Abstract
Matching of actionable tumor mutations with targeted therapy increases response rates and prolongs survival in lung cancer patients. Drug development and trials targeting genetic alterations are expanding rapidly. We describe the role of a Molecular Tumor Board (MTB) in the design of molecularly informed treatment strategies in our lung cancer patient population.
Tumor DNA was sequenced using a 50-gene targeted next-generation sequencing panel. Cases were evaluated by a multidisciplinary MTB who suggested a course of treatment based on each patient’s molecular findings.
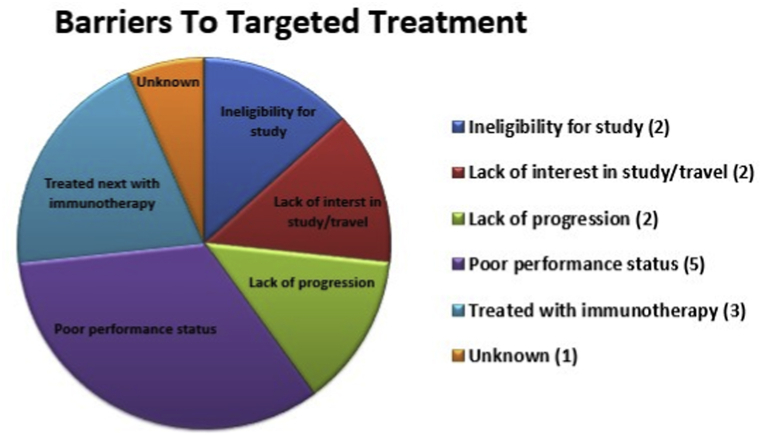
During a three-year period, 21 lung cancer patients were presented at the MTB. All patients lacked common activating EGFR mutations and ALK rearrangements. One patient had Stage IIIb disease; all others were Stage IV; 18 patients had received ≥1 prior line of therapy (range 0–5). Suggestions for treatment with a targeted therapy were made for 19/21 (90.5%) patients, and four patients (21%) underwent treatment with a targeted agent, two as part of a clinical trial. Identified barriers to treatment with targeted therapy included: ineligibility for clinical trials (n = 2), lack of interest in study/distance to travel (n = 2), lack of disease progression (n = 2), poor performance status (n = 5), decision to treat next with immunotherapy (n = 3), and unknown (n = 1).
For the majority of lung cancer patients, the MTB provided recommendations based on tumor genetic profiles. Identified barriers to treatment suggest that presentation to the MTB at earlier stages of disease may increase the number of patients eligible for treatment with a genetically informed targeted agent.
Keywords: Lung cancer, Molecular tumor board, Tumor genotyping, Personalized medicine, Targeted therapies
1. Introduction/Background
While cytotoxic chemotherapeutic agents have long been the foundation of systemic cancer treatment, the development of more specific targeted agents has rapidly progressed in the last 15 years. In many cases, these advances have resulted in superior outcomes for cancers which had a previously dismal prognosis. The beginning of molecular therapeutics can be traced to the 1960’s with the discovery of the Philadelphia Chromosome in chronic myeloid leukemia (CML). However, it took over 30 years to develop the tyrosine kinase inhibitor imatinib to target the resulting BCR-ABL fusion oncogene. Imatinib was FDA-approved in 2001, turning once-rapidly fatal CML into a chronic disease.
Lung cancer is estimated to account for 225,000 new cases and 158,000 cancer deaths annually in the U.S [1]. This is expected to represent 26.5% of all cancer deaths in 2016 [1]. Fortunately, molecular therapeutics continue to play an increasingly important role in the treatment of lung cancer as the pace of drug development to approval has increased. At the time of this cohort analysis current molecular testing guidelines for the selection of therapy in patients with lung adenocarcinoma include at minimum, EGFR and ALK testing [2]. Subsequently, ROS1, BRAF, NTRK, MET and RET have been added due to the availability of recently approved drugs.
While a relatively small proportion of tumors harbor molecular alterations targetable by FDA-approved agents, an in silico prescription strategy, based on identification of the driver alterations and their druggability options suggests that up to 70% of tumors could potentially respond to treatments currently under clinical investigation [3]. A study from M.D. Anderson Cancer Center evaluated patients with advanced cancer that harbored genetic alterations, and compared the outcomes of those enrolled into genetically matched (n = 175) versus non-matched (n = 116) clinical trials [4]. The matched group had a higher overall response rate (27% vs. 5%; P < 0.0001), as well as longer overall survival (median, 13.4 vs. 9.0 months; P = 0.017). In July 2015, the phase II NCI-MATCH [Molecular Analysis for Therapy Choice (EAY131)] trial opened to determine whether genetically-informed targeted therapies would be effective regardless of cancer subtype. In this new area of molecular therapeutics, there is an increasing need for oncologists to have access to experts in molecular diagnostics and molecular biology to interpret molecular alterations. Herein, we describe the role of a Molecular Tumor Board (MTB) in the design of molecularly informed treatment strategies for our lung cancer patient population.
