Highlights
-
•
Uniform demyelination can be seen in POEMS syndrome and in CD.
-
•
POEMS syndrome has greater distal CMAP duration in median and ulnar nerves compared to CD.
-
•
Detailed electrophysiological analysis of distal CMAP duration may help in distinguishing POEMS syndrome and CD.
Abbreviations: CD, Castleman disease; CIDP, Chronic inflammatory demyelinating polyneuropathy; CMAP, Compound muscle action potentials; M Protein, Monoclonal protein; MGUS, Monoclonal gammopathy of undetermined significance; ms, milliseconds; NCS, Nerve conduction study; POEMS syndrome, Polyneuropathy, Organomegaly, Endocrinopathy, Monoclonal protein and Skin changes Syndrome; SNAP, Sensory nerve action potentials; TLI, Terminal latency index; VEGF, Vascular endothelial growth factor
Keywords: POEMS syndrome, Castleman disease, Demyelination, CMAP duration, Temporal Dispersion, Paraproteinemic neuropathy
Abstract
Objective
We detailed the electrophysiological patterns of peripheral nerve temporal dispersion across spectrum of POEMS syndrome and Castleman disease (CD).
Methods
Compound muscle action potentials (CMAP) duration of 3 patients with POEMS syndrome and 2 with hyaline vascular type CD without clonal plasma cell dyscrasia were retrospectively analysed.
Results
Median and ulnar nerves distal CMAP duration were prolonged in all patients irrespective of plasma cell dyscrasia or M protein. All lower limbs distal CMAP responses were absent. Greatest distal CMAP duration prolongation was observed in median nerves for POEMS syndrome (17.0 ms, 158% upper limit normal) and in ulnar nerves for CD (9.8 ms, 47% upper limit normal). Distal/proximal CMAP duration ratio of <0.7 were seen in 33% of median and ulnar nerves studied among POEMS syndrome. Among nerves with ratio >0.7, all had distal CMAP duration prolongation (Range 7%–158% of upper limit normal).
Conclusions
Abnormal distal CMAP dispersion is not uncommon in POEMS syndrome and CD without clonal plasma cell dyscrasia or M protein. POEMS syndrome has greater distal CMAP duration in median and ulnar nerves, particularly in median nerve that can reach up to 150% of upper limit normal, compared to <50% in CD.
Significance
Detailed electrophysiological analysis of distal CMAP duration may help in distinguishing POEMS syndrome and CD.
1. Introduction
Demyelinating type peripheral neuropathy associated with plasma cell dyscrasia is rare, often found in patients with monoclonal gammopathy of undetermined significance (MGUS), and across spectrum between POEMS (Polyneuropathy, Organomegaly, Endocrinopathy, Monoclonal protein and Skin changes) syndrome and Castleman variant of POEMS (Dispenzieri, 2019, Raheja et al., 2015, Kim et al., 2017). In contrast, patients with Castleman disease (CD) but with no clonal plasma cell dyscrasia typically have little or no peripheral neuropathy, and when present it is more often sensory (Dispenzieri, 2019). The underlying pathomechanism behind demyelination process remains unclear (Dispenzieri, 2019, Raheja et al., 2015). Endothelial injury caused by an abnormal activation of endothelial cells by angiogenic factors such as vascular endothelial growth factor (VEGF) overexpression in the nerves has been postulated (Scarlato et al., 2005). The presence of M protein in majority of the patients with POEMS syndrome may results in paraproteinemic demyelinating neuropathy similar to that in chronic inflammatory demyelinating polyneuropathy (CIDP) (Raheja et al., 2015, Kim et al., 2017). However, up to 15% of POEMS syndrome patients have no detectable M protein when tested by immunofixation (Dispenzieri et al., 2003). Whether the presence of M protein alters demyelination pattern of peripheral nerve in POEMS syndrome and CD remains unknown.
Demyelinating neuropathy in POEMS syndrome comes with few distinctive characteristics. It has a more diffusely distributed pattern compared to those with typical CIDP, with predominant nerve conduction slowing in the intermediate and proximal segments (Sung et al., 2002, Mauermann et al., 2012, Sobue et al., 1992). Other electrophysiological features differentiating POEMS from CIDP includes fewer conduction blocks and higher terminal latency index (TLI) (Sung et al., 2002). In addition, less (13%) temporal dispersion was observed in POEMS compared to CIDP, defined by duration of the distal potential/duration of the proximal potential <0.7 (Mauermann et al., 2012). However, in these studies, distal compound muscle action potential (CMAP) duration was not evaluated. Comparison of electrophysiological patterns of demyelination between POEMS syndrome and CD has not been performed.
