Abstract
Background:
We studied the relationship between resting heart rate (HR), chronotropic response to exercise, and clinical outcomes in patients with heart failure (HF) across the spectrum of left ventricle ejection fraction (LVEF).
Methods and Results:
Resting HR and chronotropic index (CIx) were assessed in 718 patients with HF (53 ± 14 years of age, 66% male) referred for exercise testing. Associations with the composite outcome of left ventricular assist device implantation, transplantation, or death (151 events, 4.4 [range 3.0 – 5.8] years of follow-up) were assessed with the use of Cox models adjusted for age, sex, HF etiology, diabetes, LVEF, beta-blocker use, device therapy, estimated glomerular filtration rate, and peak oxygen uptake. Resting HR was 73 ± 15 beats/min, CIx was 0.60 ± 0.26, LVEF was 34% ± 15%, and 39% had an LVEF 40%. Resting HR correlated poorly with CIx (r = 0.08; P = .04) and did not predict (P = .84) chronotropic incompetence (CIx <0.60). Both higher resting HR (per 5 beats/min increase: adjusted hazard ratio [HR] – 1.05, 95% confidence interval [CI] 1.00 − 1.11) and CIx (per SD change: adjusted HR–0.77, 95% CI 0.62–0.94) were independent prognostic markers. No heterogeneity of effect was noted based on LVEF (P >.05).
Conclusion:
Higher resting HR and lower CIx are both associated with more severe HF, but correlated poorly with each other. They provide independent and additive prognostic information in HF across the LVEF spectrum.
Keywords: heart failure, heart rate, chronotropic index, exercise, prognosis
Higher resting heart rate (HR) and increases in HR over time predict adverse outcomes in patients with heart failure (HF).1–3 Resting HR is considered to be a modifiable risk factor because pharmacologic treatments that lower HR also reduce cardiovascular events in patients with HF.4,5 Chronotropic incompetence, broadly understood as an abnormal HR response to exercise, is commonly found in patients with HF and is associated with greater functional limitation and worse outcomes in this syndrome.6 However, there are conflicting data regarding its independent prognostic value in HF.7–10 Between-study differences in the definition of chronotropic incompetence as well as differences in the HF populations studied may partially explain these discordant findings. Despite the bulk of evidence on each of these HR-related variables individually, data on how resting HR and chronotropic response to exercise relate to each other regarding prognosis are limited. Although chronotropic incompetence is one of the most consistent pathophysiologic abnormalities described in patients with HF with preserved ejection fraction (HFpEF)11,12 and correlates with functional impairment,13 most prognostic studies of chronotropic response evaluated patients with significantly reduced left ventricular ejection fraction (LVEF). We studied the relationship between resting HR and chronotropic response to exercise and examined their prognostic value in patients with HF across the LVEF spectrum.
Methods
Study Population
This study included 946 patients with a diagnosis of HF referred for cardiopulmonary exercise testing (CPET) with the use of a cycle ergometer at 1 referral quaternary care hospital from July 2007 to June 2013. We excluded 191 patients because they had ventricular pacing at rest and 37 patients with a submaximal test defined by a peak respiratory exchange ratio <1.0. Patient demographics, coexisting medical conditions, current medications, exercise variables, and gas-exchange variables were prospectively collected, and retrospective chart review was performed for additional clinical characteristics (see below) and laboratory values at the time of testing. The Partners Human Research Committee approved this study and waived the requirement for informed consents.
Clinical Variable Definition
Ischemic cardiomyopathy etiology was defined based on chart review. Symptomatic status was defined if patients were in New York Heart Association (NYHA) functional class 2 or greater as determined by the referring physician or had a history of hospitalization for decompensated HF. Antiarrhythmic medications included amiodarone or digoxin. Glomerular filtration rate was estimated (eGFR) with the use of the CKD-EPI formula,14 with chronic kidney disease defined as eGFR <60 mL min−1 1.73 m−2. LVEF data was abstracted from the transthoracic echocardiography report that was most contemporaneous to the CPET date (median time difference 0 days, interquartile range [IQR] 0 – 10 days). Anemia was defined if plasma hemoglobin concentration was <12 g/dL in women and 13 g/dL in men. Medication use was assessed through patient self-report at the time of the exercise test. Angiotensin-converting enzyme inhibitor (ACEi) and angiotensin receptor blocker (ARB) were coded in a single variable (ACEi/ARB). Cardiac resynchronization therapy or implantable cardioverter-defibrillator were coded in a single variable (CRT/ICD). To estimate the degree of β-blockade, we calculated the percentage of the maximal guideline-recommended dose of β-blocker dosing prescribed at the time of CPET (Supplemental Table 1).15 No patient was on ivabradine at the time of CPET. Natriuretic peptide levels were not available or uniformly assessed in this population.
Exercise Protocol
All exercise tests were performed in the Brigham and Women’s Hospital cardiopulmonary exercise laboratory using an upright cycle ergometer (Lode, Groningen, Netherlands) with the subject breathing room air. Symptom-limited CPET was performed on all subjects. All pharmacologic therapy was continued before and through exercise testing. The equipment was calibrated daily in standard fashion with the use of reference gases. Minute ventilation (VE), oxygen uptake (VO2), and carbon dioxide output (VCO2) were acquired breath by breath and averaged over a 10-second interval, using a ventilatory expired gas analysis system (MGC Diagnostics, Saint Paul, Minnesota). Exercise ventilatory and gas exchange data were averaged over every 10-second interval. Peak VO2 was defined as the highest 10-second average VO2 during the last stage of the symptom-limited exercise test. VE/VCO2 slope was taken from rest to the gas exchange at peak exercise. Rhythm was monitored by means of continuous 12-lead electrocardiography.
Heart Rate Variables
Resting HR was measured in the supine position before the patient started exercise. Age-predicted maximal HR (APMHR) was estimated with the use of the Astrand formula16: 220 – age (years). Chronotropic index (CIx) was calculated as (peak HR – resting HR)/(APMHR – resting HR). Percentage of predicted peak HR was calculated as (peak HR/APMHR) × 100. Abnormal CIx was defined as <0.60 based on previous studies.7
Outcomes
The main outcome of this analysis was a composite of death, heart transplantation, or left ventricular assist device (LVAD) implantation. LVAD implantation and heart transplantation were assessed by means of chart review through December 2014. All-cause death was determined with the use of the National Death Index with complete follow-up through December 31, 2014.
Statistical Analysis
Continuous variables are expressed as mean ± SD for normally distributed data and as median (interquartile range [IQR]) for nonnormally distributed data. Categoric variables are expressed as n (%). Comparisons between groups were performed with the use of 2-sided unpaired or paired t tests and Wilcoxon rank-sum test for normally and nonnormally distributed data, respectively. Fisher exact test was applied to compare proportions. One-way analysis of variance with Bonferroni correction was used to perform multiple group comparisons. Correlations between hemodynamic and metabolic variables were determined with the use of Pearson and Spearman correlation for normally and nonnormally distributed data, respectively. Multivariable linear regression was used to study independent predictors of resting HR and CIx, and included all covariates that were significantly associated with the HR measures (P < .05) in univariate analysis. Covariates included in multivariable models for resting HR were age, sex, ischemic etiology, diabetes, β-blocker therapy, device therapy, eGFR, LVEF, and peak VO2. Covariates included in multivariable models for CIx were age, sex, LVEF, device therapy, eGFR, resting HR, and peak VO2.
