:mTFP-YFP double+:mTFP-mCherry double+:mCherry-YFP double+:triple+ = 73.4% ± 2.8%:13.7% ± 1.5%:12.4% ± 1.8%:0%:0.4% ± 0.2%:0.3% ± 0.2%:0%, mean ± SEM, n = 910 granule neurons, N = 4 mice; 1,500 nL dosage, mCherry+:mTFP+: YFP+:mTFP-YFP double+:mTFP-mCherry double+:mCherry-YFP double+:triple+ = 71.6% ± 3%:11.7% ± 1.8%:16.2% ± 1.9%:0%:0.1% ± 0.1%:0.4% ± 0.2%:0%, mean ± SEM, n = 1,127 granule neurons, N = 4 mice (Figure 3, Figure S6).
Figure 3.
BATTLE-2.1: Triple Splitting Allocation of Transgenes Using the Battle of the Recombinases
(A) In BATTLE-2.1 technology, Cre, FLPO, and Dre are expressed under the control of CamKIIa promoter. Cre excises FLPO and Dre transgenes, whereas FLPO excises Cre and Dre transgenes. Dre excises FLPO and Cre transgenes. Using AAVs, Cre induces mCherry expression and FLPO induces YFP expression. In addition, Dre induces mTFP expression.
(B) A representative maximum intensity projection image of hippocampus infected with the BATTLE-2.1 system when the ratio of Dre:Cre:FLPO was 1:1:1. Scale bar, 100 um.
(C) Magnified maximum intensity projection image (6 μm thickness) of the boxed area in (B). Red color shows mCherry expression.
(D) Magnified maximum intensity projection image of the boxed area in (B). Yellow color shows YFP expression.
(E) Magnified maximum intensity projection image of the boxed area in (B). Blue color shows mTFP expression.
(F) Superposed maximum intensity projection image of the boxed area in (B). Red, yellow, and blue show mCherry, YFP and mTFP, respectively. Scale bar, 10 μm.
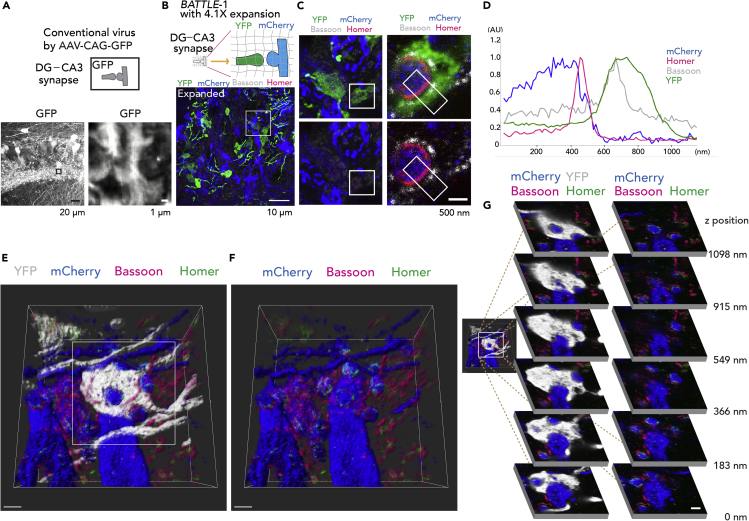
High-Resolution Imaging of Whole Synaptic Structures Using Multicolor Immunohistochemistry with BATTLE-1EX
Conventional methods using AAV-expressing GFP did not clearly reveal whole synaptic structures (Figure 4A). To visualize whole structures of synaptic connections clearly, we combined BATTLE-1 with ExM (BATTLE-1EX), which is a high-resolution method (Figure 4B, top). Hippocampal slices infected by BATTLE-1 were expanded linearly by 4.1 times (Figure 4B bottom, Figure S7, 4.1 ± 0.0, mean ± SEM, n = 5 slices). Therefore, a lens with a diffraction limit of ∼280 nm (60X, 1.2 numerical aperture water objective from Olympus) would attain an effective resolution of ∼280 nm/4.1 ≈ 70 nm. In this area (44.3 μm × 44.3 μm × 6.8 μm), we observed that 14 YFP-positive presynaptic terminals stochastically formed the whole synapse with mCherry-positive postsynaptic spines of CA3 neurons. Other cases consisting of mCherry-positive presynaptic terminals with YFP-positive postsynaptic spines were also observed (see Figure 5B).