In this report, we characterized the demyelinating neuropathy in patients with POEMS syndrome and CD using different temporal dispersion measurements as demyelinating parameters.
2. Methods
We included patients with diagnosis of peripheral neuropathy associated with POEMS syndrome and CD from our department database of patients with chronic dysimmune neuropathies registered with Medical Research and Ethics Committee, Ministry of Health, Malaysia (NMRR-17-947-35560). All clinical and laboratory data were retrospectively collected and analysed. Their clinical characteristics were evaluated in reference to the criteria for diagnosis of POEMS syndrome (Dispenzieri, 2019). Diagnosis of CD was based on histopathological features of lymph node biopsy.
Results of nerve conduction study (NCS) were analysed for both sensory nerve action potentials (SNAP) and compound muscle action potentials (CMAPs) as per routine study protocols in our neurophysiology department. All NCS were performed during the diagnostic work-up prior to immunotherapy. A standard high-pass filter setting of 10 Hz and a low-pass filter setting of 10 kHz were used for all NCS recordings.
The duration of evoked distal and proximal CMAPs was measured for median, ulnar, tibial, and peroneal nerves. Distal CMAP duration was defined as the time period from onset of the first negative deflection to return to baseline of the last negative deflection of the distal CMAP, so as to not underestimate the duration of desynchronized distal CMAP (Cleland et al., 2006). Distal CMAP duration was measured manually for each nerve at a sensitivity of 500 µV/cm for precise cursor markings (Cleland et al., 2006). Cut-off for distal CMAP duration are as follow: median ≥ 6.6 ms, ulnar ≥ 6.7 ms, peroneal ≥ 7.6 ms, and tibial ≥ 8.8 ms (Isose et al., 2009). This cut-off was based on filter settings of 20 Hz to 20 kHz. We chose the above cut-off due to limited availability of reliable normative reference using our NCS machine setting. For each motor nerves, we calculated the percentage increase in the duration of the waveform dispersion and ratio between proximal and distal stimulation sites to determine the presence of temporal dispersion in the forearm or foreleg nerve segments (Mauermann et al., 2012). We then determined the number of nerves fulfilling definition of temporal dispersion, defined as duration of the distal potential/duration of the proximal potential <0.7, representing >30% of the proximal CMAP duration compared with the distal duration for each nerve segment (Thaisetthawatkul et al., 2002). TLI was calculated for median and ulnar motor study using the following formula: TLI = terminal distance (mm)/(distal latency (ms) × MCV (m/s). Results of temporal dispersion based on above definitions were compared for POEMS syndrome and CD.
Statistical analysis for differences within each group was performed with the Mann–Whitney U test using SPSS version 20 for Windows.
3. Results
We identified 5 patients: Two POEMS syndrome with monoclonal protein (M protein), one without M protein and two with hyaline vascular type CD without clonal plasma cell dyscrasia. One patient with POEMS syndrome did not have monoclonal protein detected despite immunofixation. Clinical characteristics of 5 patients are summarized in Table 1. There were 4 (80%) males and 1 (20%) female. Age of 2 POEMS with M protein patients was 36 and 58 years-old and for CD was 34 and 60 years-old respectively. Age of POEMS without M Protein was 41 years. The average duration of neuropathic symptoms onset to NCS was 36.0 weeks (median 40, range 12–52).
Table 1.
Clinical characteristics of 5 patients.