We used univariate and multivariable Cox proportional hazard regression models to assess the unadjusted and adjusted association of resting HR and CIx with the composite outcome of LVAD implantation, heart transplantation, and all-cause mortality. Covariates for the multivariable model studying the prognostic significance of resting HR were selected by using a forward stepwise selection procedure (retention P < .20) with age, sex, β-blocker therapy, and peak VO2 forced into the model and with the use of baseline clinical variables that differed significantly across resting HR quartiles as candidate covariates. Covariates included in the multivariable analyses were age, sex, cardiomyopathy etiology, diabetes, LVEF, β-blocker use, presence of CRT/ICD, and peak VO2. The same methodology was used for CIx, and the following covariates were included in the final multivariate model: age, sex, eGFR, presence of CRT/ICD, resting HR, LVEF, β-blocker use, and peak VO2. The proportional hazards assumption was assessed by visual inspection of Schoenfeld residuals. We also performed 2 subgroup analysis: (1%) restricted to patients with LVEF ≥40%; (2%) restricted to patients in atrial fibrillation at the time of CPET. The incremental value of CIx when added to the clinical variables and resting HR, or when added to clinical variables, resting HR, and peak VO2, was assessed by comparing the C-statistic of the predictive models without versus with CIx. Statistical analysis was performed with the use of Stata software version 12.1 (Stata Corp, College Station, Texas). Continuous net reclassification improvement (NRI) and integrated discrimination improvement (IDI) associated with CIx was assessed for the composite end point at 2 years with the use of time-to-event data.17 A 2-sided P value of <0.05 was considered to be significant.
Results
Clinical Characteristics of Studied Patients
The 718 patients with HF in this analysis had a mean age of 53 ± 14 years, were predominantly male (66%) and white (83%), and had a mean LVEF of 34% ± 15% (Table 1). Hypertension was present in 56% and diabetes in 24%. Use of evidence-based therapy was high, including β-blocker treatment (83%), ACEi/ARB (78%), and CRT/ ICD (31%).
Table 1.
Clinical Characteristics and Exercise Data of Studied Population, According to Resting Heart Rate (HR; beats/min) Quartiles
| Overall | Resting HR Q1 | Resting HR Q2 | Resting HR Q3 | Resting HR Q4 | ||
|---|---|---|---|---|---|---|
| Characteristic | (n = 718) | (34–62; n= 186) | (63–70; n= 175) | (71–82; n= 191) | (82–136; n= 166) | P Value |
| Age, y | 53 ± 14 | 55 ± 14 | 53 ± 14 | 53 ± 14 | 50 ± 14 | .006 |
| Male sex | 477 (66%) | 131 (70%) | 113 (65%) | 119(62%) | 114 (69%) | .58 |
| Race | .10 | |||||
| White | 596 (83%) | 159 (86%) | 148 (85%) | 156 (82%) | 133 (80%) | |
| Black | 62 (9%) | 10 (5%) | 15 (9%) | 20(11%) | 17 (10%) | |
| Hispanic | 26 (4%) | 7 (4%) | 7 (4%) | 5 (3%) | 7 (4%) | |
| BMI, kg/m2 | 28.4 ± 5.9 | 27.9 ± 5.5 | 28.1 ± 5.4 | 28.4 ± 6.3 | 29.1 ± 6.2 | .05 |
| Cardiomyopathy | ||||||
| Ischemic | 148 (21%) | 50 (27%) | 42 (24%) | 32 (17%) | 24 (15%) | .001 |
| Symptomatic HF | 586 (82%) | 146 (79%) | 132 (75%) | 158 (83%) | 150 (90%) | .001 |
| NYHA functional class | .001 | |||||
| 1 | 253 (35%) | 68 (37%) | 75 (43%) | 65 (34%) | 45 (27%) | |
| 2 | 256 (36%) | 76 (41%) | 53 (30%) | 71 (37%) | 56 (34%) | |
| 3 | 173 (24%) | 37 (20%) | 41 (23%) | 43 (23%) | 52 (31%) | |
| 4 | 35 (5%) | 5 (3%) | 6 (3%) | 11 (6%) | 13 (8%) | |
| Comorbidities | ||||||
| Hypertension | 401 (56%) | 95 (51%) | 92 (53%) | 114 (60%) | 100 (60%) | .04 |
| Diabetes | 171 (24%) | 33 (18%) | 32(18%) | 54 (28%) | 52 (31%) | <0.001 |
| Coronary artery disease | 225 (31%) | 69 (37%) | 59 (34%) | 54 (28%) | 43 (26%) | .01 |
| Stroke | 53 (7%) | 21 (11%) | 13 (7%) | 9 (5%) | 10 (6%) | .03 |
| Atrial fibrillation | 192 (27%) | 62 (33%) | 41 (23%) | 51 (27%) | 38 (23%) | .06 |
| COPD | 57 (8%) | 11 (6%) | 13 (7%) | 16 (8%) | 17 (10%) | .13 |
| CKD | 148 (21%) | 40 (22%) | 37(21%) | 40(21%) | 31 (19%) | .54 |
| Treatment | ||||||
| β-Blockers | 596 (83%) | 162 (87%) | 147 (84%) | 158 (83%) | 129 (78%) | .02 |
| % of recommended β-blockade | 35 ± 28 | 38 ± 28 | 37 ± 29 | 34 ± 27 | 30 ± 27 | .006 |