2. Patients and methods
2.1. MTB format
All patients were evaluated and treated at the NCI-designated Norris Cotton Comprehensive Cancer Center at Dartmouth-Hitchcock Medical Center. The primary treating oncologist of record determined which patients would benefit from molecular testing and requested presentation at the Molecular Tumor Board (MTB). The MTB was formed through collaboration with medical oncologists, hematologists, molecular and anatomic pathologists, genetic counselors, and basic science researchers with expertise in cancer genetics, signaling pathways, and molecular therapeutics. Patient-specific surgical pathology, clinical history, molecular testing results, and an in-depth summary of the significance of tumor mutations were presented monthly to the MTB. After discussion, final treatment suggestions and referral recommendations were made by the MTB and documented in the patient’s medical record [5]. Tumor genetic profiles, MTB recommendations, and patient outcomes were then collected into an Institutional Review Board (IRB)-approved data set.
2.2. Tumor genetic profiling
In our institution, at the time of diagnosis of a lung adenocarcinoma, the diagnosing pathologist reflexively orders molecular testing; testing other histologic subtypes of lung cancer is by request of the treating oncologist. Testing was performed in the Clinical Laboratory Improvement Amendments-certified Dartmouth-Hitchcock Medical Center Department of Pathology and Laboratory Medicine’s Laboratory for Clinical Genomics and Advanced Technology using validated methods [6,7]. After pathologist’s review, macrodissection was performed to ensure ≥10% tumor cellularity. DNA was extracted from eight 4-μm sections of formalin-fixed paraffin-embedded tumor tissue using the Gentra PureGene Blood Kit Plus or Qiacube (Qiagen, Hilden, Germany) and quantified using PicoGreen (Promega, Madison, WI). Barcoded libraries were prepared from 10 ng of DNA using the Ion AmpliSeq Library Kit 2.0 with Ion AmpliSeq Cancer Hotspot Panel v2 per manufacturer’s protocol (Life Technologies, Rockville, MD). This method generates 207 polymerase chain reaction (PCR) amplicons covering 2855 COSMIC-catalogued mutations across 50 cancer-related genes. Libraries were sequenced using the Ion Torrent Personal Genome Machine (PGM) System and Ion 318 Chips (Life Technologies, Rockville, MD). Sequencing reads were aligned to hg19, and variants were called using Torrent Suite Variant Caller Plugin v4.0. Variant annotation was performed using Golden Helix SNP and Variation Suite software v8.2.1. Filters were applied to remove benign polymorphisms, as well as synonymous variants. Potential germline polymorphisms were filtered from the variant call file based on an internally curated list of benign polymorphisms based on 1% population frequency in available population databases. The thresholds for calling a variant were ≥5% variant allelic fraction and ≥500-fold coverage; in our facility, tumor DNA is always sequenced to ˃1000-fold average coverage. A report detailing variants detected in the tumor and resultant amino acid changes was included in each patient’s medical record. At that time, variants with FDA approved therapies or contraindications were listed as “clinically actionable” while other variants were listed as “not clinically indicated”.
2.3. Variant analysis
Upon request for presentation at the MTB by the primary treating oncologist, each case was assigned to a member of the MTB to review. Publicly available tools and databases (e.g., cBioPortal for Cancer Genomics) were queried to determine the frequency of a given mutation across patient populations. We then used databases (e.g., PubMed, COSMIC, Google, MutationAssessor, UniProt, ClinVar, OncoKB, MyCancerGenome, clinicaltrials.gov,and dbSNP) to determine A) whether a given mutation was previously observed and evaluated, B) potential for germline mutation, C) relevant pathway(s) that may be affected by the mutation, D) available drugs (approved, off-label, or experimental) targeting the affected pathway(s), and E), the Level(s) of Evidence (i.e., preclinical in vitro or in vivo, clinical case report, or clinical trial) (Table 1) [5] supporting a mutation-induced change in protein function and/or drug sensitivity. The results of this in-depth analysis are summarized and presented to the MTB for discussion and formation of treatment recommendations.
Table 1.
