Abstract
BACKGROUND
Low-grade fever during convalescence is an atypical symptom of coronavirus disease 2019 (COVID-19). Reports of such cases are rare, and the mechanism and outcome of low-grade fever during COVID-19 convalescence are not completely clear. We report 3 cases with low-grade fever during COVID-19 convalescence and highlight the main clinical, radiographic, and laboratory characteristics, thereby increasing the level of expertise in the clinical management of COVID-19 during convalescence and facilitating individualized decision-making.
CASE SUMMARY
We describe 3 patients with COVID-19, two females aged 62 and 66 years and a male 55 years, who had low-grade fever during COVID-19 convalescence. All 3 patients had no other discomfort or comorbidities during low-grade process. Lesions on computed tomography in all 3 patients had resolved during this period. Two patients tested negative on two consecutive severe acute respiratory syndrome coronavirus 2 tests with an interval of at least 24 h between tests. Body temperature in all 3 patients returned to normal after several days without treatment, and fever recurrence was not observed.
CONCLUSION
Enhancing the knowledge of low-grade fever during COVID-19 convalescence may increase the expertise in the delivery of optimal healthcare services.
Keywords: COVID-19, Convalescence, Low-grade fever, Clinical characteristics, SARS-CoV-2, Case report
Core tip: Coronavirus disease 2019 (COVID-19) has spread rapidly worldwide, and our knowledge of this infection is still insufficient in many respects. We present 3 cases with low-grade fever during COVID-19 convalescence. These cases highlight the main clinical, radiographic, and laboratory characteristics of COVID-19 and help provide guidance for frontline medical staff in terms of clinical management and individualized decision-making during convalescence.
INTRODUCTION
Since the outbreak in December 2019, coronavirus disease 2019 (COVID-19) has spread worldwide[1]. The number of infected cases and the death toll have markedly increased[2]. Studies have confirmed that COVID-19 is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) via human to human transmission[3]. Although there have been reports on the epidemiological and clinical characteristics of COVID-19[1,4], our knowledge on this infection is still insufficient in many respects, such as symptoms, trends, prognosis, etc. In particular, if disease fluctuations or recurrence develop during convalescence, which refers to recovered non-febrile patients without respiratory symptoms[5], a patient's treatment may fail and the patient may spread the virus to others. Thus, we report 3 clinical cases of low-grade fever (axillary temperature of 37-38°C) during COVID-19 convalescence, and highlight the main clinical, radiographic, and laboratory characteristics. We hope that this report will enhance the knowledge of COVID-19 and help increase the level of expertise in frontline medical staff with regard to clinical management and individualized decision-making during convalescence.
CASE PRESENTATION
Chief complaints
Patient A was a 62-year-old female who had a fever, cough, and anorexia for 1 wk, Patient B was a 66-year-old female with a fever and cough for 2 d, and Patient C was a 55-year-old male with a fever, cough, and fatigue for 4 d.