| ACEi/ARBs | 562 (78%) | 145 (78%) | 138 (79%) | 146 (76%) | 133 (80%) | .79 |
| Diuretics | 439(61%) | 97 (52%) | 100 (57%) | 119(62%) | 123 (74%) | <.001 |
| MRA | 169 (24%) | 38 (20%) | 48 (27%) | 41 (22%) | 42 (25%) | .54 |
| Antiarrhythmic | 183 (26%) | 60 (32%) | 45 (26%) | 33 (17%) | 45 (27%) | .07 |
| CRT/ICD | 220 (31%) | 69 (37%) | 50 (29%) | 51 (27%) | 50 (30%) | .12 |
| Laboratory tests and echocardiography | ||||||
| Hemoglobin, g/dL | 13.5 ± 1.8 | 13.7 ± 1.6 | 13.4 ± 1.9 | 13.4 ± 1.7 | 13.6 ± 1.8 | .61 |
| Anemia | 168 (23%) | 40 (22%) | 36 (21%) | 49 (26%) | 43 (26%) | .20 |
| Creatinine, g/dL | 1.2 ± 1.0 | 1.2 ± 0.7 | 1.2 ± 1.0 | 1.2 ± 1.0 | 1.2 ± 1.1 | .82 |
| eGFR, mL. min−1·1.73 m−2 | 78 ± 25 | 76 ± 24 | 77 ± 24 | 78 ± 27 | 80 ± 25 | .15 |
| LVEF, % | 34 ± 15 | 40 ± 13 | 34 ± 13 | 35 ± 16 | 28 ± 14 | <.001 |
| CPET data | ||||||
| Resting SBP, mm Hg | 117 ± 20 | 121 ± 20 | 118 ± 21 | 117 ± 18 | 114 ± 19 | .02 |
| Resting DBP, mm Hg | 75 ± 11 | 74 ± 11 | 75 ± 12 | 75 ± 11 | 75 ± 11 | .80 |
| Peak SBP, mm Hg | 145 ± 29 | 151 ± 29 | 146 ± 30 | 143 ± 29 | 139 ± 29 | .004 |
| Peak DBP, mm Hg | 77 ± 12 | 76 ± 11 | 77 ± 13 | 76 ± 12 | 78 ± 13 | .63 |
| Resting HR, beats/min | 73 ± 15 | 57 ± 5 | 66 ± 2 | 75 ± 3 | 94 ± 10 | <0.001 |
| Peak HR, beats/min | 129 ± 27 | 119 ± 27 | 127 ± 28 | 130 ± 27 | 142 ± 21 | <.001 |
| Change in HR, beats/min | 57 ± 27 | 63 ± 28 | 61 ± 28 | 54 ± 26 | 48 ± 21 | <.001 |
| Peak HR, % of predicted | 77 ± 15 | 72 ± 14 | 76 ± 15 | 78 ± 15 | 84 ± 13 | <.001 |
| Chronotropic index | 0.60 ± 0.26 | 0.57 ± 0.22 | 0.60 ± 0.26 | 0.60 ± 0.28 | 0.64 ± 0.30 | .08 |
| Chronotropic index <0.60 | 379 (53%) | 107 (58%) | 89 (51%) | 97 (51%) | 86 (52%) | .51 |
| Peak RER | 1.21 ± 0.11 | 1.22 ± 0.12 | 1.21 ± 0.11 | 1.20 ± 0.11 | 1.21 ± 0.11 | .64 |
| PeakVO2, mL·min−1·kg−1 | 16.5 ± 6.4 | 18.3 ± 7.1 | 16.9 ± 6.6 | 16.0 ± 6.2 | 14.5 ± 4.6 | <.001 |
| Peak VO2, % pred | 64 ± 20 | 71 ± 19 | 66 ± 20 | 63 ± 20 | 57 ± 18 | <.001 |
| Peak O2 pulse, mL/beat | 11 ± 4 | 13 ± 4 | 11 ± 4 | 10.1 ± 3.4 | 8.9 ± 2.9 | <.001 |
| VE/VCO2 slope | 31.9 ± 7.9 | 29.7 ± 6.4 | 32.1 ± 9.0 | 32.1 ± 7.2 | 33.8 ± 8.7 | <.001 |
Values presented as mean ± SD or n (%). ACEi, angiotensin-converting-enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; DBP, diastolic blood pressure; FC, functional class; eGFR, estimatedglomerular filtration rate; HF, heart failure; HR, heart rate; ICD, implantable cardioverter-defibrillator; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; RER, respiratory exchange ratio; SBP, systolic blood pressure; VCO2, carbon dioxide output; VE, minute volume; VO2, oxygen uptake.
Predictors of Resting Heart Rate and Chronotropic Response to Exercise
The mean resting HR was 73 ± 15 beats/min. Patients with higher resting HR had worse NYHA functional class, higher prevalence of diuretic treatment, lower LVEF, higher prevalence of diabetes, and less likelihood of being on β-blockers or having CRT/ICD (Table 1). A higher resting HR was associated with lower peak VO2 and higher VE/ VCO2 slope (Table 1). In multivariate analysis, younger age, higher body mass index, symptomatic status, diabetes, lower LVEF, and absence of β-blocker treatment were independent predictors of a higher resting HR (Supplemental Table 2).
Mean CIx was 0.60 ± 0.26. Lower CIx was associated with lower LVEF, higher prevalence of comorbidities, and higher prevalence of β-blocker use (Table 2). At exercise testing, lower CIx was associated with lower peak VO2 and higher VE/VCO2 slope. Independent predictors of reduced chronotropic response were higher body mass index, symptomatic status, diabetes, lower eGFR, and lower resting HR, but not β-blocker treatment (Supplemental Table 3).
Table 2.
Clinical Characteristics and Exercise Data of Studied Population, According to Chronotropic Index (CIx) Quartiles
| Characteristic | Overall (n = 718) | CIx Q1 (0.03–0.41; n = 180) | CIx Q2 (0.41–0.58; n = 177) | CIx Q3 (0.58–0.78; n = 180) | CIx Q4 (0.78–1.84; n = 179) | P Value |
|---|---|---|---|---|---|---|
| Age, y | 53 ± 14 | 56 ± 14 | 52 ± 14 | 53 ± 14 | 52 ± 13 | .02 |
| Male sex | 477 (66%) | 132 (73%) | 109 (62%) | 124 (69%) | 111 (62%) | .09 |
| Race | .001 | |||||
| White | 596 (83%) | 147 (82%) | 128 (72%) | 160 (89%) | 159 (89%) | |