| Characteristics | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 |
|---|---|---|---|---|---|
| Diagnosis | POEMS Syndrome with M protein | POEMS Syndrome with M protein | POEMS Syndrome without M protein | Castleman Disease (Hyaline vascular type) |
Castleman Disease (Hyaline vascular type) |
| Age (years) | 58 | 36 | 41 | 34 | 60 |
| Gender | Female | Male | Male | Male | Male |
| Mandatory Major Criteria | |||||
| Polyneuropathy | ✓ | ✓ | ✓ | ✓ | ✓ |
| Monoclonal plasma cell proliferative disorder (almost always lambda) | ✓ | ✓ | − | − | − |
| Other major criteria (one required) | |||||
| Castleman Disease | − | − | − | ✓ | ✓ |
| Sclerotic bone lesion | ✓ | ✓ | ✓ | ✓ | ✓ |
| VEGF elevation | ND | ND | ND | ND | ND |
| Minor criteria | |||||
Organomegaly
|
✓ ✓ ✓ |
− − − |
✓ ✓ ✓ |
✓ ✓ − |
✓ − ✓ |
Extravascular volume overload
|
✓ ✓ ✓ |
− − − |
✓ − |
− − − |
− − ✓ |
Endocrinopathy
|
− − − − − ✓ |
− − − − − − |
− ✓ − − − ✓ |
− − − − − − |
− − − − − − |
Skin changes
|
− − − − − − − − – |
− − − − ✓ − − ✓ ✓ |
✓ − − − ✓ − ✓ ✓ − |
− − − − − − − − ✓ |
− − − − − − − − − |
| Thrombocytosis/polycythaemia | |||||
Other symptoms & signs
|
− − − − − − − |
− − − − − − − |
✓ ✓ − − − − − |
− − − − − − − |
− ✓ − − − − − |
| Treatment Received | Dexamethasone + Melphalan |
Dexamethasone + Melphalan |
Dexamethasone + Melphalan |
Autologous Bone Marrow Transplant | No treatment |
ND: Not done.
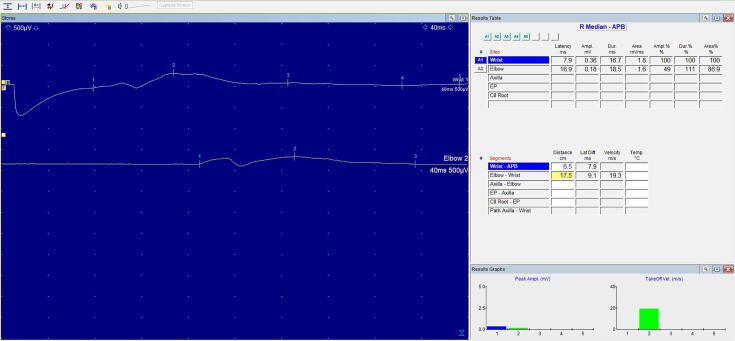
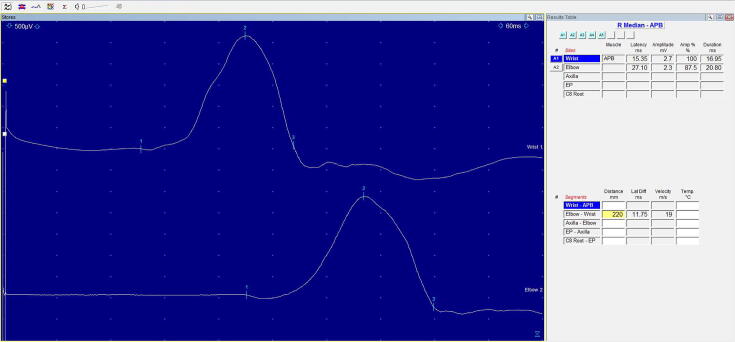
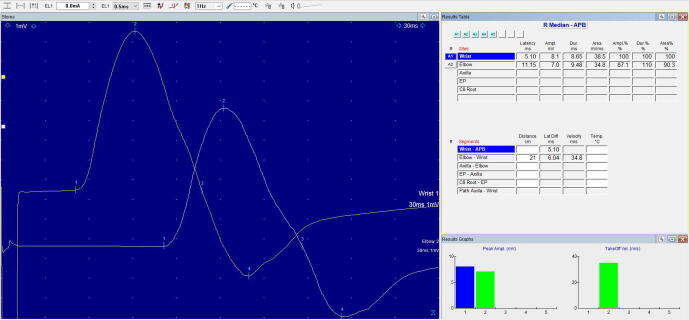
Analysis of temporal dispersion are summarized in Table 2. Original CMAP recordings of right median nerves for patients with POEMS syndrome, POEMS syndrome without M protein and CD are illustrated in Fig. 1, Fig. 2, Fig. 3 respectively. In the upper limbs, the durations of the distal CMAPs for the median and ulnar nerves were prolonged in all patients with POEMS and CD irrespective of plasma cell dyscrasia or M protein status. In contrast, CMAPs in the lower limbs were absent in all patients. For POEMS syndrome, greatest distal CMAP duration prolongation was observed in median nerves (17.0 ms, 158% of upper limit normal), whilst for CD patients, greatest distal CMAP duration prolongation was observed in ulnar nerve (9.8 ms, 47% of upper limit normal). In comparison, greatest distal CMAP duration prolongation of ulnar nerve in patients with POEMS syndrome was up to 12.6 ms, 87.3% of upper limit normal. POEMS syndrome without M protein had the most prolonged distal CMAP duration in both median and ulnar nerves compared to those with M protein and CD.