Figure 4.
BATTLE-1EX: Three-Dimensional (3D) High-Resolution Imaging of Whole Synaptic Structures
(A) (Top) Illustration of DG-CA3 synapse visualized using AAV-CAG-GFP. (Left) Representative maximum intensity projection image in the CA3 region infected with conventional virus AAV-CAG-GFP. (Right) Magnified confocal image of boxed area to the left. Note unclear morphologies of whole synaptic structures of DG-CA3 synapses.
(B) (Top) Illustration showing BATTLE-1EX. BATTLE-1-infected slices are expanded using 4.1× expansion microscopy (ExM). (Bottom) Representative maximum intensity projection image of CA3 stratum lucidum of BATTLE-1EX slices.
(C) (Top left) Magnified maximum intensity projection image of boxed area in (B). (Top, right) Magnified confocal images of boxed area in (C) top left.
(D) Cross-sectional line profiles of fluorescent intensities in boxed region in (C) right.
(E) Representative volume-rendering 3D image of the whole synaptic structure of DG-CA3 synapses. Scale bar, 732 nm.
(F) Same volume-rendering 3D image in (D) without YFP.
(G) (Left) Magnified images of (E). (Right) the sequential z stack images of the boxed area in (left). Scale bar, 500 nm.
Figure 5.
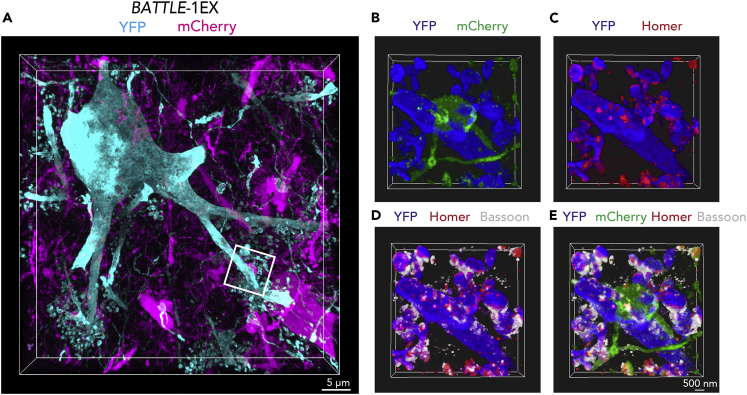
BATTLE-1EX: Three-Dimensional (3D) High-Resolution Imaging of Hilar Mossy Cells in the Hippocampus
(A) Representative maximum intensity projection 3D image of hilar mossy cells of BATTLE-1EX.
(B–E) Volume-rendering 3D image of box area in (A). Blue, Green, Red, and White show YFP, mCherry, Homer, and Bassoon respectively. Note that mCherry-positive presynaptic terminals form a whole synapse with YFP-positive postsynaptic spines of a mossy cell in the hippocampus.
Using BATTLE-1EX, whole synaptic structures consisting of YFP-positive presynaptic terminals from the dentate gyrus and mCherry-positive postsynaptic spines of CA3 pyramidal neurons were clearly visualized (Figures 4C and S8). Furthermore, the localizations of Bassoon and Homer, which are presynaptic and postsynaptic proteins, respectively, were also simultaneously identified in whole synaptic structures (Figure 4C and 4D). We performed volume rendering, offering 3D whole synaptic structures and 3D localization of Bassoon and Homer of DG-CA3 synapses in the hippocampus (Figures 4E and 4F, Video S2). High-resolution imaging of the whole synaptic structures was performed at sequential z stacks (Figure 4G). We further performed volume rendering (Figures 5A–5E) to visualize the 3D high-resolution whole synaptic structures of synapses from granule neurons to hilar mossy cells.
