Abstract
BACKGROUND
In December 2019, an ongoing outbreak of coronavirus disease 2019 (COVID-19) was first identified in Wuhan, China. The characteristics of COVID-19 patients treated in local hospitals in Wuhan are not fully representative of patients outside Wuhan. Therefore, it is highly essential to analyze the epidemiological and clinical characteristics of COVID-19 in areas outside Wuhan or Hubei Province. To date, a limited number of studies have concentrated on the epidemiological and clinical characteristics of COVID-19 patients with different genders, clinical classification, and with or without basic diseases.
AIM
To study the epidemiological and clinical characteristics of COVID-19 patients in Hengyang (China) and provide a reliable reference for the prevention and control of COVID-19.
METHODS
From January 16 to March 2, 2020, a total of 48 confirmed cases of COVID-19 were reported in Hengyang, and those cases were included in this study. The diagnostic criteria, clinical classification, and discharge standard related to COVID-19 were in line with the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7) released by National Health Commission and National Administration of Traditional Chinese Medicine. The presence of SARS-CoV-2 in pharyngeal swab specimens was detected by quantitative reverse transcription polymerase chain reaction. All the data were imported into the excel worksheet and statistically analyzed by using SPSS 25.0 software.
RESULTS
A total of 48 cases of COVID-19 were collected, of which 1 was mild, 38 were moderate, and 9 were severe. It was unveiled that there were 31 (64.6%) male patients and 17 (35.4%) female patients, with a female-to-male ratio of 1.82:1. The range of age of patients with COVID-19 was dominantly 30-49 years old [25 (52.1%) of 48], followed by those aged over 60 years old [11 (22.9%)]. Besides, 29.2% (14 of 48) of patients had basic diseases, and 57.2% (8 of 14) of patients with basic diseases were aged over 60 years old. The occupations of 48 COVID-19 patients were mainly farmers working in agricultural production [15 (31.5%) of 48], rural migrant workers from Hengyang to Wuhan [15 (31.5%)], and service workers operating in the service sector [8 (16.7%)]. The mean latent period was 6.86 ± 3.57 d, and the median was 7 [interquartile range (IQR): 4-9] d. The mean time from onset of symptoms to the first physician visit was 3.38 ± 2.98 (95%CI: 2.58-9.18) d, with a median of 2 (IQR: 1-5) d, and the mean time from hospital admission to confirmed diagnosis was 2.29 ± 2.11 (95%CI: 1.18-6.42) d, with a median of 2 (IQR: 1-3) d. The main symptoms were fever [43 (89.6%) of 48], cough and expectoration [41 (85.4%)], fatigue [22 (45.8%)], and chills [22 (45.8%)]. Other symptoms included poor appetite [13 (27.1%)], sore throat [9 (18.8%)], dyspnea [9 (18.8%)], diarrhea [7 (14.6%)], dizziness [5 (10.4%)], headache [5 (10.4%)], muscle pain [5 (10.4%)], nausea and vomiting [4 (8.3%)], hemoptysis [4 (8.3%)], and runny nose [1 (2.1%)]. The numbers of peripheral blood leukocytes, lymphocytes, and eosinophils were significantly reduced in the majority of the patients. The levels of C-reactive protein, fibrinogen, blood glucose, lactate dehydrogenase, D-dimer, alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (γ-GT), myoglobin (MB), and creatine kinase (CK) were increased in 64.6%, 44.7%, 43.2%, 37.0%, 29.5%, 22.9%,20.8%, 21.6%, 13.6%, and 12.8% of patients, respectively. The incidence of ALT elevation in male patients was remarkably higher than that in females (P < 0.01), while the incidences of AST, CK, and blood glucose elevations in severe patients were remarkably higher than those in moderate patients (P < 0.05, respectively). Except for the mild patients, chest computed tomography showed characteristic pulmonary lesions. All the patients received antiviral drugs, 38 (79.2%) accepted traditional Chinese medicine, and 2 (4.2%) received treatment of human umbilical-cord mesenchymal stem cells. On March 2, 2020, 48 patients with COVID-19 were all cured and discharged.
CONCLUSION
Based on our results, patients with COVID-19 often have multiple organ dysfunction or damage. The incidences of ALT elevation in males, and AST, CK, and blood glucose elevations in severe patients are remarkably higher.
Keywords: Novel coronavirus pneumonia, COVID-19, SARS-CoV-2, Epidemiology, Hengyang, Coronavirus disease 2019
Core tip: To study the epidemiological and clinical characteristics of coronavirus disease 2019 (COVID-19) patients with different genders, clinical classification, and with or without basic diseases could provide more information for COVID-19 prevention and control. Our research found that many patients with COVID-19 had multiple organ dysfunction or damage. The higher attention of the government and residents on the prevention and control of COVID-19 was the key factor to control the development of COVID-19 epidemic. Wearing masks and home isolation could effectively cut off transmission routes and prevent the spread of the novel coronavirus.
INTRODUCTION
On December 31, 2019, an unexplained pneumonia was reported in Wuhan, China[1]. It was found that this pneumonia was caused by a 2019 novel coronavirus (2019-nCoV)[2], which was named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by the International Committee on Taxonomy of Viruses. The disease caused by SARS-CoV-2 was called coronavirus disease 2019 (COVID-19)[3].
Some previous articles reported the epidemiological and clinical characteristics of COVID-19 in Wuhan, and even throughout mainland China; however, from the geographical perspective, the data were mainly from the epidemic centers of Hubei Province, especially Wuhan[4,5]. However, the characteristics of patients treated in local hospitals in Wuhan are not fully representative of patients outside Wuhan. Therefore, it is highly essential to analyze the epidemiological and clinical characteristics of COVID-19 in areas outside Wuhan or Hubei Province. At the same time, there have been limited reports about the differences in the epidemiological and clinical characteristics of COVID-19 patients with different genders, clinical classification, and with or without basic diseases.
Since the first COVID-19 patient was discovered on January 16, 2020, a total of 48 confirmed cases of COVID-19 have been reported in Hengyang (Hunan Province), a close neighbor city of Hubei. Until 18:00 on March 2, 2020, all the 48 confirmed cases were cured and discharged.
At present, COVID-19 is affecting many countries and territories worldwide[6]. The present study aimed to summarize the epidemiological and clinical characteristics of COVID-19 patients in Hengyang (China) and provide a reliable reference for the prevention and control of COVID-19.
MATERIALS AND METHODS
Patients with COVID-19
According to the arrangements by the Hengyang Municipal People's Government, all the COVID-19 patients were admitted centrally to the Affiliated Nanhua Hospital of the University of South China and the First Affiliated Hospital of the University of South China without selectivity. From January 16 to March 2, 2020, all the 48 patients with COVID-19 who were treated at these two hospitals were included in the current research.
Epidemiological investigation
According to the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7) released by National Health Commission and National Administration of Traditional Chinese Medicine[7], the epidemiological history can be divided into the following five conditions: (1) History of travel to or residency in an epidemic area within 14 d prior to onset of illness; (2) History of contact with patients with COVID-19 within 14 d before onset; (3) History of contact with patients with fever or respiratory symptoms from epidemic areas within 14 d prior to onset; (4) Agglomeration; and (5) An unclear epidemic history.
Diagnosis and treatment
The diagnostic criteria, clinical classification, and discharge standard related to COVID-19 were in line with the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7)[7].
