Dear Editor,
We read with interest the letter by Jiang and colleagues who described the spread of SARS-CoV-2 in a family cluster in China.1 Herein, we describe the epidemiological, clinical and biological characteristics of 30 households in close contact with SARS-CoV-2-infected members, living in a confined environment during the French national lockdown. This report highlights important issues about transmission.
Two families (26 and 4 members, respectively) were included in the study. During the 5 days before the French national lockdown, both families moved from their usual Parisian residence to a closed property in the countryside, composed by 3 neighboring houses (A, B and C) in a park. House A was inhabited by 8 persons from a single family, including 3 couples who shared their bed for at least 4 days after the onset of symptoms. In house B, there were 2 families composed of 9 persons. There were 2 married couples who shared their bed during all the time, even in presence of symptoms. In house C, there were 2 families composed of 13 persons. There were 4 married couples who shared the bed until the hospitalization of the 84-year-old subject for severe SARS-CoV-2 infection. With the exception of the elderly couple who already lived in House C, all the remaining people arrived there between March 12 and 16, before the beginning of the national lockdown (March 17, 2020). Thereafter, all residents were not allowed to quit until the end of lockdown (May 11, 2020). During the first week of cohabitation there were regular and close contacts among households from the 3 houses. Since the occurrence of the first 3 symptomatic cases, contacts among the 3 houses were reduced although they indirectly continued through children displacements.
Within their stay, all residents were clinically examined at least once. RT-PCR testing of SARS-CoV-2 was performed for symptomatic cases.2 Serologic testing using the approved COVID-PRESTO® rapid diagnostic test3 (AAZ, Boulogne-Billancourt, France), detecting both IgM and IgG, was performed on whole blood finger-stick more than 45 days after the onset of symptomatic cases on all the 30 subjects. Population characteristics are detailed in Table 1 .
Table 1.
Clinical characteristics of population, results of RT-PCR on nasopharyngeal swab specimen and rapid diagnostic tests.
| Resident number, sex, age (years) | Kinship | Symptoms | Date of onset of symptoms | RT-PCR results | Ct positivity (for RdRP, N and E genes) | COVID-19 IgM | COVID-19 IgG | Smoking status |
|---|---|---|---|---|---|---|---|---|
| House A (one family) | ||||||||
| 1. Male, 672. Female, 653. Male, 344. Female, 335. Male, 336. Female, 337. Female, 28. Male, 35 | Husband case 2Wife case 1Husband case 4Wife case 3Husband case 6Wife case 5Daughter case 5Son case 1 and 2 | YesMildYesNoNoNoNoNoNo | March 13March 16March 20NANANANANA | PositiveNegativePositiveNegativeNegativeNDNDND | 20, 17, 1622,19,18 | PositiveNegativePositiveNegativeNegativeNegativeNegativeNegative | PositiveNegativeNegativeNegativeNegativePositiveNegativeNegative | NoNoYesNoYesYesNoYes |
| House B (two families) | ||||||||
| 9. Male, 3710. Female, 3711. Male, 612. Male, 413. Male, 214. Male, 4915. Female, 3516. Female, 617. Female, 4 | Husband case 10Wife case 9Son case 9 and 10Son case 9 and 10Son case 9 and 10Husband case 15Wife case 14Daughter case 14/15Daughter case 14/15 | YesNoNoNoMildNoNoNoNo | March 12NANANANANANANANA | NDNDNDNDNDNDNDNDND | NegativeNegativeNegativeNegativeNegativeNegativeNegativeNegativeNegative | PositiveNegativeNegativeNegativeNegativeNegativeNegativeNegativeNegative | YesYesNoNoNoYesYesNoNo | |
| House C (one family) | ||||||||
| 18. Male, 8419. Female, 7520. Male, 4821. Male, 2722. Female, 3723. Male, 3824. Male, 725. Male, 326. Male, 4727. Female, 4628. Male, 1629. Male, 1430. Male, 8 | Husband case 19Wife case 18Son case 18–19,Husband case 20Daughter case 19/20Husband case 22Son case 22/23Son case 22/23Husband case 27Wife case 26Son case 26/27Son case 26/27Son case 26/27 | Yes, severeYesYesNoYesMildNoNoNoNoNoNoNo | March 21March 24March 24NAMarch 10March 14NANANANANANANA | PositiveNDNDNDNDNDNDNDNDNDNDNDND | 27, 28, 26 | PositiveNegativeNegativeNegativeNegativeNegativeNegativeNegativeNegativeNegativeNegativeNegativeNegative | PositivePositivePositiveNegativePositivePositiveNegativeNegativeNegativeNegativeNegativeNegativeNegative | NoNoNoNoYesYesNoNoYesNoNoNoNo |
Abbreviations: Ct = cycle threshold; NA = not applicable; ND = not done.