Representative volume-rendering 3D movie shows whole synaptic structure of DG-CA3 synapse. White, blue, red, and green show YFP, mCherry, Bassoon, and Homer, respectively. Scale bar, 1.2 μm.
Discussion
The concept, battle of transgenes, offers a theoretical and experimental base for the progress of research in the various scientific fields including life science, biotechnology, bioengineering technology, and artificial intelligence technology. BATTLE-recombinase systems, generated on this concept, are transgenic strategies for wide-scale studies of cellular and synaptic interactions in the life sciences and have two key advantages. First, they allow genetically engineered recombinases to “fight,” and only the winning recombinase survives in the infected cells to induce its specific transgene allocation. Unlike other systems (Zong et al., 2005, Saunders et al., 2012, Lao et al., 2012, Tasic et al., 2012, Pontes-Quero et al., 2017), the subsequent mutually exclusive transgene allocation is flexible and tunable by simply changing the ratio of mixed BATTLE-recombinase viruses without re-designing basic constructs of the viral vectors. The interleaved strategy is based on the competition between two recombinases and is useful for splitting transgene expression in transgenic animals, but it has less flexibility than a battle strategy and lacks tunability (He et al., 2017). BATTLE-recombinase systems have considerable potential to be applied to functional studies such as optogenetics (Fenno et al., 2011, Boyden, 2011, Kohara et al., 2014) and pharmacogenetics (Roth, 2016).
In contrast, other systems using blended multiple transgenes such as Brainbow systems (Livet et al., 2007, Weissman and Pan, 2015, Sakaguchi et al., 2018) are not suitable for these applications. The other combinatorial targeting strategies and tools are useful and versatile for functional and developmental studies of a small population of cells, like inhibitory neurons (He et al., 2016). However, they do not have either the split-tunability or sparseness-tunability of multiple transgene allocation, in contrast to BATTLE-recombinase systems. In addition, they are mainly based on double- or triple-transgenic mice, which are more expensive, and it takes a relatively long time to start experiments. Second, BATTLE-recombinase strategies produce flexible, intense, and tunable multiple sparse allocation of transgenes. Other sparse expression systems have limited expression levels (Zong et al., 2005, Tasic et al., 2012, Young et al., 2008), flexibility (Zong et al., 2005, Saunders et al., 2012, Young et al., 2008, Luo et al., 2016, Mikuni et al., 2016), and applicability; mastering the techniques is difficult (dal Maschio et al., 2012, Schubert et al., 2018). Notably, BATTLE-recombinase strategies have high versatility and are independent of leaky feedback expression systems based on the TetO system (Luo et al., 2016, Lin et al., 2018). Consequently, these systems have the potential to be combined with large-scale conditional TetO transgenic mice (Lewandoski, 2001).
Cre and FLP systems are widely popular, and over 1,000 Cre- and FLP-dependent AAV vectors and numerous pre-made Cre-dependent AAVs are commercially available from companies and institutions (such as Addgene). Remarkably, the BATTLE-recombinase system can be quickly applied and widely custom-built to standard and pre-existing Cre- and FLP-dependent AAV resources, offering a huge variety. In addition, there are also considerable floxed transgenic mouse resources for region-specific knockout studies (Lewandoski, 2001) and single-cell gene knockout studies (Kohara et al., 2007, Young et al., 2008, Luo et al., 2016, Mikuni et al., 2016). BATTLE-2 technology has great potential to be applied to single-cell gene knockout studies.
We combined the BATTLE-recombinase strategy with ExM to create BATTLE-1EX that enabled 3D high-resolution imaging of whole synaptic structures. Brainbow technology using multiple colors is mostly incompatible with immunohistochemistry of endogenous proteins and synaptic proteins because it uses multiple colors (Livet et al., 2007, Weissman and Pan, 2015, Sakaguchi et al., 2018). Therefore, it is insufficient to perform unambiguous identifications and dissections of whole synaptic structures and synaptic proteins. In this study, we visualized and dissected the whole synaptic structures of local circuits in the hippocampus (DG-CA3 circuits and DG-mossy cell circuits), and BATTLE-1EX has the potential to be extended to other regions.