Levels of evidence supporting targeted therapies recommended by Molecular Tumor Board [5].
| Level | Definition |
|---|---|
| 1 | FDA-approved agent for given indication; demonstration that patients with tumors bearing specific genetic alterations are more likely to respond than those without such alterations. |
| 2 | Agent met a clinical endpoint in a trial (objective response, PFS, or OS) with evidence of target inhibition; plausible evidence that tumors bearing a specific genetic alteration are predicted to respond. |
| 3 | Agent demonstrated evidence of clinical activity with evidence of target inhibition; some evidence that tumors bearing a specific genetic alteration are predicted to respond. |
| 4 | Preclinical evidence of anti-tumor activity and evidence of target inhibition; hypothesis that tumors bearing a specific alteration will respond. |
3. Results
3.1. Tumor types and mutated genes
The MTB at Dartmouth-Hitchcock Medical Center was formed in November 2013. This data set was locked for analysis in November 2016. During that three-year period, a total of 88 patients were presented to the MTB. Of these, 21 were lung cancer patients (23.9%). Tumor types included adenocarcinoma (n = 18), squamous (n = 1), and other (n = 2).
3.2. Impact on patient treatment and survival
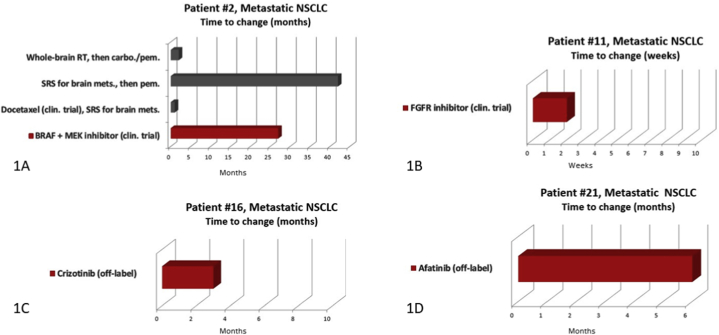
Of the 21 lung cancer cases presented (Table 2), all patients lacked common (i.e., indicated for an FDA-approved drug) activating EGFR mutations and ALK rearrangements. One patient had Stage IIIb disease; all others were Stage IV; 18 patients had previously received ≥1 prior line of therapy (range 0–5). Suggestions for treatment with a targeted therapy were made for 19/21 (90.5%) patients, and four patients underwent treatment with a MTB-recommended targeted agent (21.1%), two as part of a clinical trial. Herein, we provide treatment histories for the four patients to illustrate how rational drug-mutation matching has impacted outcome (Fig. 2).
Table 2.
Lung cancer patients presented to the Molecular Tumor Board, mutations present, final recommendations, and barriers to treatment.
| Patient Number | Tumor Board Date | Pathology | Stage | Mutations | AA change | DNA mutation | Prior Treatments | TB Recommendations - final recs | Targeted Therapy | Level of evidence | Barriers |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 12/19/13 | Adenocarcinoma | IV | EGFR | p.E709_710 delinsD | c.2126_2129 delinsA | 0 | First line chemotherapy | no | n/a | n/a |
| PIK3CA | p.E545K | c.1633 g > A | |||||||||
| 2 | 12/19/13 | Adenocarcinoma | IV | BRAF | p.V600E | c.1799T > A | 1 | Consider BRAF inhibitor trial. | yes | 3 | n/a |
| MET | p.T9921 | c. 2975C > T | |||||||||
| 3 | 12/18/14 | Adenocarcinoma | IV | KRAS | p.G12R | c.34G > C | 2 | NCT01798485 if willing to travel, CDK4/6 inhibitor trial for KRAS mutation NSCLC after progression on 2nd line therapy. | no | 4 | 1 |
| SMO | p.C193Y | c.578G > A | |||||||||
| 4 | 12/19/13 | Adenocarcinoma | IV | TP53 | p.Y88C | c.263A > G | 1 | Patient with poor performance status thus second line chemotherapy recommended, not fit for clinical trial. | n/a | n/a | n/a |
| JAK3 | p.V722I | c.2164G > A | |||||||||
| 5 | 06/19/14 | Adenocarcinoma | IV | MET | p.P814L | c.2441C > T | 3 | Continue maintenance chemotherapy, phase I MET inhibitor trial at progression. followed by MET inhibitor on trial at progression. | No | 4 | 5 |
| TP53 | p.G134E | c.401G > A | |||||||||
| 6 | 07/17/14 | Sarcomatoid | IV | TP53 | p.G134E | c.401G > A | 0 | Consider EGFR inhibitor as second line therapy. | No | n/a | 4 |
| No | |||||||||||