| Black | 62 (9%) | 14 (8%) | 29 (16%) | 10 (6%) | 9 (5%) | |
| Hispanic | 26 (4%) | 11 (6%) | 8 (5%) | 4 (2%) | 3 (2%) | |
| BMI, kg/m2 | 28.4 ± 5.9 | 29.2 ± 6.4 | 29.1 ± 6.0 | 27.9 ± 5.2 | 27.2 ± 5.5 | <.001 |
| Cardiomyopathy | ||||||
| Ischemic | 148 (21%) | 60 (33%) | 38 (22%) | 44 (24%) | 6 (3%) | <.001 |
| Symptomatic HF | 586 (82%) | 174 (97%) | 152 (86%) | 142 (79%) | 116(65%) | <.001 |
| NYHA functional class | <.001 | |||||
| 1 | 253 (35%) | 21 (12%) | 55 (31%) | 72 (40%) | 104 (58%) | |
| 2 | 256 (36%) | 68 (38%) | 70 (40%) | 63 (35%) | 55 (31%) | |
| 3 | 173 (24%) | 71 (39%) | 44 (25%) | 38 (21%) | 19(11%) | |
| 4 | 35 (5%) | 20(11%) | 7 (4%) | 7 (4%) | 1 (1%) | |
| Comorbidities | ||||||
| Hypertension | 401 (56%) | 122 (68%) | 108 (61%) | 87 (48%) | 83 (46%) | <.001 |
| Diabetes | 171 (24%) | 63 (35%) | 48 (27%) | 37 (21%) | 21 (12%) | .001 |
| Coronary artery disease | 225 (31%) | 83 (46%) | 58 (33%) | 58 (32%) | 26 (15%) | <.001 |
| Stroke | 53 (7%) | 20(11%) | 8 (5%) | 19(11%) | 6 (3%) | .05 |
| Atrial fibrillation | 192 (27%) | 71 (39%) | 31 (18%) | 43 (24%) | 47 (26%) | <.001 |
| COPD | 57 (8%) | 17 (9%) | 20(11%) | 19(11%) | 6 (3%) | .02 |
| CKD | 148 (21%) | 67 (37%) | 36 (20%) | 43 (24%) | 13 (7%) | <.001 |
| Treatment | ||||||
| β-Blockers | 596 (83%) | 155 (86%) | 159 (90%) | 151 (84%) | 131 (73%) | <.001 |
| % of recommended β-blockade | 35 ± 28 | 41 ± 30 | 33 ± 24 | 33 ± 28 | 31 ± 29 | .002 |
| ACEi/ARBs | 562 (78%) | 136 (76%) | 140 (79%) | 147 (82%) | 138 (77%) | .60 |
| Diuretics | 439 (61%) | 152 (84%) | 119(67%) | 98 (54%) | 69 (39%) | <.001 |
| MRA | 169 (24%) | 63 (35%) | 45 (25%) | 41 (23%) | 20(11%) | <.001 |
| Antiarrhythmic | 183 (26%) | 68 (38%) | 44 (25%) | 41 (23%) | 30 (17%) | <.001 |
| CRT/ICD | 220(31%) | 80 (44%) | 60 (34%) | 59 (33%) | 21 (12%) | <.001 |
| Laboratory tests and echocardiography | ||||||
| Hemoglobin, g/dL | 13.5 ± 1.8 | 13.1 ± 1.9 | 13.3 ± 1.7 | 13.7 ± 1.8 | 13.9 ± 1.6 | <.001 |
| Anemia | 168 (23%) | 64 (36%) | 44 (25%) | 33 (18%) | 26 (15%) | <.001 |
| Creatinine, g/dL | 1.2 ± 1.0 | 1.5 ± 1.6 | 1.2 ± 0.9 | 1.1 ± 0.4 | 1.0 ± 0.3 | <.001 |
| eGFR, mL·min−1·1.73 m −2 | 78 ± 25 | 68 ± 28 | 79 ± 26 | 79 ± 23 | 85 ± 20 | <.001 |
| LVEF, % | 34 ± 15 | 32 ± 16 | 33 ± 14 | 34 ± 13 | 38 ± 14 | <.001 |
| CPET data | ||||||
| Resting SBP, mm Hg | 117 ± 20 | 117 ± 20 | 117 ± 21 | 119 ± 19 | 117 ± 18 | .67 |
| Resting DBP, mm Hg | 75 ± 11 | 74 ± 11 | 75 ± 12 | 75 ± 11 | 74 ± 10 | .85 |
| Peak SBP, mm Hg | 145 ± 29 | 131 ± 26 | 141 ± 29 | 152 ± 28 | 156 ± 27 | <.001 |
| Peak DBP, mm Hg | 77 ± 12 | 74 ± 12 | 76 ± 13 | 78 ± 13 | 78 ± 12 | .002 |
| Resting HR, beats/min | 73 ± 15 | 72 ± 15 | 74 ± 16 | 70 ± 14 | 75 ± 15 | .005 |
| Peak HR, beats/min | 129 ± 27 | 98 ± 15 | 121 ± 13 | 136 ± 12 | 161 ± 16 | <.001 |
| Change in HR, beats/min | 57 ± 27 | 26 ± 11 | 47 ± 11 | 67 ± 15 | 86 ± 20 | <.001 |
| Peak HR, % of predicted | 77 ± 15 | 60 ± 7 | 72 ± 5 | 82 ± 4.1 | 96 ± 9 | <.001 |
| Chronotropic index | 0.60 ± 0.26 | 0.28 ± 0.10 | 0.50 ± 0.05 | 0.68 ± 0.06 | 0.94 ± 0.17 | <.001 |
| Chronotropic index <0.60 | 379 (53%) | |||||
| Peak RER | 1.21 ± 0.11 | 1.18 ± 0.12 | 1.20 ± 0.11 | 1.22 ± 0.10 | 1.24 ± 0.11 | <.001 |
| PeakVO2, mL·min−1·kg−1 | 16.5 ± 6.4 | 12.1 ± 3.6 | 14.6 ± 3.7 | 17.9 ± 5.6 | 21.4 ± 7.5 | <.001 |
| Peak VO2, % pred | 64 ± 20 | 49 ± 14 | 59 ± 15 | 68 ± 17 | 80 ± 20 | <.001 |
| Peak O2 pulse, mL/beat | 11 ± 4 | 11 ± 4 | 11 ± 4 | 11 ± 4 | 11 ± 4 | .85 |
| VE/VCO2 slope | 31.9 ± 7.9 | 34.6 ± 8.4 | 32.4 ± 9.3 | 30.8 ± 7.1 | 29.7 ± 5.9 | <.001 |
Values presented as mean ± SD or n (%). Abbreviations as in Table 1
Resting HR and CIx correlated poorly with each other (r = 0.08; P = .04). A significant, though modest, correlation was noted between resting HR and CIx (r = 0.24; P = .03) among the subgroup of patients in the highest quartile of B-blockade dose. Patients in the higher quartiles of resting HR had greater absolute and percent-predicted peak HR than those with lower resting HR (Table 1). Resting HR did not predict (P = .84) the presence of chronotropic incompetence (CIx <0.60). Accordingly, the prevalence of chronotropic incompetence did not differ across the resting HR quartiles (P = .51).