Many brain diseases are known to be accompanied by abnormal morphologies in dendrites (Irie et al., 2014) and synaptic structures (van Spronsen and Hoogenraad, 2010, Penzes et al., 2011). However, considerable efforts have been made to develop technologies for visualizing whole synaptic structures using light microscopy to understand the functions of normal mouse brains as well as the pathogenic mechanisms in the brains of disease mouse models. BATTLE-recombinase technologies could provide opportunities for the dissections of whole synaptic structures of the local circuits of mouse models of disease.
Recently developed and growing all-optical electrophysiology technologies (Hochbaum et al., 2014, Kannan et al., 2018, Fan et al., 2018) have great potential to reveal electrophysiological interactions between neurons in the local circuits. Because these technologies require mutually exclusive transgene expressions in many cases (Kannan et al., 2018, Fan et al., 2018), more flexible and tunable associated technologies might further facilitate the expansion of their use. Therefore, BATTLE-recombinase strategies might have a considerable and flexible potential to promote all-optical electrophysiology studies.
For a wide range of studies in the life sciences, both the CamKIIa promoter and other systems such as synapsin and actin promoters (Chan et al., 2017, Huda et al., 2014) could be applied to BATTLE-recombinase. In mouse studies, region-specific transgenic mice are well developed, but require large animal facilities and are expensive. Furthermore, the region-specific mice are insufficient to understand the homo relationship of intra-regions and intra-cell types. The BATTLE-recombinase systems might offer more flexible approaches to understanding the local circuits and cell interactions in the regions and cell groups.
Limitations of the Study
We acknowledge that in the current study the BATTLE-recombinase systems could be applied to local regions, like the hippocampus, but not global regions. Future studies may update BATTLE-recombinase systems to enable split-tunable allocations of multiple transgenes at the whole-body level.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Keigo Kohara (koharake@hirakata.kmu.ac.jp).
Materials Availability
All unique/stable reagents generated in this study are available from the Lead Contact with a completed Materials Transfer Agreement.
Data and Code Availability
This study did not generate/analyze datasets and code.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank Y. Tatsumi, T. Unzai, E. Oiki, K. Nitta, A. Konno, A. Onishi, and N. McCullough for their assistance with the experiments. We thank H. Matsuda for her encouragement. This research was partially supported by the program for Brain Mapping by Integrated Neurotechnologies for Disease Studies (Brain/MINDS) from the Japan Agency for Medical Research and Development, AMED under the Grant Number JP19 dm0207057. This study was supported by Center for Medical Research and Education, Graduate School of Medicine, Osaka University, and grants from JSPS (16H03304, 19H03534, and 19K22583) and the Takeda Science Foundation to K.K.
Author Contributions
K.K. conceived, designed, and performed the BATTLE-recombinase experiments. A.I., T.K., and K.K. performed the ExM experiments. H.H. packaged the lentiviruses and AAVs. K.K. and M.M. performed the viral molecular experiments. Y.N., R.B., and C.K. performed the experiments using mice. K.K, C.K., and A.I. performed quantitative analysis. K.K and A.I. wrote the manuscript. K.K. supervised the entire project.
Declaration of Interests
The authors declare no competing interests.
Published: June 26, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101248.