| 7 | 09/18/14 | Adenocarcinoma | IV | RET | p.S653C | c.1958C > G | 2 | Evaluate tumor for KIF5B-RET fusion. Consider RET inhibitor. | No | 4 | 4 |
| TP53 | p.L111R | c.332T > G | |||||||||
| 8 | 10/16/14 | Adenocarcinoma | IV | KRAS | p.G12C | c.34G > T | 1 | Continue first line chemotherapy. On progression, recommend treatment with RET/multi-kinase inhibitor Dovitinib. | No | 4 | 2 |
| RET | p.E884V | c.2651A > T | |||||||||
| 9 | 12/18/14 | Adenocarcinoma | IV | KRAS | p.G12R | c.34G > C | 1 | Downstream KRAS target with Ganetespib clinical trial. | no | 4 | 6 |
| SMO | p.C193Y | c.578G > A | |||||||||
| 10 | 02/19/15 | Adenocarcinoma | IIIB | CDKN2A | Exon 2 loss | c.∗74-1G > T | 1 | Treatment with CDK4/6 inhibitor, on clinical trial such as LEE011. | no | 3 | 1 |
| TP53 | p.C110F | c.725G > T | |||||||||
| 11 | 03/19/15 | Adenocarcinoma | IV | FGFR1 | p.A268P | c.802G > C | 3 | Continue chemotherapy, upon progression consider Signature trial arm for cancer FGFR dysregulation | yes | 4 | n/a |
| TP53 | p.A159P | c.475G > C | |||||||||
| 12 | 05/21/15 | Adenocarcinoma | IV | EGFR | p.L747S | c.2240T > C | 2 | EGFR mutation likely not activating and associated with erlotinib resistance. Consider off label treatment with JAK3 inhibitor. (V722I subsequently ckassified as polymorphism) | no | 4 | 5 |
| JAK3 | p.V722I | c.2164G > A | |||||||||
| 13 | 05/21/15 | Poorly differentiated carcinoma | IV | APC | p.E1299Q | c.3895G > C | 2 | APC and SMO mutations likely not drivers. Consider TP 53 clinical trials if patient is willing to travel. | no | 4 | 3 |
| SMO | p.G529A | c.1586G > C | |||||||||
| TP53 | p.S241Y | c.722C > A | |||||||||
| 14 | 09/24/15 | Adenocarcinoma | IV | BRAF | p.G469V | c.1406G > T | 2 | PIK3CA inhibitor + MEK inhib combination trial, if available, otherwise MATCH EAY 131-R trial. | no | 3 | 4 |
| PICK3CA | p.E545K | c.1633G > A | |||||||||
| STK11 | p.F204fs | c.612delC | |||||||||
| 15 | 10/22/15 | Squamous | IV | PIK3CA | p.E542K | c.1624G > A | 3 | Continue immunotherapy, consider combination trial of PIK3CA/EGFR inhibitors or MATCH trial at progression | no | 4 | 5 |
| 16 | 11/19/15 | Adenocarcinoma | IV | KRAS | p.G12V | c.35G > T | 1 | MET change likely SNP, consider FISH for MET amplification, if present F15153 trial, Phase IB NCT01999972, or crizotinib. If MET amplification not present consider ATR or PARP inhibitor. | yes | 4 | n/a |
| MET | p. R970C | c.2908C > T | |||||||||
| ATM | p.D2725E | c.8175T > C | |||||||||
| 17 | 11/19/15 | Adenocarcinoma | IV | MET | p.T992I | c.2975C > T | 2 | MET change is likely a SNP. Consider bavituximab or CDK4/6 inhibitor clinical trial. | no | 3 | 4 |
| CDKN2A | p.L63R | c.188T > G | |||||||||
| TP53 | p.C242F | c.725_726 GC > TT | |||||||||
| 18 | 01/28/16 | Adenocarcinoma | IV | SMAD4 | p.D351G | c.1052A > G | 5 | Continue immunotherapy, consider traveling to a site where NCT02423343 open (Nivolumab + TGFBR1 inhibitor). | no | 4 | 4 |
| 19 | 04/28/16 | Adenocarcinoma | IV | MET | p.P814L | c.2441C > T | 2 | Consider phase I cMET inhibitor trial, EFGR inhibitor with pre-clinical data of higher response due to MET variant. | no | 4 | 3 |
| 20 | 09/22/16 | Adenocarcinoma | IV | PI3K | p.E542K | c.1624G > A | 3 | Consider PIK3CA inhibitors, GDC-0023 (taselisib) or MATCH trial. | no | 3 | 2 |
| 21 | 11/17/16 | Adenocarcinoma | IV | EGFR | p.S768I | c.2303G > T | 0 | Continue afatinib, at progression consider CDK4/6 given CDKN2A, consider p53-refolding drugs such as PRIMA-1 (APR246). | yes | 2 | n/a |
| EGFR | p.V774 M | c.2320G > A | |||||||||
| CDKN2A | p.P81H | c.242C > A | |||||||||
| TP53 | p.G245C | c.733G > T | |||||||||
Fig. 2.