The presence of SARS-CoV-2 in pharyngeal swab specimens was detected by quantitative reverse transcription polymerase chain reaction (PCR). The SARS-CoV-2 nucleic acid diagnostic kit (PCR-Fluorescence Probing) was provided by Hunan Shengxiang Biotechnology Co., Ltd. (Changsha, China). This test utilizes the 2019-nCoV ORF 1ab and the specific conserved sequence of the gene coding for nucleocapsid protein N as the target regions, which were designed for the conserved sequence of the double-target genes, in order to detect sample RNA through fluorescent signal changes. The sequences are: Target 1 (ORF1ab)[8]: Forward primer, CCCTGTGGGTTTTACACTTAA and reverse primer, ACGATTGTGCATCAGCTGA, and the probe 5′-VIC-CCGTCTGCGGTATGTGGAAAGGTTATGBHQG-1-3′; Target 2 (N)[8]: Forward primer, GGGGAACTTCTCCTGCTAGAAT and reverse primer, CAGACATTTTGCTCTCAAGCTG, and the probe 5′-FAM-TTGCTGCTGCTTGACAGATT-TAMRA-3′. Conditions for the amplifications were 50°C for 30 min, 95°C for 1 min, followed by 45 cycles of 95°C for 15 s and 60°C for 30 s. The patients diagnosed with COVID-19 were positive for the SARS-CoV-2 nucleic acid test, and all the patients released from isolation and discharged from hospital were tested for SARS-CoV-2 nucleic acid at least twice.
All the patients received antiviral, traditional Chinese medicine (TCM), and symptomatic therapy according to the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7)[7].
Statistical analysis
The patients’ data were collected or supplemented by interviews with patients and relatives, and all the data (including epidemiological, demographic, clinical, laboratory, treatment, and outcome data) were imported into an excel worksheet and statistically analyzed by using SPSS 25.0 software (IBM, Armonk, NY, United States). All the data were checked by two physicians (Huang J and Lin HL), and any difference in interpretation between the two physicians was adjudicated by a third researcher (Yang XF).
Numerical variables are described using the mean ± SD, 95% confidence interval (95%CI), median, and interquartile range (IQR) values. Categorical variables are described as percentages and were compared using the χ2 test. P < 0.05 was considered statistically significant.
RESULTS
Patients’ demographic characteristics
A total of 48 cases of COVID-19 were collected, of which 1 was mild, 38 were moderate, and 9 were severe. The mean age of the 48 patients was 44.35 ± 15.76 (95%CI: 3.42-48.24) years old, and the median age was 41 (IQR: 34.75-53.75) years old; for moderate cases, the mean age was 43.95 ± 16.24 (95%CI: 12.64-75.36) years old, and the median age was 41 (IQR: 11.64-74.36) years old; for severe cases, the mean age was 49.11 ± 13.45 (95%CI: 22.54-75.46) years old, and the median age was 47 (IQR: 38-65) years old. Among all the patients, the maximum and minimum ages were 76 and 11 years old, respectively. The range of age of patients with COVID-19 was dominantly 30-49 years old [25 (52.1%) of 48], followed by those aged over 60 years old [11 (22.9%) of 48]. There were no children who were aged under 10 years old. Besides, 29.2% (14 of 48) of patients had basic diseases, and 57.2% (8 of 14) of patients with basic diseases were aged over 60 years old. The prevalence of COVID-19 in patients over 60 years old with basic diseases was significantly higher than that in patients without basic diseases (P < 0.05).
It was unveiled that there were 31 (64.6%) male patients and 17 (35.4%) female patients, with a female-to-male ratio of 1.82:1. The occupations of 48 COVID-19 patients were mainly farmers working in agricultural production [15 (31.5%) of 48], rural migrant workers from Hengyang to Wuhan [15 (31.5%)], and service workers operating in the service sector [8 (16.7%)]. The patients’ demographic characteristics are shown in Table 1.
Table 1.
Demographic characteristic of 48 coronavirus disease 2019 patients at the time of admission (%)
| Constituent (%) | T (n = 48) | A (n = 31) | B (n = 17) | χ2 (P value1) | C (n = 14) | D (n = 34) | χ2 (P value2) | E (n = 38) | F (n = 9) | χ2 (P value3) |
| Age (years old) | ||||||||||
| Over 60 | 22.9 (11) | 19.4 (6) | 29.4 (5) | 0.1882 (> 0.5) | 57.2 (8) | 8.8 (3) | 10.514 (< 0.005) | 21.1 (8) | 33.3 (3) | 0.119 (> 0.5) |
| 50-59 | 10.4 (5) | 9.7 (3) | 11.8 (2) | 0.0715 (> 0.75) | 7.1 (1) | 11.8 (4) | 0.00 (> 0.9) | 10.5 (4) | 11.1 (1) | 0.003 (> 0.9) |
| 40-49 | 25.0 (12) | 19.4 (6) | 35.3 (6) | 0.759 (> 0.25) | 14.3 (2) | 29.4 (10) | 0.538 (> 0.25) | 26.3 (10) | 22.2 (2) | 0.000 (> 0.9) |
| 30-39 | 27.1 (13) | 29 (9) | 23.5 (4) | 0.001 (> 0.9) | 21.4 (3) | 29.4 (10) | 0.043 (> 0.75) | 26.3 (10) | 22.2 (2) | 0.000 (> 0.9) |
| 20-29 | 10.4 (5) | 16.1 (5) | 0 (0) | 1.576 (> 0.1) | 0 (0) | 14.7 (5) | 0.992 (> 0.25) | 10.5 (4) | 11.1 (1) | 0.000 (> 0.9) |
| 10-19 | 4.2 (2) | 6.4 (2) | 0 (0) | 0.1 (> 0.5) | 0 (0) | 5.9 (2) | 0.018 (> 0.9) | 5.3 (2) | 0 (0) | 0.000 (> 0.9) |
| Under 10 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Occupation | ||||||||||
| Technical professionals | 2.1 (1) | 3.2 (1) | 0 (0) | 0.0949 (> 0.75) | 7.1 (1) | 0 (0) | 0.215 (> 0.25) | 2.6 (1) | 0 (0) | 0.000 (> 0.9) |
| Office staff | 8.3 (4) | 12.9 (4) | 0 (0) | 1.0 (> 0.25) | 0 (0) | 11.8 (4) | 0.587 (> 0.25) | 10.5 (4) | 0 (0) | 0.125 (> 0.5) |
| Service workers | 16.7 (8) | 16.1 (5) | 17.7 (3) | 0.07 (> 0.75) | 14.3 (2) | 17.6 (6) | 0.000 (> 0.9) | 13.2 (5) | 33.3 (3) | 0.912 (>0 .25) |
| Farmers | 31.2 (15) | 19.4 (6) | 52.9 (9) | 5.76 (< 0.025) | 50 (7) | 23.5 (8) | 2.119 (> 0.1) | 31.6 (12) | 22.2 (2) | 0.021 (> 0.75) |
| Rural migrant workers | 31.2 (15) | 35.5 (11) | 23.5 (4) | 0.73 (> 0.25) | 28.6 (4) | 32.4 (11) | 0.000 (> 0.9) | 31.6 (12) | 33.3 (3) | 0.000 (> 0.9) |
| Students | 6.3 (3) | 9.7 (3) | 0 (0) | 0.49 (> 0.25) | 0 (0) | 8.8 (3) | 0.242 (> 0.5) | 7.9 (3) | 0 (0) | 0.000 (> 0.9) |
| Others | 4.2 (2) | 3.2 (1) | 5.9 (1) | 0.1 (> 0.5) | 0 (0) | 5.9 (2) | 0.000 (> 0.9) | 2.6 (1) | 11.1 (1) | 0.046 (> 0.25) |
Indicates differences between male and female patients.
Denotes differences between patients with and without basic diseases.