Table 2.
Analysis of temporal dispersion.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Motor NCS | POEMS Syndrome with M protein |
POEMS Syndrome with M protein |
POEMS Syndrome without M protein |
Castleman Disease |
Castleman Disease |
||||||||||||||||
| Nerve/Sites | Normal (ms) | Duration (ms) | % prolonged | Distal/Proximal Duration | TLI 60 | Duration (ms) | % prolonged | Distal/Proximal Duration | TLI65 | Duration (ms) | % prolonged | Distal/Proximal Duration | TLI60 | Duration (ms) | % prolonged | Distal/Proximal Duration | TLI65 | Duration (ms) | % prolonged | Distal/Proximal Duration | TLI |
| Right MEDIAN – Abductor Pollicis Brevis | |||||||||||||||||||||
| 1. Wrist | 6.6 | 8.00 | 21.2 | 0.43* | 0.13 | 16.67 | 152.6 | 0.87 | 0.40 | 17.00 | 157.6 | 0.82 | 0.17 | 8.65 | 31.1 | 0.91 | 0.64 | 7.50 | 13.6 | 0.77 | 0.44 |
| 2. Elbow | 18.80 | 19.06 | 20.80 | 9.48 | 9.79 | ||||||||||||||||
| Left MEDIAN – Abductor Pollicis Brevis | |||||||||||||||||||||
| 1. Wrist | 6.6 | 7.65 | 15.9 | 0.63* | 0.42 | 13.18 | 99.7 | 0.60* | 0.34 | 16.10 | 143.9 | 0.82 | 0.23 | 8.49 | 28.6 | 0.92 | 0.55 | 9.53 | 44.4 | 0.87 | 0.62 |
| 2. Elbow | 12.10 | 22.03 | 19.70 | 9.22 | 10.99 | ||||||||||||||||
| Right ULNAR – Adductor Digiti Minimi | |||||||||||||||||||||
| 1. Wrist | 6.7 | 7.25 | 8.2 | 0.76 | 1.06 | 8.02 | 19.7 | 0.86 | 0.65 | 12.55 | 87.3 | 0.82 | 0.39 | 9.84 | 46.9 | 0.97 | 0.34 | 7.19 | 7.3 | 0.81 | 0.57 |
| 2. B. Elbow | 9.05 | 9.38 | 15.35 | 10.16 | 8.91 | ||||||||||||||||
| 3. A. Elbow | 10.00 | 10.52 | 15.75 | 10.05 | 13.75 | ||||||||||||||||
| Left ULNAR – Adductor Digiti Minimi | |||||||||||||||||||||
| 1. Wrist | 6.7 | 8.30 | 23.9 | 6.93 | 3.4 | 0.93 | 0.49 | 12.40 | 85.1 | 0.66* | 0.40 | 9.58 | 43.0 | 0.93 | 0.31 | 8.96 | 33.7 | 0.85 | 0.58 | ||
| 2. B. Elbow | 7.45 | 18.80 | 10.26 | 10.57 | |||||||||||||||||
| 3. A. Elbow | 9.06 | 18.25 | 10.89 | 12.19 | |||||||||||||||||
ms: millisecond; NCS, Nerve Conduction Study; TLI: Terminal latency index.
*Represent distal/proximal CMAP duration ratio of <0.7.
Fig. 1.
Right Median Nerve CMAP recording of patient with POEMS Syndrome. Sensitivity: 500 µV/cm.
Fig. 2.
Right Median Nerve CMAP recording of patient with POEMS Syndrome without M Protein. Sensitivity: 500 µV/cm.
Fig. 3.
Right Median Nerve CMAP recording of patient with Castleman Disease. Sensitivity: 1000 µV/cm (Due to large CMAP amplitude).