Barriers to targeted treatment.
Patient 2 was a 51-year-old male diagnosed with Stage IV lung adenocarcinoma with symptomatic brain metastases. In 2013, molecular profiling of the primary lung tumor revealed mutations in BRAF (p.V600E) and MET (p.T992I). At the time, case reports and interim results of Phase II trials indicate that BRAF p.V600E-mutant lung cancers frequently respond to BRAF inhibition [[8], [9], [10], [11]]. The MTB recommended treatment with BRAF and MEK inhibitors per clinical trial F12214: A Phase II study of the Selective BRAF Kinases Inhibitor GSK2118436 in Subjects With Advanced Non-small Cell Lung Cancer and BRAF Mutations [11]. The patient remained on therapy for 2 years and 3 months before progressing (Fig. 1A). He was next treated with the anti-PD1 antibody nivolumab. Of note, BRAF V600E became a FDA-approved indication with breakthrough designation of the combination of dabrafenib plus trametinib in 2015 followed by regular approval in 2017.
Fig. 1.
Clinical course of four patients who received targeted therapies.
Patient 11 was a 77-year-old female diagnosed with Stage IV lung adenocarcinoma with lymph node involvement and bilateral pulmonary metastases. Molecular profiling of a lymph node biopsy with immunohistochemistry consistent with her primary lung tumor revealed mutations in FGFR1 (p.A268P c.802G > C) and TP53 (p.A159P). Although there were no data found on the A268P mutation, A268S variant had been reported in three colorectal cancer cell lines (COSM2960508). The MTB considered this a variant of uncertain significance, although the patient may be eligible for a FGFR inhibitor trial upon screening. She was enrolled in NCT02160041 a phase II trial of BGJ398, a selective FGFR 1/2/3 inhibitor, for patients with tumors with FGFR genetic alterations. (Fig. 1B). Unfortunately, the trial protocol designated that the patient terminate her participation due to the development of Grade III hypercalcemia and hypoglycemia shortly after initiation of treatment with the targeted agent. She was next treated with the oral EGFR inhibitor erlotinib as it was FDA-approved for second line therapy regardless of EGFR mutation status. However, she developed debilitating rash and diarrhea and discontinue erlotinib after one month. Subsequently, she was treated with nivolumab until disease progression. She died of disease eight months later.
Patient 16 was a 51-year-old female with metastatic adenocarcinoma of the lung with pleural-based and contralateral pulmonary metastasis. Molecular profiling of a lymph node biopsy metastasis revealed mutations in KRAS (p.G12V), MET (exon 14, p. R970C), and ATM (p.D2725E). This case highlights the benefits of reviewing and interpreting molecular findings in the context of a MTB. The MET p.R970C variant was included in the final report as “not clinically indicated”. However, being in exon 14, this was initially interpreted by the treating oncologist as a MET exon 14 skipping mutation and the patient initiated crizotinib therapy prior to presentation at the MTB. The MTB noted that this MET variant was likely a single nucleotide polymorphism (SNP) and review of databases and the literature did not support this being an oncogenic variant. The MTB also noted that ATM variant may confer sensitivity to ATR or PARP inhibitors [12,13]. This led to recommendations for expanded testing to also include MET amplification. If MET amplification was identified, consider F15153 (INC280) trial, Phase IB NCT01999972, or crizotinib and if not present consider ATR or PARP inhibitor trial. Expanded testing was ultimately not performed. At the next follow up, approximately 3 months later, crizotinib was discontinued due to disease progression (Fig. 1C). This patient was then treated with the anti-PD1 antibody nivolumab.
Patient 21 was a 68-year-old female with metastatic adenocarcinoma of the lung with brain metastases. Molecular profiling of a lymph node biopsy with immunohistochemistry consistent with her primary lung tumor revealed mutations in EGFR (p.S768I and p.V774 M), CDKN2A (p.P81H), and TP53 (p.G245C). The MTB concluded that the EGFR S768I mutation likely conferred sensitivity to the EGFR/HER2 dual inhibitor afatinib [14], while the V774 M mutation was predicted to not affect drug sensitivity. The CDKN2A P81H mutation was found to be rare and without functional data. However, CDKN2A alterations were noted to predict sensitization to CDK4/6 inhibition in preclinical studies. Finally, the MTB noted that the TP53 G245C mutation has been shown to confer sensitivity to p53-refolding drugs such as PRIMA-1 (APR246) [15]. Concluding MTB recommendations were for first-line treatment with afatinib (which has subsequently been approved for these EGFR mutations). The patient was initiated on afatinib with primary tumor shrinkage noted on her initial two-month restaging exam. The patient remained on targeted treatment for 6 months duration (Fig. 1D) when she was found to have progression. She was next treated with carboplatin and pemetrexed with good response. At last follow up she remained on pemetrexed maintenance therapy.