Represents differences between moderate and severe patients. P < 0.05 was considered statistically significant. T: All patients; A: Male; B: Female; C: With basic diseases; D: Without basic diseases; E: Moderate; F: Severe.
Epidemiological history
Among 48 COVID-19 patients, there was no history of wildlife exposure (including direct contact with animals and visits to the wildlife market) within 14 d, and 58.3% (28 of 48) had a history of travel to or residency in Wuhan and surrounding areas. In addition, 31.3% (15 of 48) had a history of contact with patients with COVID-19 within 14 d; 10.4% (5 of 48) had a history of contact with patients with fever or respiratory symptoms from the epidemic area within 14 d prior to onset; 22.9% (11 of 48) had clustered onset, which occurred in 10 families and 1 community; 8.3% (4 of 48) of patients had an unclear epidemic history.
Date distribution of confirmed cases
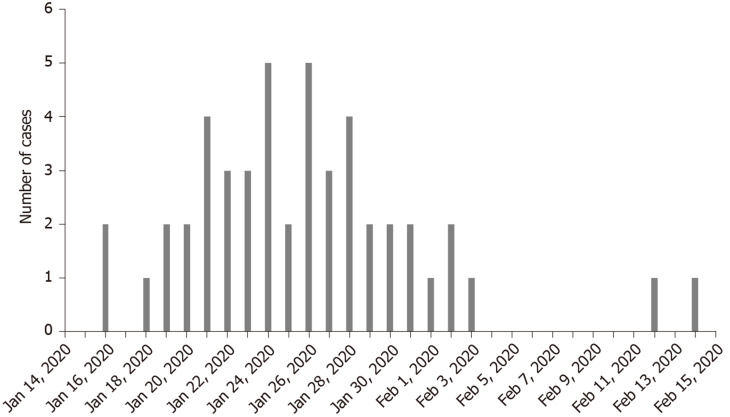
The first onset of COVID-19 occurred on January 16, 2020. Figure 1 shows the distribution of confirmed cases of COVID-19 by date of onset. It was uncovered that there were no new confirmed cases in Hengyang after February 14, 2020.
Figure 1.
Distribution of confirmed cases of coronavirus disease 2019 by date of onset.
Latent period
The latent period was defined as the time from exposure to the onset of symptoms, which was estimated among patients who could provide the exact date of close contact with individuals from Wuhan and other epidemic areas with confirmed or suspected SARS-CoV-2 infection. The longest latent period was 15 days and the shortest was 1 day. The mean latent period was 6.86 ± 3.57 d, and the median was 7 (IQR: 4.25-9.00) d.
Mean time of first physician visit and confirmed diagnosis
The mean time from onset of symptoms to the first physician visit was 3.38 ± 2.98 (95%CI: 2.58-9.18) d, with a median of 2 (IQR: 1-5) d, and the mean time from hospital admission to confirmed diagnosis was 2.29 ± 2.11 (95%CI: 1.18-6.42) d, with a median of 2 (IQR: 1-3) d.
Basic diseases
It was uncovered that 29.2% (14 of 48) of patients had basic diseases (also known as Co-morbidities), including 2 cases of coronary disease, 1 case of hypertension, 1 case of coronary disease with hypertension, diabetes, and cerebral infarction, 2 cases of diabetes with hypertension and hyperlipidemia, 1 case of diabetes with hypertension and gout, and 7 cases of chronic hepatitis B. In the 7 cases of chronic hepatitis B, the serum markers of hepatitis B were positive for hepatitis B surface antigen, hepatitis B “e” antibody, and hepatitis B “c” antibody, 6 cases had HBV-DNA titers < 500 IU/mL, and 1 case had an HBV-DNA titer > 500 IU/mL. Except for 1 case with elevated alanine aminotransferase (ALT) and 1 case with elevated aspartate aminotransferase (AST), the remaining 5 patients had normal liver function, and 7 patients did not take antiviral drugs.
Symptoms and signs
The most common symptoms at the time of admission were fever [43 (89.6%) of 48], cough and expectoration [41 (85.4%)], chills [22 (45.8%)], and fatigue [22 (45.8%)]. Other symptoms included poor appetite [13 (27.1%)], sore throat [9 (18.8%)], dyspnea [9 (18.8%)], diarrhea [7 (14.6%)], dizziness [5 (10.4%)], headache [5 (10.4%)], muscle pain [5 (10.4%)], nausea and vomiting [4 (8.3%)], hemoptysis [4 (8.3%)], and runny nose [1 (2.1%)]. At the time of admission, the mean body temperature was 38.3 ± 0.8 °C, and respiratory frequency was 21.17 ± 1.78 times/min. The clinical symptoms are summarized in Table 2.
Table 2.
The incidence of symptoms in patients with coronavirus disease 2019 at the time of admission (%)
| Symptom (%) | T (n = 48) | A (n = 31) | B (n = 17) | χ2 (P value1) | C (n = 14) | D (n = 34) | χ2 (P value2) | E (n = 38) | F (n = 9) | χ2 (P value3) |
| Fever | 89.6 (43) | 93.5 (29) | 82.4 (14) | 0.5189 (> 0.25) | 71.4 (10) | 67.6 (23) | 0.0073 (> 0.9) | 92.1 (35) | 88.9 (8) | 0.1238 (> 0.5) |
| Cough and expectoration | 85.4 (41) | 87.1 (27) | 82.4 (14) | 0.0013 (> 0.9) | 71.4 (10) | 91.2 (31) | 1.722 (> 0.1) | 86.8 (33) | 88.9 (8) | 0.0276 (> 0.5) |
| Chill | 45.8 (22) | 51.6 (16) | 35.3 (6) | 1.1777 (> 0.25) | 42.9 (6) | 47.1 (16) | 0.0705 (> 0.75) | 47.4 (18) | 44.4 (4) | 0.0455 (> 0.5) |
| Fatigue | 45.8 (22) | 45.2 (14) | 47.1 (8) | 0.7802 (> 0.25) | 50 (7) | 44.1 (15) | 0.1382 (> 0.75) | 44.7 (17) | 55.6 (5) | 0.0455 (> 0.5) |
| Poor appetite | 27.1 (13) | 25.8 (8) | 29.4 (5) | 1.1868 (> 0.25) | 35.7 (5) | 23.5 (8) | 0.2562 (> 0.5) | 23.7 (9) | 44.4 (4) | 0.9938 (> 0.25) |
| Sore throat | 18.8 (9) | 16.1 (5) | 23.5 (4) | 0.0584 (> 0.25) | 21.4 (3) | 17.6 (6) | 0.0103 (> 0.9) | 18.4 (7) | 11.1 (1) | 0.00099 (> 0.9) |
| Dyspnea | 18.8 (9) | 16.1 (5) | 23.5 (4) | 0.0584 (> 0.25) | 14.3 (2) | 20.6 (7) | 0.0103 (> 0.9) | 13.2 (5) | 44.4 (4) | 2.8017 (> 0.05) |
| Diarrhea | 14.6 (7) | 6.5 (2) | 29.4 (5) | 4.494 (> 0.25) | 14.3 (2) | 14.7 (5) | 0.1701 (> 0.5) | 7.9 (3) | 33.3 (3) | 2.2526 (> 0.1) |
| Dizziness | 10.4 (5) | 6.5 (2) | 17.6 (3) | 0.5189 (> 0.25) | 21.4 (3) | 5.9 (2) | 1.1726 (> 0.25) | 10.5 (4) | 11.1 (1) | 0.3025 (> 0.5) |
| Headache | 10.4 (5) | 12.9 (4) | 5.9 (1) | 0.1413 (> 0.25) | 0 (0) | 14.7 (5) | 0.9925 (> 0.25) | 7.9 (3) | 22.2 (2) | 0.4255 (> 0.1) |
| Muscle ache | 10.4 (5) | 3.2 (1) | 23.5 (4) | 2.9184 (> 0.05) | 14.3 (2) | 8.8 (3) | 0.0019 (> 0.9) | 10.5 (4) | 11.1 (1) | 0.3025 (> 0.9) |
| Nausea and vomiting | 8.3 (4) | 6.5 (2) | 11.8 (2) | 0.0083 (> 0.5) | 7.1 (1) | 8.8 (3) | 0.1467 (> 0.9) | 10.5 (4) | 0 (0) | 0.1248 (> 0.5) |
| Hemoptysis | 8.3 (4) | 6.5 (2) | 11.8 (2) | 0.0083 (> 0.05) | 7.1 (1) | 8.8 (3) | 0.1467 (> 0.9) | 7.9 (3) | 11.1 (1) | 0.1248 (> 0.5) |
| Runny nose | 2.1 (1) | 3.2 (1) | 0 (0) | 0.0949 (> 0.5) | 0 (0) | 2.9 (1) | 0.2146 (> 0.5) | 2.6 (1) | 0 (0) | 0.6281 (> 0.25) |
Indicates differences between male and female patients.