Supplemental Information
References
- Ai H.W., Henderson J.N., Remington S.J., Campbell R.E. Directed evolution of a monomeric, bright and photostable version of Clavularia cyan fluorescent protein: structural characterization and applications in fluorescence imaging. Biochem. J. 2006;400:531–540. doi: 10.1042/BJ20060874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastassiadis K., Fu J., Patsch C., Hu S., Weidlich S., Duerschke K., Buchholz F., Edenhofer F., Stewart A.F. Dre recombinase, like Cre, is a highly efficient site-specific recombinase in E. coli, mammalian cells and mice. Dis. Model Mech. 2009;2:508–515. doi: 10.1242/dmm.003087. [DOI] [PubMed] [Google Scholar]
- Arenkiel B.R., Ehlers M.D. Molecular genetics and imaging technologies for circuit-based neuroanatomy. Nature. 2009;461:900–907. doi: 10.1038/nature08536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano S.M., Gao R., Wassie A.T., Tillberg P.W., Chen F., Boyden E.S. Expansion microscopy: protocols for imaging proteins and RNA in cells and tissues. Curr. Protoc. Cell Biol. 2018;80:e56. doi: 10.1002/cpcb.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson D.L., Isackson P.J., Gall C.M., Jones E.G. Contrasting patterns in the localization of glutamic acid decarboxylase and Ca2+/calmodulin protein kinase gene expression in the rat central nervous system. Neuroscience. 1992;46:825–849. doi: 10.1016/0306-4522(92)90188-8. [DOI] [PubMed] [Google Scholar]
- Boyden E.S. A history of optogenetics: the development of tools for controlling brain circuits with light. F1000 Biol. Rep. 2011;3:11. doi: 10.3410/B3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K.Y., Jang M.J., Yoo B.B., Greenbaum A., Ravi N., Wu W.L., Sánchez-Guardado L., Lois C., Mazmanian S.K., Deverman B.E. Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat. Neurosci. 2017;20:1172–1179. doi: 10.1038/nn.4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Tillberg P.W., Boyden E.S. Expansion microscopy. Science. 2015;347:543–548. doi: 10.1126/science.1260088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang K., Nguyen E., Sergeev Y., Badea T.C. Novel heterotypic rox sites for combinatorial Dre recombination strategies. G3 (Bethesda) 2015;6:559–571. doi: 10.1534/g3.115.025841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dal Maschio M., Ghezzi D., Bony G., Alabastri A., Deidda G., Brondi M., Sato S.S., Zaccaria R.P., Di Fabrizio E., Ratto G.M. High-performance and site-directed in utero electroporation by a triple-electrode probe. Nat. Commun. 2012;3:960. doi: 10.1038/ncomms1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawkins R. Oxford University Press; 1976. The Selfish Gene. [Google Scholar]
- Fan L.Z., Nehme R., Adam Y., Jung E.S., Wu H., Eggan K., Arnold D.B., Cohen A.E. All-optical synaptic electrophysiology probes mechanism of ketamine-induced disinhibition. Nat. Methods. 2018;15:823–831. doi: 10.1038/s41592-018-0142-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenno L., Yizhar O., Deisseroth K. The development and application of optogenetics. Annu. Rev. Neurosci. 2011;34:389–412. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Nicola D., Riecken K., Fehse B., Perry V.H. In-vivo RGB marking and multicolour single-cell tracking in the adult brain. Sci. Rep. 2014;4:7520. doi: 10.1038/srep07520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner B.M. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- He M., Tucciarone J., Lee S., Nigro M.J., Kim Y., Levine J.M., Kelly S.M., Krugikov I., Wu P., Chen Y. Strategies and tools for combinatorial targeting of GABAergic neurons in mouse cerebral cortex. Neuron. 2016;91:1228–1243. doi: 10.1016/j.neuron.2016.