History of present illness
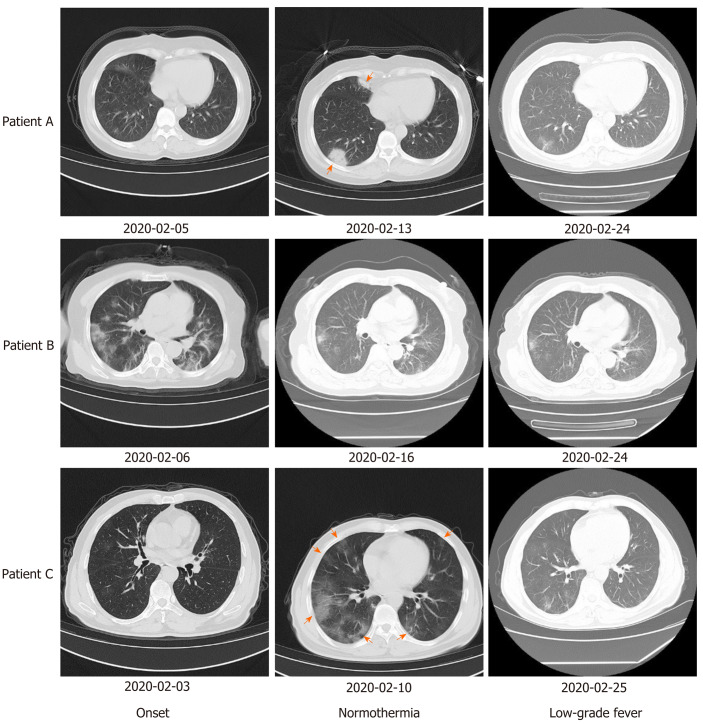
Patient A: From disease onset on 1 February 2020, the patient had a fever with a maximum axillary temperature of 39.8°C, which was accompanied by a cough and anorexia (details shown in Table 1). The patient was considered to have a common upper respiratory tract infection and was treated with antibiotics and ribavirin. Isolation and preventive measures were not taken. The patient's condition did not improve, and COVID-19 was confirmed on February 7 by positive SARS-CoV-2 oropharyngeal swab test at our local Center for Disease Control (CDC). She was transferred to an isolation ward, and antiviral treatment with arbidol was administered. Her temperature returned to normal on February 10, but new lesions on computed tomography (CT) were observed on February 13 (Figure 1). Antibiotics and arbidol were discontinued on February 18 and February 20, respectively, and no drugs were used since then. During convalescence, the patient's cough and anorexia were completely relieved, and two consecutive SARS-CoV-2 tests were negative with an interval of at least 24 h between tests. However, she developed low-grade fever for 3 d from February 23 to 25 without any other discomfort or comorbidities, and her CT lesions were resolved (Figure 1). Her temperature returned to normal without treatment.
Table 1.
Information of the 3 patients at different time points
|
Patient A |
Patient B |
Patient C |
||||||
| Onset, 02-07 | Normothermia, 02-18 | Low-grade fever, 02-23 | Onset, 02-05 | Normothermia, 02-14 | Low-grade fever, 02-23 | Onset, 02-03 | Normothermia, 02-14 | Low-grade fever, 02-24 |
| Age in yr | ||||||||
| 62 | 66 | 55 | ||||||
| Female | Female | Male | ||||||
| Fever | ||||||||
| Yes | No | Yes | Yes | No | Yes | Yes | No | Yes |
| Cough | ||||||||
| ++ | + | No | ++ | + | No | ++ | + | No |
| Expectoration | ||||||||
| No | No | No | ++ | + | No | + | + | No |
| Anorexia | ||||||||
| ++ | + | No | No | No | No | + | No | No |
| Fatigue | ||||||||
| + | + | No | No | No | No | ++ | + | No |
| Pharyngalgia | ||||||||
| No | No | No | Yes | No | No | Yes | No | No |
| Dyspnea | ||||||||
| No | No | No | No | No | No | No | No | No |
| Diarrhea | ||||||||
| No | No | No | ++ | + | No | No | No | No |
| Constipation | ||||||||
| No | No | No | No | No | No | + | No | No |
| Insomnia | ||||||||
| + | + | No | No | No | No | + | No | No |
| WBC: 4-10 × 109/L | ||||||||
| 3.5 | 4.8 | 3.8 | 2.9 | 4.0 | 3.5 | 3.3 | 5.8 | 3.2 |
| RBC: 3.5-5.5 × 1012/L | ||||||||
| 3.2 | 3.1 | 2.5 | 3.6 | 3.4 | 3.1 | 4.6 | 3.2 | 3.8 |
| Hb: 110-160 g/L | ||||||||
| 97.0 | 89.0 | 73.0 | 112.0 | 105.0 | 96.0 | 145.0 | 99.0 | 115.0 |
| NEUT: 2-7 × 109/L | ||||||||
| 1.7 | 2.2 | 1.8 | 1.7 | 2.7 | 1.8 | 2.2 | 3.8 | 1.1 |
| LYM: 0.8-4 × 109/L | ||||||||
| 1.6 | 1.7 | 1.6 | 0.8 | 1.0 | 1.2 | 0.9 | 1.4 | 1.6 |
| PCT: 0.5-1.5 ng/mL | ||||||||
| NA | 0.07 | 0.05 | NA | 0.11 | 0.05 | NA | 0.05 | 0.05 |
| CRP: ≤ 10 mg/L | ||||||||
| 3.4 | 0.9 | 0.8 | 7.2 | 1.6 | 0.8 | 68.7 | 0.8 | 0.8 |
| SAA: ≤ 10 mg/L | ||||||||
| NA | 10.3 | 9.0 | NA | 11.3 | 9.0 | NA | 8.2 | 9.1 |
| SARS-CoV-2 test | ||||||||
| P | P | N | P | P | P | P | P | N |
++: Severe; +: Mild or moderate. CRP: C-reactive protein; Hb: Hemoglobin; NA: Not available; N: Negative; NEUT: Neutrophils; P: Positive; PCT: Procalcitonin; PLT: Platelet; SAA: Serum amyloid A; RBC: Red blood cell; WBC: White blood cell.