Heart Rate, Chronotropic Incompetence, and Prognosis
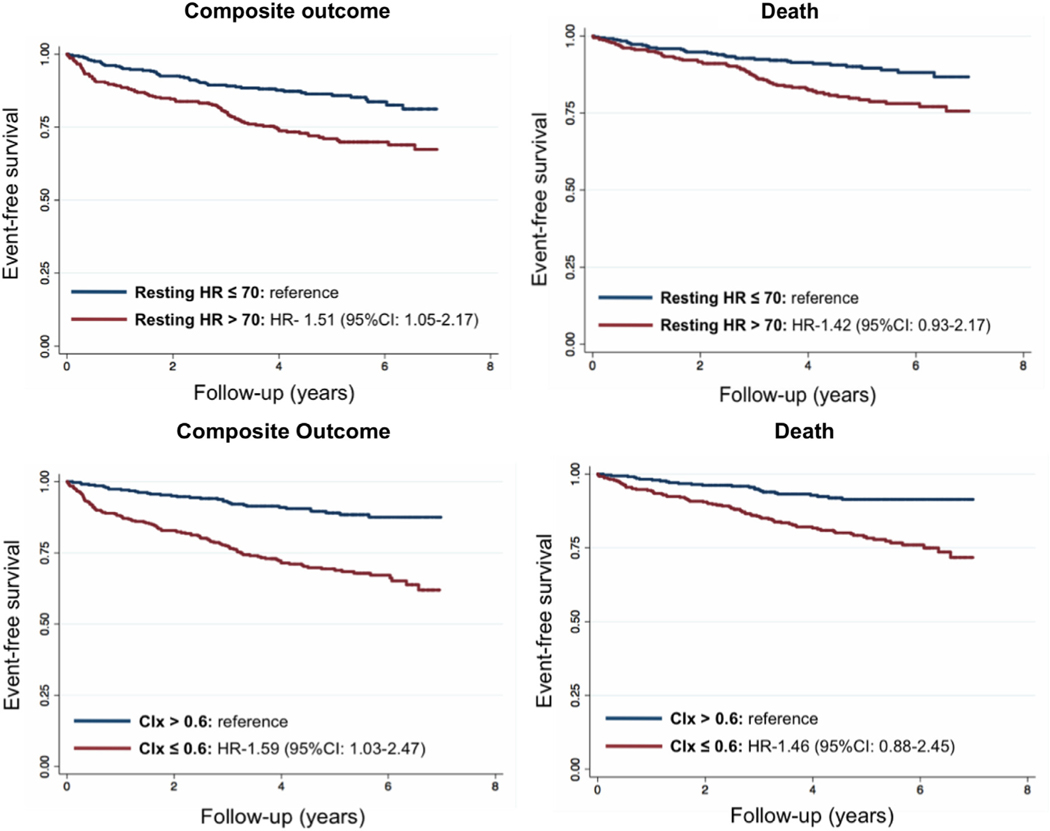
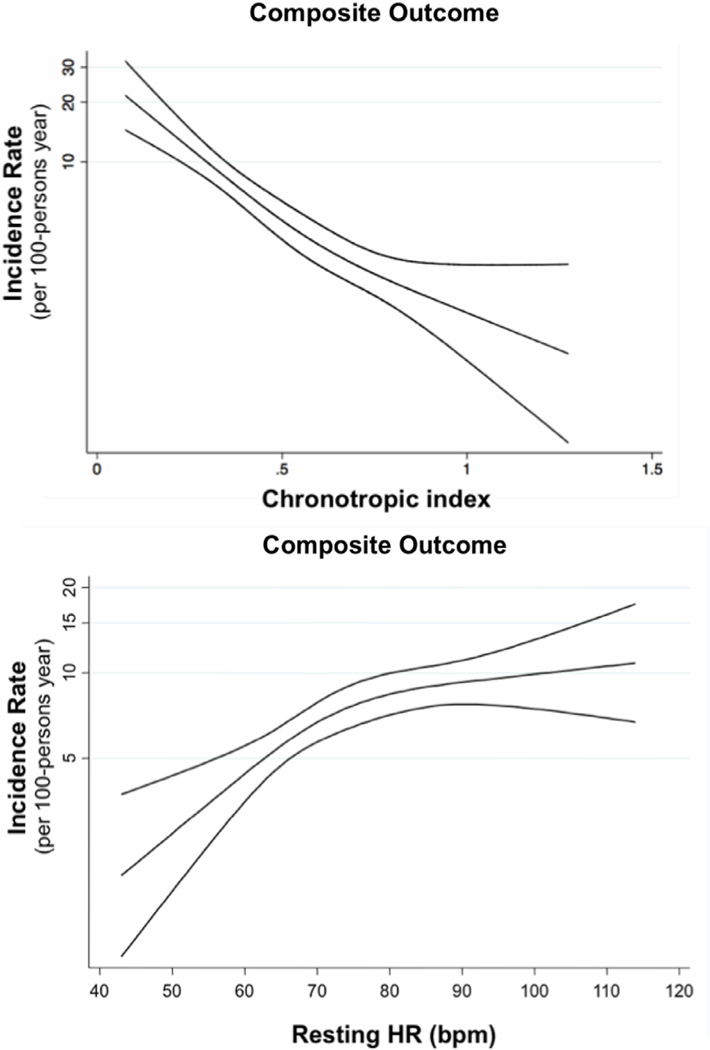
During a median follow-up of 4.4 (IQR 3.0 – 5.8) years, there were 107 (15%) deaths and 151 (21%) composite events. After adjusting for age, sex, cardiomyopathy etiology, diabetes, LVEF, β-blocker use, presence of CRT/ICD, and peak VO2, each 5 beats/min increase in resting HR was associated with a 5% higher risk of the composite outcome (HR 1.05, 95% CI 1.00 – 1.11; P = .08). Similar associations were observed between resting HR and outcomes after further adjustment for eGFR (Supplemental Table 2). Patients with HF with a resting HR >70 beats/min had 51% higher risk of composite outcome than those with <70 beats/min (Table 3; Fig. 1). In multivariable analysis adjusting for age, sex, eGFR, presence of CRT/ICD, resting HR, LVEF, β-blocker use, and peak VO2, CIx was an independent predictor of composite outcome (per SD change: hazard ratio [HR] 0.77, 95% CI 0.62 – 0.94; P = .01) and showed a linear relationship with clinical events (Fig. 2). Compared with patients with CIx >0.60, those with a CIx <0.60 had a 60% higher risk of the composite clinical event (Table 3; Fig. 1). Resting HR (HR 1.05, 95% CI 0.99 – 1.12, per each 5 beats/-min increase; P = .13) and CIx (HR 0.76, 95% CI 0.59 – 0.97, per SD change; P = .03) demonstrated similar effect estimates for the end point of all-cause mortality but did not achieve statistical significance (Table 3).
Table 3.
Multivariable Adjusted Hazard Ratios for Incident Death or Cardiovascular Events Associated With Resting HR and CIx
| Outcome | No. of Events/ Person-Time-at-Risk | Event Rate Per 100 Person-Years (95% CI) | Unadjusted Hazard Ratio (95% CI) | Adjusted Hazard Ratio (95% CI) |
|---|---|---|---|---|
| Resting HR* | ||||
| Death | ||||
| Resting HR ≤70 beats/min | 38/1673 | 2.27 (1.65–3.12) | Reference | Reference |
| Resting HR >70 beats/min | 69/1566 | 4.41 (3.48–5.58) | 1.93 (1.30–2.87) | 1.42 (0.93–2.17) |
| Composite outcome | ||||
| Resting HR ≤70 beats/min | 53/1625 | 3.26 (2.49–4.27) | Reference | Reference |
| Resting HR >70 beats/min | 98/1452 | 6.75 (5.54–8.23) | 2.03 (1.46–2.85) | 1.51 (1.05–2.17) |
| Chronotropic Index† | ||||
| Death | ||||
| CIx >0.60 | 27/1601 | 1.69 (1.16–2.46) | Reference | Reference |
| CIx ≤0.60 | 80/1638 | 4.89 (3.92–6.08) | 2.91 (1.88–4.50) | 1.46 (0.88–2.45) |
| Composite outcome | ||||
| CIx >0.60 | 36/1570 | 2.29 (1.66–3.18) | Reference | Reference |
| CIx ≤0.60 | 115/1508 | 7.63 (6.35–9.16) | 3.29 (2.26–4.79) | 1.59(1.03–2.47) |
CI, confidence interval; other abbreviations as in Table 1.