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L., Li Y., Li Y., Pu W., Huang X., Tian X., Wang Y., Zhang H., Liu Q., Zhang L. Enhancing the precision of genetic lineage tracing using dual recombinases. Nat. Med. 2017;23:1488–1498. doi: 10.1038/nm.4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochbaum D.R., Zhao Y., Farhi S.L., Klapoetke N., Werley C.A., Kapoor V., Zou P., Kralj J.M., Maclaurin D., Smedemark-Margulies N. All-optical electrophysiology in mammalian neurons using engineered microbial rhodopsins. Nat. Methods. 2014;11:825–833. doi: 10.1038/nmeth.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huda F., Konno A., Matsuzaki Y., Goenawan H., Miyake K., Shimada T., Hirai H. Distinct transduction profiles in the CNS via three injection routes of AAV9 and the application to generation of a neurodegenerative mouse model. Mol. Ther. Methods Clin. Dev. 2014;6:14032. doi: 10.1038/mtm.2014.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie T., Matsuzaki Y., Sekino Y., Hirai H. Kv3.3 channels harbouring a mutation of spinocerebellar ataxia type 13 alter excitability and induce cell death in cultured cerebellar Purkinje cells. J. Physiol. 2014;592:229–247. doi: 10.1113/jphysiol.2013.264309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan M., Vasan G., Huang C., Haziza S., Li J.Z., Inan H., Schnitzer M.J., Pieribone V.A. Fast, in vivo voltage imaging using a red fluorescent indicator. Nat. Methods. 2018;15:1108–1116. doi: 10.1038/s41592-018-0188-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohara K., Yasuda H., Huang Y., Adachi N., Sohya K., Tsumoto T. A local reduction in cortical GABAergic synapses after a loss of endogenous brain-derived neurotrophic factor, as revealed by single-cell gene knock-out method. J. Neurosci. 2007;27:7234–7244. doi: 10.1523/JNEUROSCI.1943-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohara K., Pignatelli M., Rivest A.J., Jung H.Y., Kitamura T., Suh J., Frank D., Kajikawa K., Mise N., Obata Y. Cell type-specific genetic and optogenetic tools reveal hippocampal CA2 circuits. Nat. Neurosci. 2014;17:269–279. doi: 10.1038/nn.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lao Z., Raju G.P., Bai C.B., Joyner A.L. Mosaic mutant Analysis with Spatial and Temporal control of Recombination (MASTR): a new technique for determining the fate of mutant cells using conditional floxed alleles in mice. Cell Rep. 2012;2:386–396. doi: 10.1016/j.celrep.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandoski M. Conditional control of gene expression in the mouse. Nat. Rev. Genet. 2001;2:743–755. doi: 10.1038/35093537. [DOI] [PubMed] [Google Scholar]
- Lin R., Wang R., Yuan J., Feng Q., Zhou Y., Zeng S., Ren M., Jiang S., Ni H., Zhou C. Cell-type-specific and projection-specific brain-wide reconstruction of single neurons. Nat. Methods. 2018;15:1033–1036. doi: 10.1038/s41592-018-0184-y. [DOI] [PubMed] [Google Scholar]
- Livet J., Weissman T.A., Kang H., Draft R.W., Lu J., Bennis R.A., Sanes J.R., Lichtman J.W. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature. 2007;450:56–62. doi: 10.1038/nature06293. [DOI] [PubMed] [Google Scholar]
- Luo W., Mizuno H., Iwata R., Nakazawa S., Yasuda K., Itohara S., Iwasato T. Supernova: a versatile vector system for single-cell labeling and gene function studies in vivo. Sci. Rep. 2016;6:35747. doi: 10.1038/srep35747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikuni T., Nishiyama J., Sun Y., Kamasawa N., Yasuda R. High-throughput, high-resolution mapping of protein localization in mammalian brain by in vivo genome editing. Cell. 2016;165:1803–1817. doi: 10.1016/j.cell.2016.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A. Cre recombinase: the universal reagent for genome tailoring. Genesis. 2000;26:99–109. [PubMed] [Google Scholar]
- Nimchinsky E.A., Sabatini B.L., Svoboda K. Structure and function of dendritic spines. Annu. Rev. Physiol. 2002;64:313–353. doi: 10.1146/annurev.physiol.64.081501.160008. [DOI] [PubMed] [Google Scholar]