Denotes differences between patients with and without basic diseases.
Represents differences between moderate and severe patients. P < 0.05 was considered statistically significant. T: All patients; A: Male; B: Female; C: With basic diseases; D: Without basic diseases; E: Moderate; F: Severe.
Laboratory results
In the first blood routine examination, the majority [29 (60.4%) of 48] of patients were in normal range, and the levels of leucocytes were reduced in about one third of patients [18 (37.5%)]. Neutrophil count was in normal range in the majority of patients [38 (79.2%)], and it was decreased in some [7 (14.6%)] and increased in a limited number of patients [3 (6.2%)]. The lymphocyte count was reduced in the majority of patients [34 (70.8%) of 48], was in normal range in about one third of patients [13 (27.1%)], and was elevated in a small number of cases [1 (2.1%)]. The eosinophil count was significantly reduced [48 (100.0%)].
The liver function tests showed that serum levels of ALT, AST, γ-GT, and alkaline phosphatase were increased in 22.9% (11 of 48), 20.8% (10 of 48), 21.6% (8 of 37), and 5.4% (2 of 37) of patients, respectively. The abnormal incidence of ALT elevation in male patients was significantly higher than that in female patients (P < 0.01), while the abnormal incidence of AST elevation in severe patients was significantly increased compared with moderate patients (P < 0.05). After patients with liver disease were excluded, there were 17.07% (7 of 41) of patients with elevated levels of ALT and AST, and 24.24% (8 of 33) with elevated level of γ-GT.
The serum myocardial enzyme test revealed that the levels of lactate dehydrogenase (LD-L), myoglobin (MB), and creatine kinase (CK) were increased in 37.0% (17 of 46), 13.6% (6 of 44), and 12.8% (6 of 47) of patients, respectively. The incidence of abnormal CK elevation in severe patients was significantly higher than that in moderate patients (P < 0.05). For renal function test, some patients [10 (20.9%) of 48] were found to have increased levels of uric acid. In terms of fibrinolysis detection, the levels of fibrinogen and D-dimer were increased in 44.7% (21 of 47) and 29.5% (13 of 44) of patients, respectively. Additionally, elevated serum levels of C-reactive protein and blood glucose were noted in 64.6% (31 of 48) and 43.2% (19 of 48) of patients, respectively. Compared with moderate patients, the incidence of abnormal levels of blood glucose was remarkably increased in the severe patients (P < 0.05). The results of laboratory examination are shown in Table 3.
Table 3.
Laboratory results of patients with coronavirus disease 2019 at the time of admission (%)
| T | A | B | χ2 (P value1) | C | D | χ2 (P value2) | E | F | χ2 (P value3) | |
| Routine blood parameter | ||||||||||
| Leucocytes (× 109/L) | n = 48 | n = 31 | n = 17 | n = 14 | n = 34 | n = 38 | n = 9 | |||
| Normal (4.0-10.0) | 60.4 (29) | 67.8 (21) | 47.1 (8) | 1.964 (> 0.1) | 64.3 (9) | 58.8 (20) | 0.124 (> 0.5) | 68.4 (26) | 33.3 (3) | 2.452 (> 0.05) |
| Decreased (< 4.0) | 37.5 (18) | 29 (9) | 52.9 (9) | 2.678 (> 0.1) | 35.7 (5) | 38.2 (13) | 0.027 (> 0.75) | 31.6 (12) | 55.6 (5) | 0.922 (> 0.05) |
| Increased (> 10.0) | 2.1 (1) | 3.2 (1) | 0 (0) | 0.000 (> 0.9) | 0 (0) | 3 (1) | 0.000 (> 0.9) | 0 (0) | 11.1 (1) | 0.628 (> 0.1) |
| Neutrophils (× 109/L) | n = 48 | n = 31 | n = 17 | n = 14 | n = 34 | n = 38 | n = 9 | |||
| Normal (2.0-7.0) | 79.2 (38) | 87.1 (27) | 64.7 (11) | 2.118 (> 0.1) | 78.6 (11) | 79.4 (27) | 0.106 (> 05) | 81.6 (31) | 66.7 (6) | 0.281 (> 0.5) |
| Decreased (< 2.0) | 14.6 (7) | 9.7 (3) | 23.5 (4) | 0.762 (> 0.25) | 21.4 (3) | 11.8 (4) | 0.170 (> 05) | 15.8 (6) | 11.1 (1) | 0.028 (> 0.5) |
| Increased (> 7.0) | 6.2 (3) | 3.2 (1) | 11.8 (2) | 0.298 (> 0.5) | 0 (0) | 8.8 (3) | 0.242 (> 0.5) | 2.6 (1) | 22.2 (2) | 1.970 (< 0.1) |
| Lymphocyte (× 109/L) | n = 48 | n = 31 | n = 17 | n = 14 | n = 34 | n = 38 | n = 9 | |||
| Normal (1.2-3.2) | 27.1 (13) | 32.3 (10) | 17.6 (3) | 0.562 (> 0.25) | 35.7 (5) | 23.5 (8) | 0.256 (> 0.50) | 34.2 (13) | 0 (0) | 2.718 (< 0.1) |
| Decreased (< 1.2) | 70.8 (34) | 64.5 (20) | 82.4 (14) | 0.938 (> 0.25) | 64.3 (9) | 73.5 (25) | 0.085 (> 0.75) | 63.2 (24) | 100 (9) | 3.125 (> 0.05) |
| Increased (> 3.2) | 2.1 (1) | 3.2 (1) | 0 (0) | 0.095 (>0.75) | 0 (0) | 3 (1) | 0.000 (> 0.90) | 2.6 (1) | 0 (0) | 0.000 (> 0.9) |
| Eosinophils (× 109/L) | n = 48 | n = 31 | n = 17 | n = 14 | n = 34 | n = 38 | n = 9 | |||
| Normal (0.1-0.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Decreased (< 0.1) | 100.0 (48) | 100 (31) | 100 (17) | 0.000 (> 0.9) | 100.0 (14) | 100.0 (34) | 0.000 (> 0.9) | 100 (38) | 100 (9) | 0.000 (> 0.9) |