Figure 1.
Computed tomography images of lesions in the 3 patients at different time points. New lesions appeared in patients A and C after temperature returned to normal. Lesions had resolved in all 3 patients when low-grade fever occurred. Red arrows indicate new lesions.
Patient B: From disease onset on 4 February 2020, the patient had a fever with a maximum axillary temperature of 38.1°C, accompanied by a cough (details shown in Table 1) and multiple lesions on CT (Figure 1). The patient was considered to have a common upper respiratory tract infection and was treated with the Lianhua Qingwen tablet. Isolation and preventive measures were not taken. The patient's condition did not improve, and COVID-19 was confirmed on February 5 by positive SARS-CoV-2 oropharyngeal swab test at our local CDC. She was transferred to an isolation ward and was treated with lopinavir and ritonavir tablets. Her temperature returned to normal on February 8. Lopinavir and ritonavir tablets were discontinued on February 17, and no drugs were used since then. During convalescence, the patient's cough was completely relieved, and CT lesions resolved (Figure 1), but her SARS-CoV-2 test remained positive. She developed low-grade fever for 4 d from February 23 to 26 without other discomfort, comorbidities, or new CT lesions (Figure 1). Her temperature returned to normal without treatment.
Patient C: From disease onset on 31 January 2020, the patient had a fever with a maximum axillary temperature of 39.9°C, which was accompanied by a cough and fatigue (details shown in Table 1). The patient was considered to have common upper respiratory tract infection and was treated with antibiotics. Isolation and preventive measures were not taken, and the patient's condition did not improve. COVID-19 was confirmed on February 3 by positive SARS-CoV-2 oropharyngeal swab test at our local CDC. He was transferred to an isolation ward and treated with arbidol, interferon, glucocorticoid, and expectorants. His temperature returned to normal on February 9, but multiple new CT lesions were observed on February 10 (Figure 1). All drugs were discontinued on February 13. During convalescence, the patient's cough and fatigue were completely relieved, and two consecutive SARS-CoV-2 tests were negative with an interval of at least 24 h between tests. However, he developed low-grade fever for 3 d from February 24 to 26 without any other discomfort or comorbidities, and his CT lesions resolved (Figure 1). His temperature returned to normal without treatment.
Personal and family history
Patient A had contact with a COVID-19-confirmed patient (not confirmed at that time). Patient B had no contact history in the affected regions or with infected persons. Patient C had contact with persons from Wuhan, China, where the epidemic was first identified.
Physical examination upon admission
In all 3 patients, Velcro rales were heard in both lungs at the onset and disappeared during convalescence.
Laboratory examinations
Blood cell analysis and SARS-CoV-2 test results in the 3 patients at different time points are shown in Table 1. The results of blood biochemistry (hepatic function, renal function, electrolytes, lipid, and glucose), tumor biomarkers, and coagulation function were normal and are shown in Supplementary Table 1.
Imaging examinations
Patient A: On February 5 (onset stage), ground-glass opacities (GGO) were seen in the posterior right lower lung. On February 13 (temperature returned to normal), the lesion was significantly enlarged with solid shadows and blurred edges. On February 24 (low-grade fever stage during convalescence), the lesions had been absorbed and the density was decreased.