Model adjusted for age, sex, cardiomyopathy etiology, diabetes, LVEF, β-blocker use, presence of CRT/ICD, and peak VO2.
Model adjusted for age, sex, eGFR, presence of CRT/ICD, resting HR, LVEF, β-blocker use, and peak VO2.
Fig. 1.
Prognostic implications of having a resting heart rate (HR) of >70 beats/min and chronotropic index (CIx) ≤ 0.60 in heart failure. Kaplan-Meier curves plotting event-free survival (death and composite outcome) of studied patients dichotomized according to resting HR and CIx cutoffs of 70 beats/min and 0.60, respectively.
Fig. 2.
Incidence rates of composite events across resting HR and chronotropic index spectrum. Spline regression of between resting HR and CIx and composite outcome. Abbreviations as in Fig. 1.
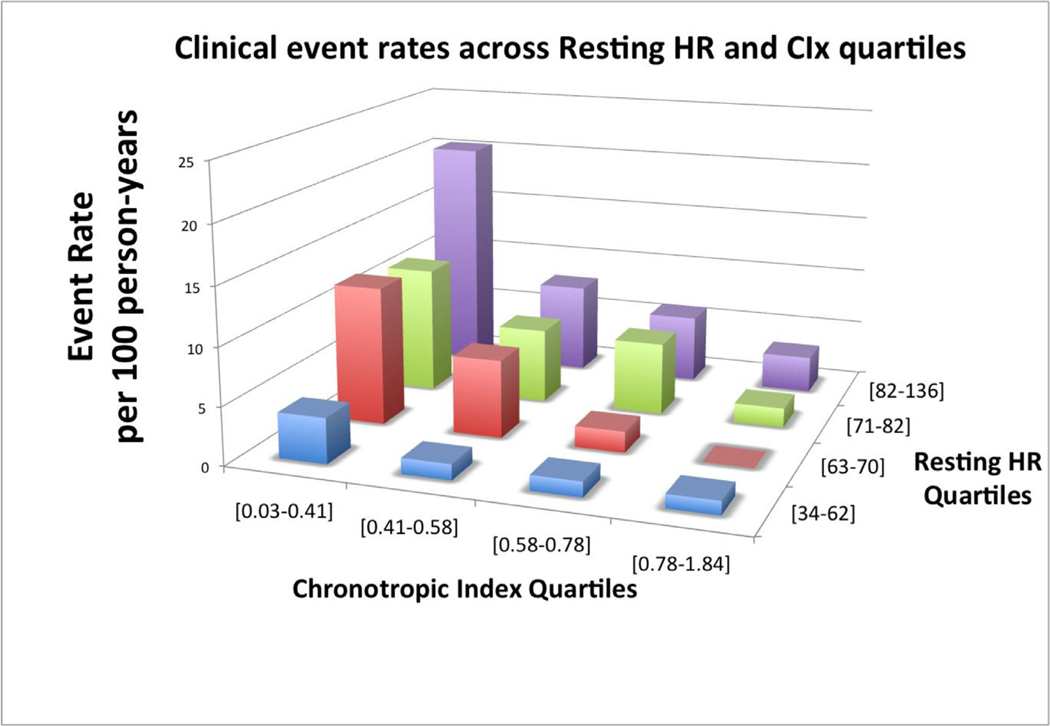
There was no interaction between resting HR and CIx for the composite outcome (P value for interaction: .36). In each quartile of resting HR, lower CIx was associated with higher event rates (Fig. 3). When added to a predictive model based on clinical characteristics (age, sex, cardiomyopathy etiology, diabetes, LVEF, eGFR, β-blockers, presence of CRT/ICD), and resting HR, CIx added significant incremental prognostic value based the C-statistic, continuous NRI, and IDI (Table 4). The incremental value of CIx was attenuated when added to a predictive model of clinical characteristics, resting HR, and peak VO2.
Fig. 3.
Unadjusted composite event rates among resting HR and CIx quartiles. Abbreviations as in Fig. 1.
Table 4.
Incremental Value of Resting HR and CIx in Predicting Adverse Clinical Events Beyond Clinical Variables
| Factors | C-Statistic (95% CI) without CIx | C-Statistic (95% CI) with CIx | P Value | NRI (95% CI) for Composite Events at 2 y | P Value | IDI (95% CI) for Composite Events at 2 y | P Value |
|---|---|---|---|---|---|---|---|
| Clinical + resting HR | 0.72 (0.68–0.76) | 0.76 (0.72–0.79) | .005 | 27.0% (10.2%–37.0%) | <.001 | 4.2% (1.5%–8.5%) | .008 |
| Clinical + resting HR + peak VO2 | 0.76 (0.72–0.79) | 0.77 (0.73–0.80) | .16 | 15.2% (0.6%–28.9%) | .024 | 1.2% (−0.2% to 3.7%) | .12 |
Clinical variables: age, sex, cardiomyopathy etiology, diabetes, LVEF, β-blocker use, presence of CRT/ICD, eGFR. IDI, integrated discrimination improvement; NRI, net reclassification improvement; other abbreviations as in Table 1.
In supplemental analyses stratified by the presence of CRT/ICD, resting heart rate was predictive of the composite outcome in patients without CRT/ICD (HR 1.08, 95% CI 1.00 – 1.16), but not among those with CRT/ICD (HR 1.01, 95% CI 0.93 – 1.10; P for interaction: .002). In contrast, CRT/ICD status did not modify the relationship between CIx and risk, with effect estimates consistent with higher CIx associated with lower risk of the composite outcome in both groups (P for interaction: .49; with CRT/ICD: HR 0.84, 95% CI 0.60 – 1.20; without CRT/ICD: HR 0.69, 95% CI 0.52 – 0.92). Among the 37 patients with respiratory exchange ratio (RER) <1.0, despite the limited statistical power, both resting heart rate and CIx demonstrated similar associations with the composite outcome as observed in those with RER ≥ 1.0 (Supplemental Table 3).