| Increased (> 0.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Liver function | ||||||||||
| ALT (U/L) | n = 48 | n = 31 | n = 17 | n = 14 | n = 34 | n = 38 | n = 9 | |||
| Normal (9-50) | 75 (36) | 61.3 (19) | 100 (17) | 6.831 (< 0.01) | 78.6 (11) | 73.5 (25) | 0.000 (>0.9) | 73.7 (28) | 77.8 (7) | 0.029 (> 0.05) |
| Decreased (< 9) | 2.1 (1) | 3.2 (1) | 0 (0) | 0.000 (>0.9) | 0 (0) | 3 (1) | 0.000 (> 0.9) | 2.6 (1) | 0 (0) | 0.000 (> 0.9) |
| Increased (> 50) | 22.9 (11) | 35.5 (11) | 0 (0) | 5.946 (< 0.01) | 21.4 (3) | 23.5 (8) | 0.049 (> 0.75) | 23.7 (9) | 22.2 (2) | 0.119 (> 0.05) |
| AST (U/L) | n = 48 | n = 31 | n = 17 | n = 14 | n = 34 | n = 38 | n = 9 | |||
| Normal (15-40) | 70.8 (34) | 71 (22) | 70.6 (12) | 0.000 (> 0.9) | 71.4 (10) | 70.6 (24) | 0.085 (> 0.75) | 81.6 (31) | 33.3 (3) | 6.225 (<0.05) |
| Decreased (< 15) | 8.4 (4) | 6.4 (2) | 11.8 (2) | 0.000 (> 0.9) | 7.2 (1) | 8.8 (3) | 0.147 (> 0.75) | 7.9 (3) | 0 (0) | 0.000 (> 0.9) |
| Increased (> 40) | 20.8 (10) | 22.6 (7) | 17.6 (3) | 0.001 (> 0.9) | 21.4 (3) | 20.6 (7) | 0.106 (> 0.5) | 10.5 (4) | 66.7 (6) | 10.547 (< 0.01) |
| ALP (U/L) | n = 48 | n = 31 | n = 17 | n = 14 | n = 34 | n = 38 | n = 9 | |||
| Normal (40-129) | 62.2 (23) | 69.2 (18) | 45.5 (5) | 0.984 (> 0.25) | 80.0 (8) | 55.6 (15) | 0.960 (> 0.25) | 71.4 (20) | 37.5 (3) | 1.808 (> 0.1) |
| Decreased (< 40) | 32.4 (12) | 23.1 (6) | 54.5 (6) | 2.205 (> 0.1) | 20.0 (2) | 37.0 (10) | 0.345 (> 0.25) | 25.0 (7) | 50.0 (4) | 0.844 (> 0.1) |
| Increased (> 129) | 5.4 (2) | 7.7 (2) | 0 (0) | 0.023 (> 0.9) | 0 (0) | 7.4 (2) | 0.004 (> 0.9) | 3.6 (1) | 12.5 (1) | 0.009 (> 0.25) |
| γ-GT (U/L) | n = 37 | n = 26 | n = 11 | n = 10 | n = 27 | n = 28 | n = 8 | |||
| Normal (7-60) | 78.4 (29) | 69.2 (18) | 100 (11) | 2.694 (> 0.05) | 80.0 (8) | 77.8 (21) | 0.000 (> 0.9) | 82.1 (23) | 62.5 (5) | 0.485 (> 0.25) |
| Decreased (< 7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Increased (> 60) | 21.6 (8) | 30.8 (8) | 0 (0) | 2.694 (> 0.05) | 20.0 (2) | 22.2 (6) | 0.000 (> 0.9) | 17.9 (5) | 37.5 (3) | 0.485 (> 0.25) |
| Renal function | ||||||||||
| Urea nitrogen (mmol/L) | n = 48 | n = 31 | n = 17 | n = 14 | n = 34 | n = 38 | n = 9 | |||
| Normal (2.76-8.07) | 75.0 36) | 80.7 (25) | 64.7 (11) | 0.759 (> 0.25) | 78.6 (11) | 73.5 (25) | 0 (> 0.9) | 76.3 (29) | 77.8 (7) | 0.119 (> 0.05) |
| Decreased (< 2.76) | 22.9 (11) | 16.1 (5) | 35.3 (6) | 1.327 (> 0.1) | 14.3 (2) | 26.5 (9) | 0.286 (> 0.5) | 23.7 (9) | 22.2 (1) | 0.141 (> 0.05) |
| Increased (> 8.07) | 2.1 (1) | 3.2 (1) | 0 (0) | 0.000 (> 0.9) | 7.1 (1) | 0 (0) | 0.215 (> 0.25) | 0 (0) | 11.1 (1) | 0.628 (> 0.1) |
| Creatinine (μmol/L) | n = 48 | n = 31 | n = 17 | n = 14 | n = 34 | n = 38 | n = 9 | |||
| Normal (41-111) | 97.9 (47) | 96.8 (30) | 100 (17) | 0.000 (>0.9) | 92.9 (13) | 100 (34) | 0.215 (>0.25) | 100 (38) | 88.9 (8) | 0.628 (> 0.1) |
| Decreased (< 40) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Increased (> 111) | 2.1 (1) | 3.2 (1) | 0 (0) | 0.000 (> 0.9) | 7.1 (1) | 0 (0) | 0.215 (> 0.25) | 0 (0) | 11.1 (1) | 0.628 (> 0.1) |
| Uric acid (μmol/L) | n = 48 | n = 31 | n = 17 | n = 14 | n = 34 | n = 38 | n = 9 | |||
| Normal (202-428) | 70.8 (34) | 64.5 (20) | 82.3 (14) | 0.938 (> 0.25) | 71.4 (10) | 70.6 (24) | 0.085 (> 0.75) | 73.7 (28) | 55.6 (5) | 0.441 (> 0.05) |
| Decreased (< 202) | 8.4 (4) | 9.7 (3) | 5.9 (1) | 0.000 (>0.9) | 7.2 (1) | 8.8 (3) | 0.147 (> 0.5) | 7.9 (3) | 11.1 (1) | 0.000 (> 0.9) |
| Increased (> 428) | 20.8 (10) | 25.8 (8) | 11.8 (2) | 0.599 (> 0.25) | 21.4 (3) | 20.6 (7) | 0.106 (> 0.5) | 18.4 (7) | 33.3 (3) | 0.515 (> 0.05) |
| Fibrinogen (g/L) | n = 48 | n = 31 | n = 17 | n = 14 | n = 34 | n = 38 | n = 9 | |||
| Normal (2.0-4.0) | 55.3 (26) | 56.7 (17) | 52.9 (9) | 0.061 (> 0.75) | 50.0 (7) | 57.6 (19) | 0.228 (> 0.5) | 62.2 (23) | 22.2 (2) | 3.184 (> 0.05) |
| Decreased (< 2.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Increased (> 4.0) | 44.7 (21) | 43.3 (13) | 47.1 (8) | 0.061 (> 0.75) | 50.0 (7) | 42.4 (14) | 0.228 (> 0.5) | 37.8 (14) | 77.8 (7) | 3.184 (> 0.05) |
| D-dimer (mg/L) | n = 48 | n = 31 | n = 17 | n = 14 | n = 34 | n = 38 | n = 9 | |||
| Normal (0-0.5) | 70.5 (31) | 76.7 (23) | 57.1 (8) | 0.936 (> 0.25) | 69.2 (9) | 71.0 (22) | 0.0610 (> 0.75) | 71.4 (25) | 62.5 (5) | 0.005 (> 0.05) |
| Increased (>0.5) | 29.5 (13) | 23.3 (7) | 42.9 (6) | 0.936 (> 0.25) | 30.8 (4) | 29 (9) | 0.061 (> 0.75) | 28.6 (10) | 37.5 (3) | 0.005 (> 0.05) |