4. Discussion
4.1. Barriers to treatment with a targeted agent
These data demonstrate that the Dartmouth-Hitchcock Medical Center MTB model made recommendations for targeted therapy in the great majority of cases (90.5%). However, overall patient attainment of targeted medication was somewhat modest (21.1%). Barriers to treatment with MTB-recommended targeted therapy are illustrated in Fig. 2 and summarized in Table 2. Interestingly, identified barriers to treatment in this patient population did not include inability to pay for an off-label drug. The most common reason for not undergoing targeted treatment was poor performance status (n = 5, 33.3%). Conversely, two patients had yet to undergo targeted therapy due to lack of disease progression on current treatment (13.3%). This highlights the need for appropriate timing in molecular testing, and highlights that patients were often brought to the MTB in later stages of disease. This is a challenging patient population which typically has undergone multiple lines of prior therapy and, by default, are referred to the MTB because their tumors lack variants with FDA-approved therapies. As a MTB, we found that level 3–4 evidence (Table 1) was frequently all that was available when considering potential eligibility for a targeted clinical trial referral.
In those patients who were able to attain targeted medication, we saw a trend towards a decreased number of prior lines of therapy. In the group that ultimately underwent treatment with a targeted therapy (n = 4), the average number of prior therapies was 1.25. In the group for which recommendations for a targeted drug were made; however barriers impeded treatment, the average number of previous therapies was 1.82. This supports our conclusion that patients are more likely to receive targeted therapy when molecular testing is utilized early in the course of disease.
An additional factor when considering treatment with a targeted agent included the recent advances in the management of NSCLC with immunotherapy. Several patients presented during the first year of MTB were ultimately treated with immunotherapy (n = 3, 20%). Immunotherapy was not yet approved for use at the time of their case MTB discussion and ultimately presented a more appealing treatment at next progression. We also now know that several genetic alterations such as STK11 co-mutation in KRAS mutant tumors as well as MSI, POLE/POLD1 and tumor mutational burden may also be considerations when selecting immunotherapy.
Enrolling patients who lived outside of the immediate geography of the medical center on clinical trials proved a significant barrier given the burden of travel and extra testing. Several patients elected to have standard second-line chemotherapy locally, citing the inconvenience of increased travel to Dartmouth-Hitchcock Medical Center or to another NCI-designated Cancer Institute for a clinical trial (n = 2, 13.3%). Finally, a minority of patients were found ineligible for available trials (n = 2, 13.3%).
5. Conclusions
The Dartmouth-Hitchcock Medical Center MTB was able to optimize molecular treatment strategies for lung cancer patients. Nevertheless, patient attainment of molecularly targeted medication was moderate at best. As genetic profiling of tumors and trials targeting genetic alterations continue to increase, our data support the need for appropriate timing and consideration of early molecular testing in lung cancer patients. In this model, genetic alterations can become a permanent part of a tumor profile, thereby impacting treatment decisions for years after profiling is performed. A limitation of the 50 gene hotspot panel used for the patients presented here, is that it is designed to target the most common targetable alterations in solid tumors; however, on a monthly basis, there are more and more emerging molecular targets entering clinical trials that may not be encompassed by a targeted panel of this scale. Also, filtering germline variants, even benign polymorphism, can be challenging in tumor only testing and rely heavily on well curated clinical grade databases. Reporting of germline variants can potentially be misinterpreted as somatic variants with clinical implications, as in the case of patient 16. Increasing awareness of molecular profiling and presentation at the MTB has expanded the number of patients receiving targeted therapy at our institution; however, clinical trial enrollment continues to be a challenge.
Given the rapid advances and accessibility of genomic testing, many institutions are implementing similar MTBs in order to create a multidiciplinary approach to inform patient care decisions using this data [[16], [17], [18], [19], [20]]. In our MTB model, the multidisciplinary group interprets molecular information at the request of the treating physician, most often in patients with advanced stage disease. Detected variants may represent new pathways for treatment. It is educational and beneficial to the oncologic community as a whole to share these experiences and struggles that MTBs encounter as we are all facing similar challenges provided the vast increase in genomic medicine.