For patients with POEMS syndrome, mean median distal CMAP duration was 13.1 ms (SD 4.3, range 7.7–17.0), corresponding to 98% above the upper limit normal. Relatively smaller mean median distal CMAP duration prolongation was observed for CD (8.5 ms, 29% upper limit normal). Mean ulnar distal CMAP duration was comparable for POEMS syndrome and CD (9.2 ms, SD 2.6, 38% upper limit normal and 8.9 ms, SD 1.1, 33% of upper limit normal respectively). Distal/proximal CMAP duration ratio of <0.7 was observed in 33% (4/12 nerves, 3 at median nerves) of median and ulnar nerves studied among POEMS syndrome patients. None of the median and ulnar nerves of CD patients demonstrated distal/proximal CMAP duration ratio of <0.7. Mean ratio was significantly higher for CD compared to POEMS syndrome (0.88, SD 0.07 vs 0.75, SD 0.15; p = 0.026). Therefore, increase in proximal CMAP duration of >30% compared to the distal for median or ulnar nerves was observed in a third of the nerves studied among POEMS syndrome patients despite prolonged distal CMAP duration in all median and ulnar nerves. Mean median TLI showed significantly more distal motor nerve segment slowing in POEMS syndrome compared to CD (0.28, SD 0.11 vs 0.56, SD 0.08; p = 0.01). In contrast, more distal segment ulnar motor nerve slowing was observed in CD compared to POEMS syndrome, but this was statistically insignificant (0.60, SD 0.25 vs 0.45, SD 0.13; p = 0.413).
All SNAP responses were absent in both upper and lower limbs for both POEMS syndrome and CD.
4. Discussions
Evaluation of electrophysiological feature of temporal dispersion by measuring CMAP duration is a useful parameter in characterizing chronic demyelinating neuropathy (Thaisetthawatkul et al., 2002). To our knowledge, analysis of distal CMAP duration has not been performed in patients with POEMS syndrome and CD.
Our analysis suggested 3 important clinical findings. First, uniform demyelination can be seen in POEMS syndrome as well as in patients with CD. Castleman disease which has no clonal plasma cell dyscrasia has often been associated with little or no peripheral neuropathy, which is more often sensory when present (Dispenzieri, 2019). From our analysis, both our patients with CD had prolonged distal CMAP duration as well as higher distal/proximal CMAP duration ratio compared to patients with POEMS syndrome. This may be the differentiating features between the 2 entities.
Secondly, we observed heterogenous patterns of demyelination in patients with typical POEMS syndrome. Some patients may demonstrate greater distal CMAP duration prolongation in both median and ulnar nerves, particularly the median distal CMAP duration that can reach up to 150% upper limit normal, compared to <50% prolongation in CD. Median nerve distal CMAP duration was more severely affected compared to ulnar. On the other hand, some POEMS patients with M protein may also demonstrated more proximal segment demyelination, evidence by the present of more nerve with distal/proximal CMAP duration ratio <0.7. This represented a more prominent proximal dispersion, with relatively less distal CMAP duration prolongation, consistent with typical demyelinating pattern of POEMS syndrome previously reported (Sung et al., 2002, Mauermann et al., 2012).
Thirdly, we noted the limitations of assessing temporal dispersion based on ratio of duration between distal/proximal CMAP potential of <0.7 or >30% duration increase between the proximal and distal negative peak CMAP. Previous study reported 13.3% of nerve tested in POEMS syndrome patients had temporal dispersion based on this ratio (Mauermann et al., 2012). In our series, a third of the nerves studied in POEMS syndrome patients have ratio of distal and proximal CMAP duration of <0.7, but among the nerves with ratio >0.7, all had associated distal CMAP duration prolongation and in some nerves, up to 150% of upper limit normal. This means ratio of distal/proximal CMAP potential of <0.7 fails to detect homogenous dispersion in case of significant prolongation of distal CMAP duration. In another words, defining temporal dispersion based on >30% increase in proximal duration compared to the distal may yield negative results. Likewise, none of the nerves studied in CD had distal and proximal CMAP duration of <0.7, suggesting of a more homogenous demyelination throughout entire nerves.
Consistent with existing knowledge, loss of lower limb CMAP amplitudes and SNAP responses were common features among POEMS syndrome (Sung et al., 2002, Mauermann et al., 2012). This was also observed in CD, representing length-dependent nature of the disease spectrum irrespective of clonal plasma cell dyscrasia. However, our study has few limitations beginning with our small cohort of patients and their heterogenous clinical presentations. Data analysis was performed retrospectively due to rarity of this disease and therefore subjected to its limitations. One important technical aspect of determining CMAP duration is the filter settings on NCS machines particularly the high pass filter. In our neurophysiology laboratory, the standard high-pass filter was set at 10 Hz and a low-pass filter setting at 10 kHz. This filter settings were lower compared to the filter setting of 20 Hz to 20 kHz used in previous reports (Isose et al., 2009, Thaisetthawatkul et al., 2002). Therefore, our measured CMAP duration could be longer than that measured by a 20 Hz high-pass setting, and direct comparison cannot be performed.