| Myocardial enzymes | ||||||||||
| LD-L (U/L) | n = 48 | n = 31 | n = 17 | n = 14 | n = 34 | n = 38 | n = 9 | |||
| Normal (120-250) | 58.7 (27) | 53.3 (16) | 68.8 (11) | 1.023 (> 0.25) | 61.5 (8) | 57.6 (19) | 0.060 (> 0.75) | 69.4 (25) | 11.1 (1) | 7.794 (< 0.01) |
| Decreased (< 120) | 4.3 (2) | 3.4 (1) | 6.2 (1) | 0.000 (> 0.9) | 7.7 (1) | 3.0 (1) | 0.000 (> 0.5) | 0 (0) | 22.2 (2) | 3.957 (> 0.05) |
| Increased (> 250) | 37 (17) | 43.3 (13) | 25 (4) | 1.505 (> 0.1) | 30.8 (4) | 39.4 (13) | 0.043 (> 0.75) | 30.6 (11) | 66.7 (6) | 2.606 (> 0.1) |
| Creatine kinase (U/L) | n = 48 | n = 31 | n = 17 | n = 14 | n = 34 | n = 38 | n = 9 | |||
| Normal (20-200) | 87.2 (41) | 90 (27) | 82.4 (14) | 0.090 (> 0.75) | 84.6 (11) | 88.2 (30) | 0.024 (> 0.75) | 94.6 (35) | 55.6 (5) | 6.590 (< 0.05) |
| Decreased (< 20) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Increased (> 200) | 12.8 (6) | 10 (3) | 17.6 (3) | 0.090 (> 0.75) | 15.4 (2) | 11.8 (4) | 0.0243 (> 0.75) | 5.4 (2) | 44.4 (4) | 6.590 (< 0.05) |
| Myoglobin (ng/mL) | n = 48 | n = 31 | n = 17 | n = 14 | n = 34 | n = 38 | n = 9 | |||
| Normal (0-70) | 86.4 (38) | 88.9 (24) | 82.4 (14) | 0.027 (> 0.75) | 92.3 (12) | 83.9 (26) | 0.069 (> 0.75) | 91.2 (31) | 66.7 (6) | 1.812 (> 0.05) |
| Increased (> 70) | 13.6 (6) | 11.1 (3) | 17.6 (3) | 0.027 (> 0.75) | 7.7 (1) | 16.1 (5) | 0.069 (> 0.75) | 8.8 (3) | 33.3 (3) | 1.812 (> 0.05) |
| C-reactive protein (mg/L) | n = 48 | n = 31 | n = 17 | n = 14 | n = 34 | n = 38 | n = 9 | |||
| Normal (0-6) | 35.4 17) | 38.7 (12) | 29.4 (5) | 0.415 (> 0.5) | 50.0 (7) | 29.4 (10) | 1.048 (> 0.25) | 39.5 (15) | 11.1 (1) | 1.497 (> 0.05) |
| Increased (> 6) | 64.6 (31) | 61.3 (19) | 70.6 (12) | 0.415 (> 0.5) | 50 (7) | 70.6 (24) | 1.048 (> 0.25) | 60.5 (23) | 88.9 (8) | 1.281 (> 0.05) |
| Blood glucose (mmol/L) | n = 48 | n = 31 | n = 17 | n = 14 | n = 34 | n = 38 | n = 9 | |||
| Normal (3.9-6.1) | 52.3 (23) | 55.2 (16) | 46.7 (7) | 0.287 (> 0.5) | 33.3 (4) | 59.4 (19) | 2.372 (> 0.1) | 58.3 (21) | 0 (0) | 4.861 (< .05) |
| Decreased (< 3.9) | 4.5 (2) | 6.9 (2) | 0 (0) | 0.077 (> 0.5) | 16.7 (2) | 0 (0) | 2.406 (> 0.05) | 5.6 (2) | 0 (0) | 0.000 (> 0.9) |
| Increased (> 6.1) | 43.2 (19) | 37.9 (11) | 53.3 (8) | 0.956 (> 0.5) | 50.0 (6) | 40.6 (13) | 0.3126 (> 0.5) | 36.1 (13) | 100. (6) | 6.091 (< .025) |
Indicates differences between male and female patients.
Denotes differences between patients with and without basic diseases.
Represents differences between moderate and severe patients. P < 0.05 was considered statistically significant. T: All patients; A: Male; B: Female; C: With basic diseases; D: Without basic diseases; E: Moderate; F: Severe. ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; LD-L: Lactate dehydrogenase; ALP: Alkaline phosphatase; γ-GT: γ-glutamyl transpeptidase.
Chest computed tomography
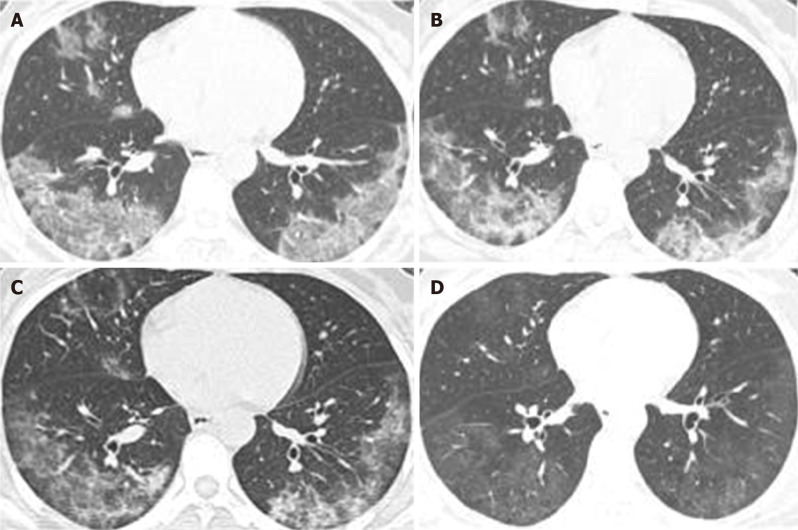
Except for 1 mild case, other patients [47 (97.92%) of 48] had pulmonary lesions, of which 8.33% (4 of 48) were single lung lesions and 89.58% (43 of 48) were double lung lesions. Pleural effusion appeared in 2 [2 (4.17%)] cases as well. Average time from onset to the first appearance of lung lesions in chest computed tomography (CT) was 3.18 ± 2.71 (95%CI: 3.031-4.63) d. Multiple small patchy shadows and interstitial changes in the early stage of lung lesions were evident in the extra-pulmonary zone; as the disease progresses, multiple ground glass shadows and invasive lesions may appear in the lungs, and in severe cases, pulmonary consolidation may occur. Figure 2 illustrates dynamic changes of chest CT in 1 case of severe COVID-19.
Figure 2.
Dynamic changes in chest computed tomography of a patient with coronavirus disease 2019. A: On day 13 of disease onset, ground glass opacities could be observed in the lower lobe of the right middle lobe and left upper lobe, and consolidation was observed in the lower lobe of both lungs, as well as consolidation of the right lung; B: On day 18 of disease onset, the consolidation of the lower lobe of both lungs was significantly reduced, and the structure was distorted. The consolidation of the lobe and ground glass was mixed; C: An obvious reduction in consolidation of both lungs and increased ground glass opacities in the lower lobe of the left lung could be observed on day 23 of disease onset; D: An obvious absorption and decreased density of bilateral lung lesions, mainly ground glass opacities and fiber strand shadow distribution, as well as bronchiectasis of the lower lobe of both lungs could be observed on day 31 of disease onset.