CRediT authorship contribution statement
Erica B. Bernhardt: Conceptualization, Methodology, Data curation, Writing - original draft. Mary D. Chamberlin: Data curation, Supervision, Writing - review & editing. Ivan P. Gorlov: Data curation. Francine B. de Abreu: Data curation, Methodology. Katarzyna J. Bloch: Supervision. Jason D. Peterson: Software, Data curation, Methodology. Gregory J. Tsongalis: Supervision. Keisuke Shirai: Methodology. Konstantin H. Dragnev: Methodology. Todd W. Miller: Data curation, Writing - original draft. Laura J. Tafe: Conceptualization, Methodology, Software, Data curation, Writing - original draft, Writing - review & editing.
Declaration of competing interest
The authors have no conflicts of interest to disclose.
Acknowledgements
This study was supported by the Norris Cotton Cancer Center in conjunction with the Laboratory for Clinical Genomics and Advanced Technology at Dartmouth-Hitchcock Medical Center, Lebanon, NH.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.plabm.2020.e00174.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Howlader N., Noone A.M., Krapcho M., Miller D., Bishop K., Altekruse S.F., Kosary C.L., Yu M., Ruhl J., Tatalovich Z., Mariotto A., Lewis D.R., Chen H.S., Feuer E.J., Cronin K.A., editors. SEER Cancer Statistics Review. National Cancer Institute; Bethesda, MD: 1975-2013. http://seer.cancer.gov/csr/1975_2013/ based on November 2015 SEER data submission, posted to the SEER web site, April 2016. [Google Scholar]
- 2.Lindeman N.I., Cagle P.T., Beasley M.B., Chitale D.A., Dacic S., Giaccone G., Jenkins R.B., Kwiatkowski D.J., Saldivar J.S., Squire J., Thunnissen E., Ladanyi M. college of American pathologists international association for the study of lung cancer and association for molecular pathology. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the college of American pathologists, international association for the study of lung cancer, and association for molecular pathology. J. Mol. Diagn. 2013 Jul;15(4):415–453. doi: 10.1016/j.jmoldx.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Rubio-Perez C. In silico prescription of anticancer drugs to cohorts of 28 tumor types reveals targeting opportunities. Canc. Cell. 2015;27(3):382–396. doi: 10.1016/j.ccell.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Tsimberidou A.M., Wen S., Hong D.S., el al Personalized medicine for patients with advanced cancer in the phase I program at M.D. Anderson: validation and landmark analyses. Clin. Canc. Res. 2012;18:6373–6383. doi: 10.1158/1078-0432.CCR-14-0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tafe L.J., Gorlov I.P., de Abreu F.B., Lefferts J.A., Liu X., Pettus J.R., Marotti J.D., Bloch K.J., Memoli V.A., Suriawinata A.A., Dragnev K.H., Fadul C.E., Schwartz G.N., Morgan C.R., Holderness B.M., Peterson J.D., Tsongalis G.J., Miller T.W., Chamberlin M.D. Implementation of a molecular tumor board: the impact on treatment decisions for 35 patients evaluated at dartmouth-hitchcock medical center. Oncol. 2015 Sep;20(9):1011–1018. doi: 10.1634/theoncologist.2015-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsongalis G.J., Peterson J.D., de Abreu F.B., Tunkey C.D., Gallagher T.L., Strausbaugh L.D., Wells W.A., Amos C.L. Routine use of the Ion torrent AmpliSeq cancer hotspot panel for identification of clinically actionable somatic mutations. Clin. Chem. Lab. Med. 2014;52(5):707–714. doi: 10.1515/cclm-2013-0883. [DOI] [PubMed] [Google Scholar]
- 7.Butler K.S., Young M.Y., Li Z., Elespuru R.K., Wood S.C. Performance characteristics of the AmpliSeq cancer hotspot panel v2 in combination with the Ion torrent next generation sequencing personal Genome machine. Regul. Toxicol. Pharmacol. 2016;74:178–186. doi: 10.1016/j.yrtph.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Schmid S., Siano M., Joerger M. Response to dabrafenib after progression on vemurafenib in a patient with advanced BRAF V600E-mutant bronchial adenocarcinoma. Lung Canc. 2014;87:85–87. doi: 10.1016/j.lungcan.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Robinson S.D., O’Shaughnessy J.A., Cowey C.L. BRAF 600E-mutation lung adenocarcinoma with metastases to the brain responding to treatment with vemurafenib. Lung Canc. 2014;85:326–330. doi: 10.1016/j.lungcan.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Planchard D., Mazieres J., Riely G.J. Interim results of phase ii study brf 113928 of dabrafenib in BRAF V600E mutation-positive non-small cell lung cancer (NSCLC) patients. J. Clin. Oncol. 2013;31 8009a. [Google Scholar]