5. Conclusions
Our analysis showed that abnormal temporal dispersion was not exclusive in POEMS syndrome but also seen in CD without clonal plasma cell dyscrasia, which may represent a spectrum of disease with VEGF playing vital role in demyelination. Presence of M protein may affect the patterns of demyelination, particularly at the more proximal segment.
Ethical approval
This study was approved by the Medical Research and Ethics Committee, Ministry of Health Malaysia and registered under the National Medical Research Registry (NMRR-17-947-35560).
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors would like to acknowledge the director general of health Malaysia for allowing us to publish this work.
References
- Cleland J.C., Malik K., Thaisetthawatkul P., Herrmann D.N., Logigian E.L. Acute inflammatory demyelinating polyneuropathy: contribution of a dispersed compound muscle action potential to electrodiagnosis. Muscle Nerve. 2006;33:771–777. doi: 10.1002/mus.20532. [DOI] [PubMed] [Google Scholar]
- Dispenzieri A. POEMS Syndrome: 2019 Update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2019;94(7):812–827. doi: 10.1002/ajh.25495. Epub 2019 May 23. [DOI] [PubMed] [Google Scholar]
- Dispenzieri A., Kyle R.A., Lacy M.Q., Rajkumar S.V., Therneau T.M., Larson D.R. POEMS syndrome: definitions and long-term outcome. Blood. 2003;101(7):2496–2506. doi: 10.1182/blood-2002-07-2299. [DOI] [PubMed] [Google Scholar]
- Isose S., Kuwabara S., Kokubun N., Sato Y., Mori M., Shibuya K. Utility of the distal compound muscle action potential duration for diagnosis of demyelinating neuropathies. J. Peripher. Nerv. Syst. 2009;14:151–158. doi: 10.1111/j.1529-8027.2009.00226.x. [DOI] [PubMed] [Google Scholar]
- Kim H., Lim Y.M., Jin J.Y., Yoon D.H., Suh C., Kim S.Y. Electrophysiologic features of POEMS syndrome compared with MGUS-related neuropathy. Muscle Nerve. 2017;56(6):E73–E77. doi: 10.1002/mus.25684. Epub 2017 May 30. [DOI] [PubMed] [Google Scholar]
- Mauermann M.L., Sorenson E.J., Dispenzieri A., Mandrekar J., Suarez G.A., Dyck P.J. Uniform demyelination and more severe axonal loss distinguish POEMS syndrome from CIDP. J. Neurol. Neurosurg. Psychiatry. 2012;83(5):480–486. doi: 10.1136/jnnp-2011-301472. Epub 2012 Mar 6. [DOI] [PubMed] [Google Scholar]
- Raheja D., Specht C., Simmons Z. Paraproteinemic neuropathies. Muscle Nerve. 2015;51(1):1–13. doi: 10.1002/mus.24471. Epub 2014 Nov 22. [DOI] [PubMed] [Google Scholar]
- Scarlato M., Previtali S.C., Carpo M., Pareyson D., Briani C., Del Bo R. Polyneuropathy in POEMS syndrome: role of angiogenic factors in the pathogenesis. Brain. 2005;128:1911–1920. doi: 10.1093/brain/awh519. Epub 2005 Jun 23. [DOI] [PubMed] [Google Scholar]
- Sobue G., Doyu M., Watanabe M., Hayashi F., Mitsuma T. Extensive demyelinating changes in the peripheral nerves of Crow-Fukase syndrome: a pathological study of one autopsied case. Acta Neuropathol. 1992;84(2):171–177. doi: 10.1007/bf00311391. [DOI] [PubMed] [Google Scholar]
- Sung J.Y., Kuwabara S., Ogawara K., Kanai K., Hattori T. Patterns of nerve conduction abnormalities in POEMS syndrome. Muscle Nerve. 2002;26(2):189–193. doi: 10.1002/mus.10182. [DOI] [PubMed] [Google Scholar]
- Thaisetthawatkul P., Logigian E.L., Herrmann D.N. Dispersion of the distal compound muscle action potential as a diagnostic criterion for chronic inflammatory demyelinating polyneuropathy. Neurology. 2002;59(10):1526–1532. doi: 10.1212/01.wnl.0000034172.47882.20. [DOI] [PubMed] [Google Scholar]