Patient B: On February 6 (onset stage), there were multiple irregular schistose GGO in both lungs (mainly under the pleura) with consolidations and strip shadows. On February 16 (temperature returned to normal) the lesions were partially absorbed, the range reduced, and the density decreased. On February 24 (low-grade fever stage during convalescence), the lesions were further absorbed and were less dense.
Patient C: On February 3 (onset stage), small patchy GGO appeared in the right lung. On February 10 (temperature returned to normal), the lesions were increased and enlarged, showing multiple irregular schistose GGO in both lungs (mainly under the pleura) and some strip shadows in the posterior left lower lung. On February 25 (low-grade fever stage during convalescence), the lesions were absorbed and reduced, with partial strip changes.
Images of lesions in all 3 patients at different time points are shown in Figure 1.
FINAL DIAGNOSIS
The 3 patients were confirmed with COVID-19 by positive SARS-CoV-2 oropharyngeal swab test at our local CDC.
TREATMENT
All 3 patients did not receive any treatment for low-grade fever, and body temperature returned to normal after several days.
OUTCOME AND FOLLOW-UP
The 3 patients had no recurrence of low-grade fever, any other discomfort, or comorbidities. Patients A and C had no recurrence of positive SARS-CoV-2 tests. SARS-CoV-2 tests in Patient B were negative on March 4 and did not change to positive.
DISCUSSION
Self-limiting low-grade fever during COVID-19 convalescence is an atypical symptom of infectious diseases. The characteristics of the 3 presented cases are summarized as follows.
All 3 patients experienced leukopenia and neutropenia during low-grade fever. Leukopenia and neutropenia often indicate low immunity[6,7] and are more common in COVID-19 patients than in non-COVID-19 patients[8]. The trend in white blood cells in the 3 patients shifted with disease onset, normothermia, and low-grade fever. That is, leukopenia occurred during the course of fever but returned to normal when the patients were normothermic. We speculate that these patients with COVID-19 had low-grade fever during convalescence because their immunity was not fully recovered at this stage. This suggests that disease fluctuations and recurrence of positive SARS-CoV-2 may occur in convalescence[9], thus surveillance cannot be relaxed at this stage.
The CT features in the 3 patients were consistent with typical COVID-19 and include involvement of both lung parenchyma and interstitium; the GGO and a single lesion appearing in early-phase of disease; coexistence of aggravation and repair of CT signs in the advanced phase; and presentation of multifocal distributions in the middle and lower lung areas and the posterior lung region[10]. Other studies have reported that during recovery from COVID-19 (without severe respiratory distress), severe lung abnormalities are seen approximately 10 d after initial onset of symptoms[11]. This suggests that changes in CT lesions are not directly related to body temperature, and these two sets of data could complement each other in risk assessment of COVID-19.
During the course of low-grade fever, the 3 patients had no other discomfort or comorbidities, and their temperature returned to normal without any treatment. We speculate that low-grade fever may be driven by SARS-CoV-2 evolution with genetic diversity. Phan[3] found mutations and deletions on coding and non-coding regions in complete or near-complete genomes of SARS-CoV-2. Therefore, the emergence of deficient genomes during mutation and recombination of the virus is not surprising[12], and this is similar to cell wall-deficient bacteria[13]. Non-dominant deficient genomes can easily escape immune attacks while dominant genomes are cleared. It is notable that this defective genome may benefit patients by interfering with complete viral replication[14] and stimulating the host's antiviral immune response[15].
It is noteworthy that two of the 3 patients had consecutive negative SARS-CoV-2 tests with an interval of at least 24 h between tests but another patient remained positive when low-grade fever occurred. To date, no research has accurately confirmed the contagious period of COVID-19. The return of a positive SARS-CoV-2 RNA test during convalescence has been reported[9]. In addition to the basic immunity of the host and the high-mutation biological characteristics of SARS-CoV-2, detection could be affected by the site at which specimens are obtained, operators’ experience, and the actual viral load[9,16]. Furthermore, SARS-CoV-2 infection mainly focuses on the lungs rather than the upper respiratory tract[17], and some studies have indicated that higher viral loads have been detected in the nose than in the throat[9]; thus, sampling during the recovery stage using throat a swab or sputum may not detect the virus.
