Background
The COVID-19 pandemic is ongoing and there is an increasing amount of evidence regarding this infectious disease. Several neurological symptoms or complications related with SARS-CoV-2 have already been reported for both mild and severe cases [1, 2]. Neurological complications have already been reported for other coronaviruses [3, 4]. Here, we report a case of a transverse myelitis (TM) which appeared 2 weeks after mild viral symptoms probably due to SARS-CoV-2.
Case
A 63-year-old male, known for obesity, active smoking, and excessive alcohol consumption, without notable medical history and taking no medication, presented a viral syndrome for 7 days (light headache, rhinorrhea, odynophagia, myalgia, and temperature until 37.8 °C). On the day 12 after the first symptoms, he reported paresthesia and hypoesthesia on his feet, progressing in 2–3 days to the abdominal area. On the day 14, he developed mild weakness of the lower limbs, progressively worsening, and on day 16, he consulted the ER.
The neurological examination revealed a moderate paresis in proximal predominance of lower limbs associated with pyramidal signs and sensory level T10. The cognitive, cranial nerves, and upper limbs’ exam as well as the rest of systems exam was unremarkable. The blood analysis showed a leukopenia and a slightly raised C-reactive protein (CRP) (Table 1). A broad panel of infectious and immunological tests was performed, with comprehensive serologies and PCR on blood and CSF, which were all negative (Supplementary Appendix). Brain and spinal cord MRI did not show any abnormality. A lumbar puncture (LP) showed slight elevated leucocytes and proteins (Table 1). The bacterial cultures and the polymerase chain-reaction (PCR) of the cerebrospinal fluid (CSF) for detection of virus and bacteria were negative (Supplementary Appendix). An electromyoneurography was normal. A new LP on the 6 day of hospitalization showed a slight elevation of leucocytes and proteins (Table 1). A second spine MRI, 7 days after admission was normal. Since his admission, the patient presented a persistent neutropenia deemed of reaction origin (infectious, toxic, and other inflammatory) after several investigations including a bone-marrow biopsy. A body CT scan revealed a ground-glass opacity appearance on both lungs (Fig. 1a), suggestive of a typical SARS-CoV-2 infiltrate. A PET-CT did not reveal any malignancy. The chest CT scan was repeated 18 days after the initial one, showing a clear decrease of the apical pulmonary infiltrates and the lymphadenopathies (Fig. 1b).
Table 1.
Laboratory findings during the first week of hospitalization
| Reference range | Admission | Day 2 | Day 5 | Day 6 | |
|---|---|---|---|---|---|
| Measure | |||||
| White-cell count (G/L) | 4.0–10.0 | 2.4a | 1.9 | 3.0a | 6.2 |
| Red-cell count (T/L) | 4.40–5.90 | 4.81 | 4.59 | 4.21a | 4.33a |
| Absolute neutrophil count (G/L) | 1.6–7.5 | – | 0.4a | 0.7a | 2.2 |
| Absolute lymphocyte count (G/L) | 1.0–4.0 | – | 1.0a | 1.6 | 2.4 |
| Platelet count (G/L) | 150–350 | 302 | 271 | 231 | 198 |
| Hemoglobin (g/L) | 133–177 | 152 | 144 | 134 | 137 |
| Hematocrit (L/L) | 0.40–0.52 | 0.44 | 0.43 | 0.39a | 0.40a |
| CRP (mg/L) | < 5 | 19.5a | 16.6a | – | 40.0a |
| Creatinine (µmol/L) | < 106 | 76 | 106a | 80 | 74 |
| Ferritin (µg/L) | 30–400 | – | – | 1380a | – |
| Lumbar puncture | |||||
| CSF aspect | Clear | Clear | |||
| White-cell count (/µL) | 0–4 | 16a | 36a | ||
| Red-cell count (/µL) | 0 | 0 | 0 | ||
| Neutrophils (%) | 0 | 0 | |||
| Monocytes (%) | 6 | 6.0 | |||
| Lymphocytes (%) | 92 | 94.0 | |||
| Proteins (mg/L) | 150–450 | 573a | 600a | ||
| Glucose (mmol/L) | 2.2–3.9 | 3.4 | 3.7 | ||
| Lactate (mmol/L) | 1.1–2.4 | 2.80 | 3.0a | ||
| Isoelectrofocusing | Normal | Normal | |||
aAltered values
Fig. 1.