Treatment effect
All the patients received antiviral drugs, including alpha-interferon (5 million units, atomization; average, 11.2 d), lopinavir/ritonavir (500 mg BID; average, 9.9 d), arbidol (200 mg TID; average, 7.8 d), and chloroquine phosphate (500 mg BID; average, 7.0 d). Moreover, 38 (79.2%) patients were given TCM based on the treatment protocol[7]. Furthermore, 2 (4.2%) patients received treatment of human umbilical-cord mesenchymal stem cells. A number of patients, mainly those with co-infection, were treated with antibiotics including moxifloxacin, cefoperazone sodium, and sulbactam sodium. Severe patients were given methylprednisolone (1-2 mg/kg per day) and immunoglobulin for 3-5 d. On March 2, 2020, all the 48 patients with confirmed COVID-19 were cured and discharged.
DISCUSSION
Analysis of epidemiological characteristics
The outbreak of COVID-19 in China is roughly divided into three phases[9,10]. The first phase occurred before the end of December 2019. COVID-19 mainly caused a local outbreak among people who had contact with seafood markets. In early January 2020, the second phase commenced, with clusters of disease in multiple communities or families in Wuhan and human-to-human transmission among close contacts[10,11]. In mid-to-late January 2020, with the approach of the Spring Festival, personnel mobility increased, and the third phase of outbreak appeared. At this time, the epidemic spread from Hubei Province to other provinces in mainland China[10].
Hengyang (Hunan Province), a close neighbor of Hubei Province, began to have an outbreak in mid-January 2020, just before commencing the traditional Chinese New Year. The first case of COVID-19 onset was found on January 16, 2020, and the onset occurred 2 d after he contacted with people from Wuhan. The distribution of confirmed cases of COVID-19 by date of onset in Hengyang was mainly concentrated from mid-January to late-January. No new cases were found in Hengyang after February 14, due to non-pharmacological interventions adopted in China, including wearing masks and home isolation, which effectively cut off the transmission route of the virus.
None of the patients in Hengyang had a history of wild animal contact within 14 d, excluding the possibility of animal infection. Approximately 58.3% of the patients had a history of travel or residency in the epidemic areas of Wuhan 14 d before onset, indicating that the early cases of Hengyang were introduced from Wuhan; 31.3% had no contact with the epidemic areas within 14 d, while there was a clear history of contact with the patients infected with SARS-CoV-2. Besides, 22.9% had clustered onset, of which 10 were in family and 1 in community. The characteristics of the clustered onset are similar to those of other regions in China[9,10].
Among the 48 COVID-19 patients, the mean time from onset of symptoms to the first physician visit was 3.78 ± 2.98 d, and the mean time from hospital admission to confirmed diagnosis was 2.29 ± 2.11 d, which was shorter than the time reported in the literature[9,10]. These are closely related to the government and residents attaching great importance to the prevention and treatment of COVID-19 and technological advancements in health care.
Analysis of clinical characteristics
Totally, 48 patients with COVID-19 were enrolled, of which 1 was mild, 38 were moderate, and 9 were severe. Compared with patients who were initially infected with SARS-CoV-2 in Wuhan[11], the severity of illness of patients in Hengyang was relatively mild.
The common symptoms of patients with COVID-19 in Hengyang were fever, cough, fatigue, and chills. Approximately 10.4% of patients did not have fever and were easily ignored in the daily temperature screening. Symptoms of the upper respiratory tract were rare, with only 1 case of runny nose, which was favorable for distinguishing the disease from the upper respiratory tract. One patient had no symptoms, positive signs, or changes in chest CT before and after hospitalization, and only SARS-CoV-2 nucleic acid test was positive, which was called asymptomatic novel coronavirus infection. The literature has reported that asymptomatic patients with novel coronavirus infection are also highly infectious[6]. Wong et al[12] found that SARS-CoV-2 can be prevented through basic infection control measures, including wearing of masks, washing hands, and environmental hygiene.
The laboratory tests showed the following findings: First, the numbers of peripheral blood leukocytes, lymphocytes, and eosinophils were significantly reduced, especially the number of eosinophils, which was remarkably decreased in all the patients. Second, it was revealed that the levels of ALT, AST, and γ-GT were increased in 22.9%, 20.8%, and 21.6% of patients, respectively. At present, it is believed that COVID-19 patients suffer from dysfunction or injury in multiple organs of the body due to the following three reasons: First, the novel coronavirus directly damages the targeted organ[13-15]; second, the novel coronavirus induces systemic inflammatory immune response in the human body[5]; third, toxic and side effects of some drugs are important during the course of COVID-19 treatment[16]. However, further research should be conducted to confirm the above-mentioned findings.
Except for one mild patient, the other 47 patients had morphological characteristic of pulmonary lesions on chest CT, which plays a pivotal role in diagnosing COVID-19[17] .
The pandemic of COVID-19 caused by SARS-CoV-2 presents an unprecedented challenge to identify effective drugs for prevention and treatment[18]. The patients in the present study were treated with a combination of Western medicine and TCM. None of the 38 ordinary patients got worse and became severe, and all 48 patients were cured and discharged. In the absence of effective drugs for COVID-19[18], TCM is a helpful treatment option.
Limitations
The current study was limited by a number of factors that require further attention. First, a small number of COVID-19 cases were involved in this study. Second, laboratory examination results of a number of patients were missed, resulting in incomplete data.
In conclusion, compared with patients initially infected with SARS-CoV-2 in Wuhan, the symptoms of patients with COVID-19 in Hengyang are relatively mild, and the mean time from onset of symptoms to the first physician visit and the mean time from hospital admission to confirmed diagnosis are shorter. COVID-19 patients in the present research have the following characteristics: First, in addition to fever and respiratory symptoms, there are other systemic symptoms; second, the numbers of peripheral blood leukocytes, lymphocytes, and eosinophils are significantly reduced, especially the number of eosinophils, which is reduced in all the patients; third, some morphological characteristics of pulmonary lesions appeared in the chest CT scan; fourth, laboratory tests revealed multiple organ abnormality or damage. Our outcomes may assist clinicians in diagnosis and treatment of COVID-19.
ARTICLE HIGHLIGHTS
Research background
At present, coronavirus disease 2019 (COVID-19) is influencing 210 countries and territories worldwide, which is becoming a severe public health concern.
Research motivation
There have been rare reports about the differences in the epidemiological and clinical characteristics of COVID-19 patients with different genders, clinical classification, and with or without basic diseases.
Research objectives
To study the differences in the epidemiological and clinical characteristics of COVID-19 patients with different genders, clinical classification, and with or without basic diseases, and provide a reliable reference for the prevention and control of COVID-19.
Research methods
From January 16 to March 2, 2020, a total of 48 confirmed cases of COVID-19 have been reported in Hengyang and were included in this study. All the data were imported into the excel worksheet and statistically analyzed by using SPSS25.0 software.
Research results
The main symptoms of COVID-19 patients in Hengyang were fever, cough and expectoration, fatigue, and chills, and there were multiple organ dysfunction or damage, such as reduced peripheral blood leukocytes, lymphocytes, and eosinophils, and increased C-reactive protein, fibrinogen, blood glucose, LD-L, D-dimer, ALT, AST, γ-GT, MB, and CK. Except for the mild patients, chest CT showed pulmonary lesions. On March 2, 2020, 48 patients with COVID-19 were all cured and discharged.
Research conclusions
The epidemiological and clinical characteristics of COVID-19 patients with different genders, clinical classification, and with or without basic diseases are not very different in most cases, and slightly different in individual cases. Clinical manifestations and laboratory tests reveal that patients with COVID-19 have multiple organ dysfunction or damage.
Research perspectives
The mechanism of COVID-19 combining multiple organ dysfunction or damage is worth further study.