Given the possibility of turning positive again for SARS-CoV-2 and to provide warnings for other COVID-19 cases, we suggest that: (1) Multiple biological sample tests should be considered, including oropharyngeal swabs, nasopharyngeal swabs, sputum, bronchoalveolar lavage fluid, blood, stool, and urine, especially for highly suspected false-negative SARS-CoV-2 candidates; (2) The number of consecutive negative SARS-CoV-2 tests with an interval of at least 24 h between tests should be increased to 3 before discharge; (3) All discharged patients should extend home quarantine for at least 21 d; and (4) Patients who have not turned negative for SARS-CoV-2 more than 1.5 mo should be closely monitored and even during convalescence should also be regularly tested for risk assessment.
CONCLUSION
Low-grade fever during convalescence is an atypical symptom of COVID-19. These cases are not rare, but the mechanism and outcome of low-grade fever during convalescence is not yet completely clear. Enhancing the knowledge on low-grade fever during COVID-19 convalescence might facilitate refined risk surveillance, precision risk assessment, and dynamic risk management, thereby increasing the level of expertise for tailored delivery of optimal healthcare services for each patient with COVID-19.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
Informed consent statement: Informed written consent was obtained from the patient for publication of this report and any accompanying images.
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Peer-review started: March 21, 2020
First decision: April 24, 2020
Article in press: May 21, 2020
P-Reviewer: Georgiev T, Khan S S-Editor: Wang JL L-Editor: Filipodia E-Editor: Zhang YL
Contributor Information
Shu-Fan Zhuang, Department of Gastroenterology, The Affiliated Hospital of Jiangxi University of Traditional Chinese Medicine, Nanchang 330006, Jiangxi Province, China.
Jia Hu, Department of Gastroenterology, The Affiliated Hospital of Jiangxi University of Traditional Chinese Medicine, Nanchang 330006, Jiangxi Province, China. 356529216003@email.ncu.edu.cn.
Nan Qiao, Department of Student Affairs, Jiangxi Institute of Economic Administrators, Nanchang 330088, Jiangxi Province, China.
Zhi-Hui Lan, Department of Respiration, The Affiliated Hospital of Jiangxi University of Traditional Chinese Medicine, Nanchang 330006, Jiangxi Province, China.
Jun-Yu Lai, Department of Cardiology, The Affiliated Hospital of Jiangxi University of Traditional Chinese Medicine, Nanchang 330006, Jiangxi Province, China.
Jian-Guang Wu, Department of Cardiology, The Affiliated Hospital of Jiangxi University of Traditional Chinese Medicine, Nanchang 330006, Jiangxi Province, China.
Xiao-Yong Wu, Department of Radiology, The Affiliated Hospital of Jiangxi University of Traditional Chinese Medicine, Nanchang 330006, Jiangxi Province, China.