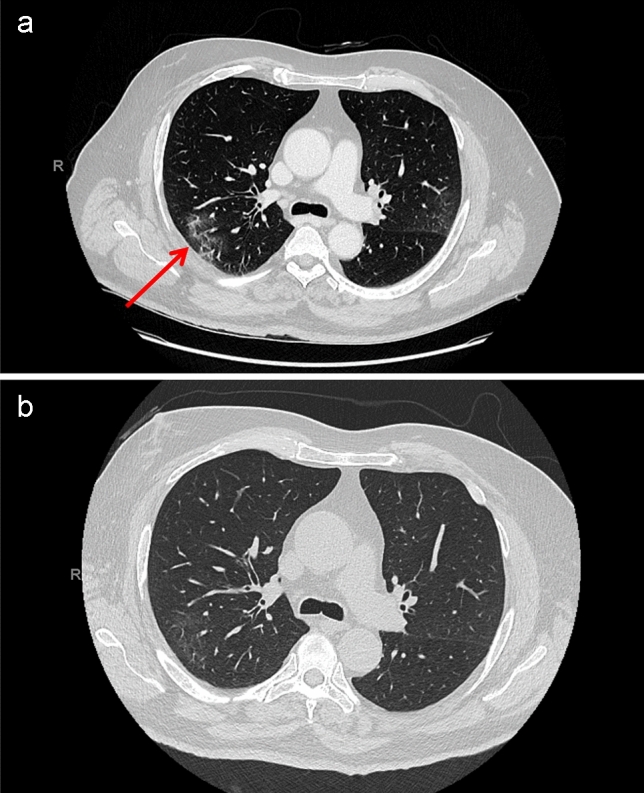
Thoracic CT imaging findings. a Thoracic CT image on day 3 from admission showing ground-glass opacity suggestive of COVID-19 (arrows). b Thoracic CT on the day 21 from admission showing almost disappearance of opacity
At admission, a nasopharyngeal smear, in the context of the ongoing COVID-19 pandemic, was negative for SARS-CoV-2. We repeated the test after the first CT results, and it was also negative. Posteriori we added a PCR for SARS-CoV-2 in the different CSF which was negative. A semi-quantitative SARS-CoV-2 serology showed the presence of both IgM and IgG at admission and at day 20 with lower IgM, suggesting a recent SARS-CoV-2 infection.
The paresis progressed rapidly to paraplegia, with total anesthesia below T10 and sphincter dysfunction. Corticosteroid treatment was considered initially, but not administered, because of SARS-CoV-2 suspicion. The patient was treated by intravenous human immunoglobulins (IVIG) 0.4 g/kg for 5 days. We did not notice any neurological improvement after the immunoglobulin treatment. Given the two negative nasopharyngeal smears of SARS-CoV-2, the absence of respiratory symptoms, and disappearance of pulmonary infiltrates, a corticosteroid therapy IV for 5 days was started the day 21 from admission.
The day 30 from his admission, the patient presented a slightly recover of his lower limbs strength and was transferred to a neurorehabilitation hospital.
Discussion
Our case fulfills the criteria of a TM of non-inflammatory origin [5], with both the LP results and the blood neutropenia suggesting a viral cause. Our complete etiologic work-up suggests that SARS-CoV-2 might probably be the pathogenic virus. The nonspecific viral symptoms before the appearance of neurological symptoms, the CT lung typical image and the presence and the evolution of IgM and IgG of SARS-CoV-2 serology, support this hypothesis. To our knowledge, this is the first case of TM after SARS-CoV-2 infection reported.
In 30–60% of idiopathic TM cases, there is an antecedent respiratory, gastrointestinal, or systemic illness [6]. We speculate that, like in most post-infectious TM, there is an immune-mediated pathogenesis [6] and the origin in our case would be an aberrant immune response. The neurological complications of SARS-CoV-2 and their pathogenesis are still to be investigated. The SARS-CoV-2 may be considered in the work-up as possible cause of post-infectious TM.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Compliance with ethical standards
Conflicts of interest
All authors report no disclosures.
Ethical standard statement
This article does not contain any studies involving human participant performed by any of the authors.
Informed consent
Written informed consent was collected from the patient for the inclusion of de-identified clinical data in a scientific publication, in accordance with the Declaration of Helsinki.
Availability of data and material
We take full responsibility for the data, the analyses, and interpretation, and that we have full access to all of the data.
References
- 1.Helms J, Kremer S, Merdji H, et al. Neurologic Features in severe SARS-CoV-2 Infection. N Engl J Med. 2020;382(23):2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nath A. Neurologic complications of coronavirus infections. Neurology. 2020;94:1–2. doi: 10.1212/WNL.0000000000009455. [DOI] [PubMed] [Google Scholar]
- 3.Hung EC, Chim SS, Chan PK, et al. Detection of SARS coronavirus RNA in the cerebrospinal fluid of a patient with severe acute respiratory syndrome. Clin Chem. 2003;49:2108–2109. doi: 10.1373/clinchem.2003.025437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arabi YM, Harthi A, Hussein J, et al. Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS-CoV) Infection. 2015;43:495–501. doi: 10.1007/s15010-015-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Transverse Myelitis Consortium Working Group Myelitis Consortium Working Group. Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology. 2002;59:499–505. doi: 10.1212/WNL.59.4.499. [DOI] [PubMed] [Google Scholar]
- 6.Krishnan C, Kaplin AI, Deshpande DM, Pardo CA, Kerr DA. Transverse Myelitis: pathogenesis, diagnosis and treatment. Front Biosci. 2004;9:1483–1499. doi: 10.2741/1351. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