- Penzes P., Cahill M.E., Jones K.A., VanLeeuwen J.E., Woolfrey K.M. Dendritic spine pathology in neuropsychiatric disorders. Nat. Neurosci. 2011;14:285–293. doi: 10.1038/nn.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontes-Quero S., Heredia L., Casquero-García V., Fernández-Chacón M., Luo W., Hermoso A., Bansal M., Garcia-Gonzalez I., Sanchez-Muñoz M.S., Perea J.R. Dual ifgMosaic: a versatile method for multispectral and combinatorial mosaic gene-function analysis. Cell. 2017;170:800–814. doi: 10.1016/j.cell.2017.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochefort N.L., Konnerth A. Dendritic spines: from structure to in vivo function. EMBO Rep. 2012;13:699–708. doi: 10.1038/embor.2012.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth B.L. DREADDs for neuroscientists. Neuron. 2016;89:683–694. doi: 10.1016/j.neuron.2016.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi R., Leiwe M.N., Imai T. Bright multicolor labeling of neuronal circuits with fluorescent proteins and chemical tags. Elife. 2018;7:e40350. doi: 10.7554/eLife.40350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders A., Johnson C.A., Sabatini B.L. Novel recombinant adeno-associated viruses for Cre activated and inactivated transgene expression in neurons. Front. Neural Circuits. 2012;6:47. doi: 10.3389/fncir.2012.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlake T., Bode J. Use of mutated FLP recognition target (FRT) sites for the exchange of expression cassettes at defined chromosomal loci. Biochemistry. 1994;33:12746–12751. doi: 10.1021/bi00209a003. [DOI] [PubMed] [Google Scholar]
- Schubert R., Trenholm S., Balint K., Kosche G., Cowan C.S., Mohr M.A., Munz M., Martinez-Martin D., Fläschner G., Newton R. Virus stamping for targeted single-cell infection in vitro and in vivo. Nat. Biotechnol. 2018;36:81–88. doi: 10.1038/nbt.4034. [DOI] [PubMed] [Google Scholar]
- van Spronsen M., Hoogenraad C.C. Synapse pathology in psychiatric and neurologic disease. Curr. Neurol. Neurosci. Rep. 2010;10:207–214. doi: 10.1007/s11910-010-0104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof T.C., Malenka R.C. Understanding synapses: past, present, and future. Neuron. 2008;60:469–476. doi: 10.1016/j.neuron.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasic B., Miyamichi K., Hippenmeyer S., Dani V.S., Zeng H., Joo W., Zong H., Chen-Tsai Y., Luo L. Extensions of MADM (mosaic analysis with double markers) in mice. PLoS One. 2012;7:e33332. doi: 10.1371/journal.pone.0033332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillberg P.W., Chen F., Piatkevich K.D., Zhao Y., Yu C.C., English B.P., Gao L., Martorell A., Suk H.J., Yoshida F. Protein-retention expansion microscopy of cells and tissues labeled using standard fluorescent proteins and antibodies. Nat. Biotechnol. 2016;34:987–992. doi: 10.1038/nbt.3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman T.A., Pan Y.A. Brainbow: new resources and emerging biological applications for multicolor genetic labeling and analysis. Genetics. 2015;199:293–306. doi: 10.1534/genetics.114.172510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young P., Qiu L., Wang D., Zhao S., Gross J., Feng G. Single-neuron labeling with inducible cre-mediated knockout in transgenic mice. Nat. Neurosci. 2008;11:721–728. doi: 10.1038/nn.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong H., Espinosa J.S., Su H.H., Muzumdar M.D., Luo L. Mosaic analysis with double markers in mice. Cell. 2005;121:479–942. doi: 10.1016/j.cell.2005.02.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A representative maximum intensity projection image of the hippocampus infected by BATTLE-2. Blue, green, and red show DAPI, YFP, and mCherry, respectively. Note that BATTLE-2 technology multi-sparsely, clearly, and brightly labeled the somata, dendrites, and axons of granule neurons.
Representative volume-rendering 3D movie shows whole synaptic structure of DG-CA3 synapse. White, blue, red, and green show YFP, mCherry, Bassoon, and Homer, respectively. Scale bar, 1.2 μm.
Data Availability Statement
This study did not generate/analyze datasets and code.