Prognostic Value in Patients With LVEF ≥ 40%
Of the overall 718 patients, 280 (39%) had LVEF ≥ 40% (mean 50% ± 8%). Compared with those with reduced LVEF, those with LVEF ≥ 40% were of similar age but were more frequently female and had a higher body mass index, better NYHA function class, and lower prevalence of ischemic cardiomyopathy and diabetes. In addition, their resting HR was lower than in patients with LVEF <40% (69 ± 13 vs 75 ± 16 bpm; P < .001). During exercise testing, patients with LVEF ≥40% demonstrated higher CIx (0.63 ± 0.25 vs 0.58 ± 0.27; P = .01) and higher peak VO2 (72% ± 20% vs 59% ± 18% of predicted; P < .001). Higher resting HR (P < .001) and lower CIx (P < .001) were both associated with lower peak VO2 in this subgroup of patients. There was no significant interaction between resting HR (P = .56) or CIx (P = .40) and LVEF for composite outcomes. In patients with LVEF ≥40%, both CIx (per SD change: HR 0.48, 95% CI 0.26 – 0.88) and resting HR (per 5 beats/min change: HR 1.12, 95% CI 0.96 – 1.29) showed an effect estimate similar to the study population overall, although only CIx remained statistically significant after adjusting for age, LVEF, and peak VO2.
Prognostic Value in Patients With Atrial Fibrillation
Seventy-eight (11%) of the studied patients had atrial fibrillation at the time of CPET. Compared with patients in sinus rhythm, patients in atrial fibrillation were older and predominantly male, had worse NYHA functional class and similar LVEF, and were more likely to be on β-blockers and antiarrhythmic medications. Their resting HR was higher (77 ± 15 vs 72 ± 15 beats/min; P = .01). During exercise testing, they demonstrated a better chronotropic response as indicated by higher peak HR (83% ± 21% vs 77% ± 14% of predicted; P < .001) and CIx (0.69 ± 0.41 vs 0.59 ± 0.24; P = .001), but lower peak VO2 (57% ± 18% vs 65% ± 20% of predicted; P < .001). There was no significant interaction between resting HR or CIx and the presence of atrial fibrillation for composite outcome (P values for interaction: .24 and .73, respectively). Effect estimates for CIx (per SD change: HR 0.73, 95% CI 0.50 – 1.06) and resting HR (per 5 beats/min change: HR 1.10, 95% CI 0.97 – 1.25) were similar to those in the overall study sample but were not statistically significant.
Discussion
Higher resting HR and lower CIx were associated with more severe HF, worse aerobic functional capacity and worse prognosis. Despite sharing common predictors, these 2 HR measures correlated weakly and resting HR did not predict the occurrence of chronotropic incompetence. Both resting HR and CIx were independently predictive of the composite outcome of LVAD implantation, heart transplantation, or all-cause mortality. Adding CIx to resting HR increased discriminative power for predicting adverse clinical outcomes. These findings persisted in the subset of patients with HF with relatively preserved LVEF.
Resting HR is influenced by intrinsic sinoatrial node properties and autonomic nervous system tone.18 It is a robust risk factor for adverse outcomes in the general population,19,20 as well as in patients with prevalent cardiac disease.6 We observed an association between higher resting HR and clinical events that was consistent with previous reports in patients with HF.3 Each 5 beats/min increase in resting HR was associated with 5% higher risk of adverse clinical events. This association was observed across the LVEF spectrum in our cohort.
The HR response to exercise is another HR-related measure commonly assessed in clinical practice. The physiologic determinants of this response include the changes in autonomic tone during the different stages of exercise, sinoatrial node responsiveness to neurohumoral stimuli, and the amount of exercise performed. Although some studies have demonstrated independent prognostic value for this measure,7,21 other studies suggest that chronotropic response to exercise is merely a surrogate marker of maximal exercise capacity.9,10 We observed an independent association between CIx and death or the composite outcome after adjusting for multiple clinical and exercise variables, including peak VO2, and found no significant effect modification of LVEF (≥ 40% vs <40%) on this relationship.
Despite sharing some predictors, resting HR and CIx weakly correlated with each other, and the association was mainly driven by patients receiving higher doses of β-blockade. Notably, diabetes was a predictor of both HR measures, supporting the autonomic nervous system as one of the putative mechanistic links between HR and prognosis.13 The absence of a strong relationship between resting HR and CIx suggest these 2 HR-related variables might signal different pathophysiologic mechanisms (inflammation and neurohumoral activation, respectively), as previously hypothesized.21 Recent studies have been questioning the causal relationship between CIx and exercise intolerance in patients with HF.22–24 Although our data do not address the causal relationship between CIx and functional capacity in HF, these fing20dings clearly support CIx as an independent prognostic factor in patients with HF across LVEF spectrum. The lack of association between resting HR and chronotropic response to exercise in HF, in combination with the independent prognostic value of these 2 HR measures, indicates that CIx is potentially a distinct therapeutic target in patients with HF—whether or not with associated exercise capacity improvement. In addition, we demonstrated that CIx provides incremental value in predicting the risk of events (Fig. 1; Table 3) beyond clinical characteristics and resting HR. The incremental prognostic value of CIx when added to a full model containing resting HR was similar to that of peak VO2, suggesting that CIx captures much of the prognosis insight given by exercise testing.
Most studies on the prognostic value of chronotropic response to exercise have evaluated patients with reduced LVEF (<35%).7,9,21 We demonstrate that low CIx predicts adverse clinical events in patients with HF including those with relatively preserved LVEF (≥ 40%). Our study design and settings preclude the generalization of these findings to patients with HFpEF. The studied patients with preserved LVEF were younger and had a male predominance, which differs from other HFpEF cohorts.25 In addition, the referral pattern to CPET might have enriched this subgroup with patients with HF with reduced ejection fraction in whom LVEF was improved after treatment. However, the consistency of the prognostic value of CIx across the LVEF spectrum suggests that chronotropic incompetence, which has been frequently noted in HFpEF,25 may also convey independent prognostic value in this HF phenotype.
Study Limitatiions
The present study has several limitations that should be noted. It was an observational study and therefore vulnerable to unmeasured confounding that may account to the observed associations. In addition, we were unable to account for the influence of changes in treatments over time on measured outcomes. Physicians who ordered CPET did not follow any standardized protocol, so different reasons may have driven exercise testing among patients with HF, and our findings may be influenced by indication bias. We tried to account for this potential concealed heterogeneity by adjusting our survival models for several important clinical characteristics. We did not have data regarding incident hospitalizations for HF, which could had given us further insight on prognostic data given by HR measures. However, by restricting the analysis to hard clinical outcomes, we were able to minimize ascertainment bias. We reviewed clinical charts at only 1 referral quaternary care hospital to assess LVAD and cardiac transplantation outcomes, although in general the frequency with which patients get these treatments at a referral institution different from where they are being longitudinally followed is low. HR variability, which is influenced by both sympathetic and parasympathetic tone, was not available, and we were therefore unable to comment on its prognostic relevance in relation to resting HR and CIx. Finally, the high prevalence of β-blockers therapy precludes the generalization of our conclusions to patients not using this recommended treatment.
Despite these limitations, this study is one of the largest of chronotropic response to exercise and clinical outcomes in optimally treated patients with HF. In contrast to most earlier studies, we included patients with a wide spectrum of LVEF. Finally, we excluded submaximal effort, an important potential confounder, as a cause of abnormal HR response to exercise with the use of cardiopulmonary data.