- 11.Planchard D., Besse B., Groen H.J.M., Souquet P.J., Quoix E., Baik C.S., Barlesi F., Kim T.M., Mazieres J., Novello S., Rigas J.R., Upalawanna A., D’Amelio A.M., Zhang P., Mookerjee B., Johnson B.E. Dabrafenib plus trametinib in patients with previously treated BRAFV600E-mutant metastatic non-small cell lung cancer: an open-label, multicenter phase 2 trial. Lancet. 2016;17(7):p984–993. doi: 10.1016/S1470-2045(16)30146-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menezes D.L., Holt J., Tang Y., Feng J., Barsanti P., Pan Y., Ghoddusi M., Zhang W., Thomas G., Holash J., Lees F., Taricani L. A synthetic lethal screen reveals enhanced sensitivity to ATR inhibitor treatment in mantel cell lymphoma with ATM loss-of-function. Nat. Chem. Biol. 2011;7(7):428–430. doi: 10.1158/1541-7786.MCR-14-0240. [DOI] [PubMed] [Google Scholar]
- 13.Kwok M., Davies N., Agathanggelou A., Smith E., Oldreive C., Petermann E., Stewart G., Brown J., Lau A., Pratt G., Parry H., Taylor M., Moss P., Hillmen P., Stankovic T. Common cancer-associated imbalances in the DNA damage response confer sensitivity to single agent ATR inhibition. Oncotarget. 2015;6(32):32396–32409. doi: 10.18632/oncotarget.6136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klughammer B., Brugger W., Cappuzzo f, Ciuleanu T., Mok T., Reck M., Tan E.H., Delmar P., Kilingelschmitt G., Yin A.Y., Spleiss O., Wu L., Shames D.S. Examining treatment outcomes with Erlotinib in patients with advanced non-small-cell lung cancer whose tumors harbor uncommon EGFR mutations. J. Thorac. Oncol. 2016;11(4):545–555. doi: 10.1016/j.jtho.2015.12.107. [DOI] [PubMed] [Google Scholar]
- 15.Muller P.A.J., Vousden K.H. Mutant p53 in cancer: new function and therapeutic opportunities. Canc. Cell. 2014;25(3):304–317. doi: 10.1016/j.ccr.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGraw S.A., Garber J., Janne P.A., Lindeman N., Oliver N., Sholl L.M., Van Allen E.M., Wagle N., Garraway L.A., Joffe S., Gray S.W. The fuzzy world of precision medicine: deliberations of a precision medicine tumor board. Per Med. 2017;14(1):37–50. doi: 10.2217/pme-2016-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwaederle M., Parker B.A., Schwab R.B., Fanta P.T., Boles S.G., Daniels G.A., Bazhenova L.A., Subramanian R., Coutinho A.C., Ojeda-Fournier H., Datnow B., Webster N.J., Lippman S.M., Kurzrock R. Molecular tumor board: the university of California-san diego moores cancer center experience. Oncol. 2014 Jun;19(6):631–636. doi: 10.1634/theoncologist.2013-0405. Epub 2014 May 5. PMID: 24797821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harada S., Arend R., Dai Q., Levesque J.A., Winokur T.S., Guo R., Heslin M.J., Nabell L., Nabors L.B., Limdi N.A., Roth K.A., Partridge E.E., Siegal G.P., Yang E.S. Implementation and utilization of the molecular tumor board to guide precision medicine. Oncotarget. 2017 Jun 14;8(34):57845–57854. doi: 10.18632/oncotarget.18471. eCollection 2017 Aug 22. Review. PMID: 28915716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bryce A.H., Egan J.B., Borad M.J., Stewart A.K., Nowakowski G.S., Chanan-Khan A., Patnaik M.M., Ansell S.M., Banck M.S., Robinson S.I., Mansfield A.S., Klee E.W., Oliver G.R., McCormick J.B., Huneke N.E., Tagtow C.M., Jenkins R.B., Rumilla K.M., Kerr S.E., Kocher J.A., Beck S.A., Fernandez-Zapico M.E., Farrugia G., Lazaridis K.N., McWilliams R.R. Experience with precision genomics and tumor board, indicates frequent target identification, but barriers to delivery. Oncotarget. 2017 Apr 18;8(16):27145–27154. doi: 10.18632/oncotarget.16057. PMID: 28423702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knepper T.C., Bell G.C., Hicks J.K., Padron E., Teer J.K., Vo T.T., Gillis N.K., Mason N.T., McLeod H.L., Walko C.M. Key lessons learned from moffitt’s molecular tumor board: the clinical genomics action committee experience. Oncol. 2017 Feb;22(2):144–151. doi: 10.1634/theoncologist.2016-0195. Epub 2017 Feb 8. PMID: 28179575. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.