References
- 1.Jiang F, Deng L, Zhang L, Cai Y, Cheung CW, Xia Z. Review of the Clinical Characteristics of Coronavirus Disease 2019 (COVID-19) J Gen Intern Med. 2020;35:1545–1549. doi: 10.1007/s11606-020-05762-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Velavan TP, Meyer CG. The COVID-19 epidemic. Trop Med Int Health. 2020;25:278–280. doi: 10.1111/tmi.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phan T. Genetic diversity and evolution of SARS-CoV-2. Infect Genet Evol. 2020;81:104260. doi: 10.1016/j.meegid.2020.104260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He F, Deng Y, Li W. Coronavirus disease 2019: What we know? J Med Virol. 2020 doi: 10.1002/jmv.25766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ling Y, Xu SB, Lin YX, Tian D, Zhu ZQ, Dai FH, Wu F, Song ZG, Huang W, Chen J, Hu BJ, Wang S, Mao EQ, Zhu L, Zhang WH, Lu HZ. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin Med J (Engl) 2020;133:1039–1043. doi: 10.1097/CM9.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hastreiter AA, Makiyama EN, Borelli P, Fock RA. Impairment of G-CSF receptor on granulocytic progenitor cells causes neutropenia in protein malnutrition. Nutrition. 2020;69:110540. doi: 10.1016/j.nut.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 7.Wen J, Yang T, Wang J, Ma X, Tong Y, Zhao Y. Kanglaite Injection Combined with Chemotherapy versus Chemotherapy Alone for the Improvement of Clinical Efficacy and Immune Function in Patients with Advanced Non-Small-Cell Lung Cancer: A Systematic Review and Meta-Analysis. Evid Based Complement Alternat Med. 2020;2020:8586596. doi: 10.1155/2020/8586596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li YX, Wu W, Yang T, Zhou W, Fu YM, Feng QM, Ye JM. [Characteristics of peripheral blood leukocyte differential counts in patients with COVID-19] Zhonghua Nei Ke Za Zhi. 2020;59:E003. [PubMed] [Google Scholar]
- 9.Chen D, Xu W, Lei Z, Huang Z, Liu J, Gao Z, Peng L. Recurrence of positive SARS-CoV-2 RNA in COVID-19: A case report. Int J Infect Dis. 2020;93:297–299. doi: 10.1016/j.ijid.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou S, Wang Y, Zhu T, Xia L. CT Features of Coronavirus Disease 2019 (COVID-19) Pneumonia in 62 Patients in Wuhan, China. AJR Am J Roentgenol. 2020:1–8. doi: 10.2214/AJR.20.22975. [DOI] [PubMed] [Google Scholar]
- 11.Pan F, Ye T, Sun P, Gui S, Liang B, Li L, Zheng D, Wang J, Hesketh RL, Yang L, Zheng C. Time Course of Lung Changes at Chest CT during Recovery from Coronavirus Disease 2019 (COVID-19) Radiology. 2020;295:715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ismail SNFB, Baharum SN, Fazry S, Low CF. Comparative genome analysis reveals a distinct influence of nucleotide composition on virus-host species-specific interaction of prawn-infecting nodavirus. J Fish Dis. 2019;42:1761–1772. doi: 10.1111/jfd.13093. [DOI] [PubMed] [Google Scholar]
- 13.Xu Y, Zhang B, Wang L, Jing T, Chen J, Xu X, Zhang W, Zhang Y, Han J. Unusual features and molecular pathways of Staphylococcus aureus L-form bacteria. Microb Pathog. 2020;140:103970. doi: 10.1016/j.micpath.2020.103970. [DOI] [PubMed] [Google Scholar]
- 14.Saira K, Lin X, DePasse JV, Halpin R, Twaddle A, Stockwell T, Angus B, Cozzi-Lepri A, Delfino M, Dugan V, Dwyer DE, Freiberg M, Horban A, Losso M, Lynfield R, Wentworth DN, Holmes EC, Davey R, Wentworth DE, Ghedin E INSIGHT FLU002 Study Group; INSIGHT FLU003 Study Group. Sequence analysis of in vivo defective interfering-like RNA of influenza A H1N1 pandemic virus. J Virol. 2013;87:8064–8074. doi: 10.1128/JVI.00240-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boergeling Y, Rozhdestvensky TS, Schmolke M, Resa-Infante P, Robeck T, Randau G, Wolff T, Gabriel G, Brosius J, Ludwig S. Evidence for a Novel Mechanism of Influenza Virus-Induced Type I Interferon Expression by a Defective RNA-Encoded Protein. PLoS Pathog. 2015;11:e1004924. doi: 10.1371/journal.ppat.1004924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Z, Yi Y, Luo X, Xiong N, Liu Y, Li S, Sun R, Wang Y, Hu B, Chen W, Zhang Y, Wang J, Huang B, Lin Y, Yang J, Cai W, Wang X, Cheng J, Chen Z, Sun K, Pan W, Zhan Z, Chen L, Ye F. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J Med Virol. 2020 doi: 10.1002/jmv.25727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]