Conclusion
In patients with HF receiving optimized treatment, HR response to exercise provides additional prognostic information beyond resting HR, regardless of LVEF. CIx is a simple and useful prognostic measure in patients with HF regardless of resting HR or LVEF.
Supplementary Material
Acknowledgments
Funding: Research grants from the Portuguese gFoundation for Science and Technology (ICJ/0013/2012; M.S.), the National Institutes of Health (grants K08HL116792, R01HL135008, and R01HL143224; A.M.S.), and a Watkins Discovery Award from the Brigham and Women’s Hospital Heart and Vascular Center (A.M.S.). The sponsors had no role in the study design, data collection, data analysis, data interpretation, or writing of the manuscript.
Footnotes
Disclosures
Dr Shah reports receiving research support from Novartis, Actelion Pharmaceuticals, and Gilead. The other authors have no disclosures.
Supplementary materials
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.cardfail.2018.09.015.
References
- 1.Fox K, Borer JS, Camm AJ, Danchin N, Ferrari R, Lopez Sendon JL, et al. Resting heart rate in cardiovascular disease. J Am Coll Cardiol 2007;50:823–30. [DOI] [PubMed] [Google Scholar]
- 2.Vazir A, Claggett B, Jhund P, Castagno D, Skali H, Yusuf S, et al. Prognostic importance of temporal changes in resting heart rate in heart failure patients: An analysis of the charm program. Eur Heart J 2015;36:669–75. [DOI] [PubMed] [Google Scholar]
- 3.Bohm M, Swedberg K, Komajda M, Borer JS, Ford I, Dubost-Brama A, et al. Heart rate as a risk factor in chronic heart failure (shift): the association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet 2010;376: 886–94. [DOI] [PubMed] [Google Scholar]
- 4.Swedberg K, Komajda M, Bohm M, Borer JS, Ford I, Dubost-Brama A, et al. Ivabradine and outcomes in chronic heart failure (shift): A randomised placebo-controlled study. Lancet 2010;376:875–85. [DOI] [PubMed] [Google Scholar]
- 5.McAlister FA, Wiebe N, Ezekowitz JA, Leung AA, Armstrong PW. Meta-analysis: beta-blocker dose, heart rate reduction, and death in patients with heart failure. Ann Intern Med 2009;150:784–94. [DOI] [PubMed] [Google Scholar]
- 6.Brubaker PH, Kitzman DW. Chronotropic incompetence: causes, consequences, and management. Circulation 2011;123:1010–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dobre D, Zannad F, Keteyian SJ, Stevens SR, Rossignol P, Kitzman DW, et al. Association between resting heart rate, chronotropic index, and long-term outcomes in patients with heart failure receiving beta-blocker therapy: data from the HF-ACTION trial. Eur Heart J 2013;34:2271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robbins M, Francis G, Pashkow FJ, Snader CE, Hoercher K, Young JB, Lauer MS. Ventilatory and heart rate responses to exercise: Better predictors of heart failure mortality than peak oxygen consumption. Circulation 1999;100: 2411–7. [DOI] [PubMed] [Google Scholar]
- 9.Witte KK, Cleland JG, Clark AL. Chronic heart failure, chronotropic incompetence, and the effects of beta blockade. Heart 2006;92:481–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Najjar Y, Witte KK, Clark AL. Chronotropic incompetence and survival in chronic heart failure. Int J Cardiol 2012;157:48–52. [DOI] [PubMed] [Google Scholar]
- 11.Santos M, Opotowsky AR, Shah AM, Tracy J, Waxman AB, Systrom DM. Central cardiac limit to aerobic capacity in patients with exertional pulmonary venous hypertension: implications for heart failure with preserved ejection fraction. Circ Heart Fail 2015;8:278–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borlaug BA, Melenovsky V, Russell SD, Kessler K, Pacak K, Becker LC, Kass DA. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation 2006;114: 2138–47. [DOI] [PubMed] [Google Scholar]
- 13.Lindman BR, Davila-Roman VG, Mann DL, McNulty S, Semigran MJ, Lewis GD, et al. Cardiovascular phenotype in hfpef patients with or without diabetes: a RELAX trial ancillary study. J Am Coll Cardiol 2014;64:541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62: e147–239. [DOI] [PubMed] [Google Scholar]
- 16.Astrand I. Aerobic work capacity in men and women with special reference to age. Acta Physiol Scand Suppl 1960;49:1–92. [PubMed] [Google Scholar]
- 17.Uno H, Tian L, Cai T, Kohane IS, Wei LJ. A unified inference procedure for a class of measures to assess improvement in risk prediction systems with survival data. Stat Med 2013; 32:2430–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verrier RL, Tan A. Heart rate, autonomic markers, and cardiac mortality. Heart Rhythm 2009;6:S68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gulati M, Shaw LJ, Thisted RA, Black HR, Bairey Merz CN, Arnsdorf MF. Heart rate response to exercise stress testing in asymptomatic women: the St. James Women Take Heart project. Circulation 2010;122:130–7. [DOI] [PubMed] [Google Scholar]
- 20.Lauer MS, Okin PM, Larson MG, Evans JC, Levy D. Impaired heart rate response to graded exercise. Prognostic implications of chronotropic incompetence in the Framingham Heart Study. Circulation 1996;93:1520–6. [DOI] [PubMed] [Google Scholar]
- 21.Benes J, Kotrc M, Borlaug BA, Lefflerova K, Jarolim P, Bendlova B, et al. Resting heart rate and heart rate reserve in advanced heart failure have distinct pathophysiologic correlates and prognostic impact: A prospective pilot study. JACC Heart Fail 2013;1:259–66. [DOI] [PubMed] [Google Scholar]
- 22.Kosmala W, Holland DJ, Rojek A, Wright L, Przewlocka-Kosmala M, Marwick TH. Effect of If-channel inhibition on hemodynamic status and exercise tolerance in heart failure with preserved ejection fraction: a randomized trial. J Am Coll Cardiol 2013;62:1330–8. [DOI] [PubMed] [Google Scholar]
- 23.Pal N, Sivaswamy N, Mahmod M, Yavari A, Rudd A, Singh S, et al. Effect of selective heart rate slowing in heart failure with preserved ejection fraction. Circulation 2015;132:1719–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jamil HA, Gierula J, Paton MF, Byrom R, Lowry JE, Cubbon RM, et al. Chronotropic incompetence does not limit exercise capacity in chronic heart failure. J Am Coll Cardiol 2016;67: 1885–96. [DOI] [PubMed] [Google Scholar]
- 25.Redfield MM, Borlaug BA, Lewis GD, Mohammed SF, Semigran MJ, Lewinter MM, et al. Phosphdiesterase-5 Inhibition to Improve Clinical Status and Exercise Capacity in Diastolic Heart Failure (RELAX) trial: rationale and design. Circ Heart Fail 2012;5:653–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.