Footnotes
Institutional review board statement: The study was conducted in accordance with the Declaration of Helsinki, and it was approved by the Ethics Committee of Affiliated Nanhua Hospital of the University of South China.
Clinical trial registration statement: This study is not a clinical trial study.
Informed consent statement: All the patients signed a written informed consent form prior to commencing the study.
Conflict-of-interest statement: All the authors declare that there is no conflict of interest.
Data sharing statement: No additional data are available.
Manuscript source: Invited manuscript
Peer-review started: March 13, 2020
First decision: March 27, 2020
Article in press: June 2, 2020
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): E
P-Reviewer: Kimura Y, Sharma K, Shen HN, Yu B S-Editor: Dou Y L-Editor: Wang TQ E-Editor: Liu JH
Contributor Information
Zhe-Feng Zhong, Department of Infectious Diseases, the Affiliated Nanhua Hospital, Hengyang Medical College, University of South China, Hengyang 421002, Hunan Province, China.
Jia Huang, Department of Gastroenterology, the Affiliated Nanhua Hospital, Hengyang Medical College, University of South China, Hengyang 421002, Hunan Province, China.
Xia Yang, Department of General Practice, the Affiliated Nanhua Hospital, Hengyang Medical College, University of South China, Hengyang 421002, Hunan Province, China.
Jin-Ling Peng, Department of General Practice, the Affiliated Nanhua Hospital, Hengyang Medical College, University of South China, Hengyang 421002, Hunan Province, China.
Xiao-Yan Zhang, Department of General Practice, the Affiliated Nanhua Hospital, Hengyang Medical College, University of South China, Hengyang 421002, Hunan Province, China.
Yang Hu, Department of Gastroenterology, the Affiliated Nanhua Hospital, Hengyang Medical College, University of South China, Hengyang 421002, Hunan Province, China.
Nian Fu, Department of Gastroenterology, the Affiliated Nanhua Hospital, Hengyang Medical College, University of South China, Hengyang 421002, Hunan Province, China.
Hai-Lian Lin, Department of Gastroenterology, the Affiliated Nanhua Hospital, Hengyang Medical College, University of South China, Hengyang 421002, Hunan Province, China.
Bo Jiang, Department of Infectious Diseases, the Affiliated Nanhua Hospital, Hengyang Medical College, University of South China, Hengyang 421002, Hunan Province, China.
Ya-Ying Tian, Department of Infectious Diseases, the Affiliated Nanhua Hospital, Hengyang Medical College, University of South China, Hengyang 421002, Hunan Province, China.
Hong-Yi Yao, Department of Intensive Medicine, the Affiliated Nanhua Hospital, Hengyang Medical College, University of South China, Hengyang 421002, Hunan Province, China.
Li-Pu Deng, Department of Intensive Medicine, the Affiliated Nanhua Hospital, Hengyang Medical College, University of South China, Hengyang 421002, Hunan Province, China.
Xiao-Qing Tang, Institute of Clinical Medicine, the First Affiliated Hospital, Hengyang Medical College, University of South China, Hengyang 421001, Hunan Province, China.
Jie-Can Zhou, Department of Infectious Diseases, the First Affiliated Hospital, Hengyang Medical College, University of South China, Hengyang 421001, Hunan Province, China.
Jian Tang, Department of Infectious Diseases, the First Affiliated Hospital, Hengyang Medical College, University of South China, Hengyang 421001, Hunan Province, China.
Xia Xie, Department of Infectious Diseases, the First Affiliated Hospital, Hengyang Medical College, University of South China, Hengyang 421001, Hunan Province, China.
Qiong Liu, Department of Infectious Diseases, the First Affiliated Hospital, Hengyang Medical College, University of South China, Hengyang 421001, Hunan Province, China.
Jing Liu, Department of Infectious Diseases, the First Affiliated Hospital, Hengyang Medical College, University of South China, Hengyang 421001, Hunan Province, China.
Cheng-Yun Dou, Department of Infectious Diseases, the First Affiliated Hospital, Hengyang Medical College, University of South China, Hengyang 421001, Hunan Province, China.
Rong-Juan Dai, Department of Infectious Diseases, the First Affiliated Hospital, Hengyang Medical College, University of South China, Hengyang 421001, Hunan Province, China.
Bo Yan, Department of Cardiology, the First Affiliated Hospital, Hengyang Medical College, University of South China, Hengyang 421001, Hunan Province, China.
Xue-Feng Yang, Department of Gastroenterology, the Affiliated Nanhua Hospital, Hengyang Medical College, University of South China, Hengyang 421002, Hunan Province, China. yxf9988@126.com.
References
- 1.Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. J Med Virol. 2020;92:401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gostic K, Gomez AC, Mummah RO, Kucharski AJ, Lloyd-Smith JO. Estimated effectiveness of symptom and risk screening to prevent the spread of COVID-19. Elife. 2020;9 doi: 10.7554/eLife.55570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao J, Tu WJ, Cheng W, Yu L, Liu YK, Hu X, Liu Q. Clinical Features and Short-term Outcomes of 102 Patients with Corona Virus Disease 2019 in Wuhan, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Released by National Health Commission National Administration of Traditional Chinese Medicine on March 3, 2020. Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7) Chin Med J (Engl) 2020;133:1087–1095. doi: 10.1097/CM9.0000000000000819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Special Expert Group for Control of the Epidemic of Novel Coronavirus Pneumonia of the Chinese Preventive Medicine Association. An update on the epidemiological characteristics of novel coronavirus pneumonia (COVID-19) Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:139–144. doi: 10.3760/cma.j.issn.0254-6450.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY, Wong JY, Xing X, Xiang N, Wu Y, Li C, Chen Q, Li D, Liu T, Zhao J, Liu M, Tu W, Chen C, Jin L, Yang R, Wang Q, Zhou S, Wang R, Liu H, Luo Y, Liu Y, Shao G, Li H, Tao Z, Yang Y, Deng Z, Liu B, Ma Z, Zhang Y, Shi G, Lam TTY, Wu JT, Gao GF, Cowling BJ, Yang B, Leung GM, Feng Z. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong SC, Kwong RT, Wu TC, Chan JWM, Chu MY, Lee SY, Wong HY, Lung DC. Risk of nosocomial transmission of coronavirus disease 2019: an experience in a general ward setting in Hong Kong. J Hosp Infect. 2020 doi: 10.1016/j.jhin.2020.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeo C, Kaushal S, Yeo D. Enteric involvement of coronaviruses: is faecal-oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol Hepatol. 2020;5:335–337. doi: 10.1016/S2468-1253(20)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chau TN, Lee KC, Yao H, Tsang TY, Chow TC, Yeung YC, Choi KW, Tso YK, Lau T, Lai ST, Lai CL. SARS-associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology. 2004;39:302–310. doi: 10.1002/hep.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Young BE, Ong SWX, Kalimuddin S, Low JG, Tan SY, Loh J, Ng OT, Marimuthu K, Ang LW, Mak TM, Lau SK, Anderson DE, Chan KS, Tan TY, Ng TY, Cui L, Said Z, Kurupatham L, Chen MI, Chan M, Vasoo S, Wang LF, Tan BH, Lin RTP, Lee VJM, Leo YS, Lye DC Singapore 2019 Novel Coronavirus Outbreak Research Team. Epidemiologic Features and Clinical Course of Patients Infected With SARS-CoV-2 in Singapore. JAMA. 2020 doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee EYP, Ng MY, Khong PL. COVID-19 pneumonia: what has CT taught us? Lancet Infect Dis. 2020;20:384–385. doi: 10.1016/S1473-3099(20)30134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19): A Review. JAMA. 2020 doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]