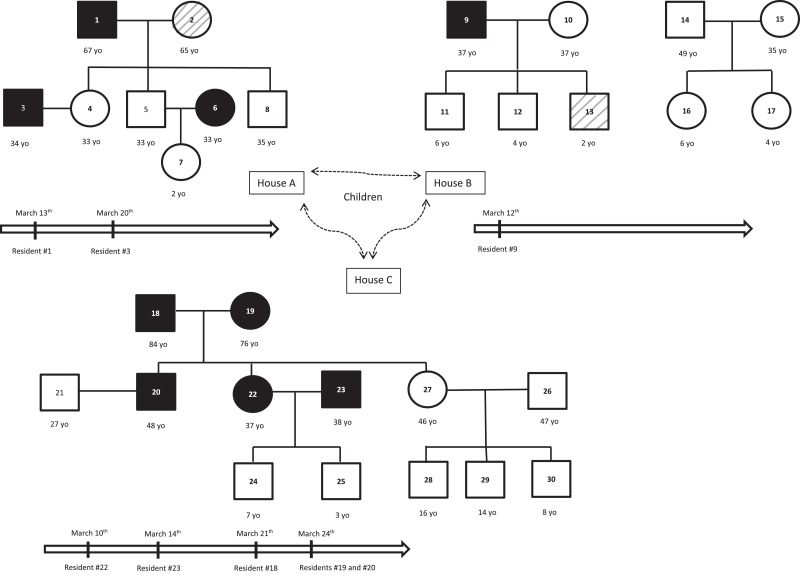
The first diagnosed case was a 67-year-old man (resident #1) living in the house A and referring to the Infectious Diseases outpatient clinic on March 17 for cough, fever and asthenia for 4 days. Investigation of the cluster began soon after resident #1 was formally diagnosed with COVID-19. This first recognized case was acquired before the arrival of resident #1, who probably transmitted the infection to resident #3 (his son-in-law) after arrival at home A. No transmission occurred between husbands and wives in house A although couples moved to separated rooms only 4 days after the onset of symptoms of the index case. As resident #6 was asymptomatic with IgG+/IgM-, it is not sure that she was infected prior or after her arrival at house A. In house B, a single resident (#9) was found to be infected with COVID-19. He probably acquired infection by the end of February after a close contact with a confirmed case in Paris. Symptoms occurred on March 12, four days before arriving at home B, with cough, asthenia, anosmia, cutaneous lesions lasting one week. As the COVID-19 cluster was unrecognized at this time, he was not separated from the rest of the residents, he continued to share the bedroom with his wife and he had remarkably close contacts with his children. Although he had close contacts with all his family and friends during all the symptomatic phase, no secondary cases occurred. In house C, resident #22 was the first (retrospectively) identified with COVID-19. She developed typical symptoms (cough, fever, headache and asthenia) on March 10 when she was in Paris. At her arrival in house C on March 13, she still had mild symptoms. She probably infected her husband, father and mother (residents #23, #18 and #19, respectively). Resident #18 developed a severe lower respiratory tract infection and was hospitalized for 2 weeks. Resident #23 developed a moderate cough for two days. Resident #20 may have been infected by resident #23, during a round trip by car on March 17, when resident #23 was already symptomatic. Resident #20 presented with diarrhea, cough and fever lasting four days. Fig. 1 shows distribution of residents in each house, kinship, confirmed COVID-19 cases and the temporal occurrence of each case.
Fig. 1.
Population's distribution in each house, kinship, confirmed COVID-19 cases and temporal occurrence of each case.
Legend: square = male; circle = female; horizontal bar = couples; vertical bar = parentage; black = confirmed COVID-19 case; white = excluded case; hatched grey = symptoms finally not related to COVID-19; vertical arrows = timelines of symptoms’ onset in COVID-19 symptomatic residents.
Abbreviation: yo = year-old.
Overall, 9 out of 30 residents (30%) were diagnosed with COVID-19. We identified 3 independent index cases (residents #1, #9, #22) that infected 6 secondary cases: 2 in house A, none in house B and 4 in house C. Therefore, the attack rate was 6 out of 27 (22.2%). Noteworthy, none of the 9 children present were infected, although they had very closed contacts with their infected parents. Among the 9 married couples who were present, all shared the same bed at least some days after the onset of symptoms or during all the time of disease. Nevertheless, partners were infected in only 3 couples, partners remained discordant in 5, and none of the partners were infected in 1. After careful questioning, discordant couples confirmed they had continued sexual activity during the period at risk of contagiousness of the infected partner.
This cluster investigation illustrates important facts concerning SARS-CoV-2 transmission. First, as no case occurred in children we are confident they did not participate in the viral transmission network, either within or between houses. This supports the hypothesis that, usually, children are not asymptomatic carriers who transmit the SARS-CoV-2.4, 5, 6 Second, there was a limited transmission of SARS-CoV-2 among couples, even though discordant couples continued to be sexually active during the contagious period of the infected partner. To our knowledge, there are no similar reports published to date. If the determinants of such a “resistance” to SARS-CoV-2 remain uncertain, some of our results (data not shown) allow us to speculate about a potential role of a genetic factor, currently under investigation.
Ethics
This study was approved by the local institutional review board. A written informed consent was obtained from all patients.
Footnotes
Conflict of interest: None.
Funding statement: Non applicable.
References
- 1.Yuening Jiang, Wuxue Niu, Qian Wang, Hua Zhao, Li Meng, Cuilian Zhang. Characteristics of a family cluster of Severe Acute Respiratory Syndrome Coronavirus 2 in Henan, China. J Infect. 2020 doi: 10.1016/j.jinf.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marty Francisco M., Kaiwen Chen, Verrill Kelly A. How to Obtain a Nasopharyngeal Swab Specimen. N Engl J Med. 2020 doi: 10.1056/NEJMvcm2010260. [DOI] [PubMed] [Google Scholar]
- 3.Prazuck T., Colin M., Giaché S. Evaluation of performance of two SARS-CoV-2 rapid whole blood finger-stick IgM-IgG combined antibody tests. PLoS ONE. 2020 doi: 10.1371/journal.pone.0237694. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gudbjartsson Daniel F., Agnar Helgason, Hakon Jonsson, Magnusson Olafur T., Pall Melsted, Norddahl Gudmundur L. et al. Spread of SARS-CoV-2 in the Icelandic population. N Engl J Med. 2020;382(24):2302–2315. doi: 10.1056/NEJMoa2006100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelvin Alyson A., Scott Halperin. COVID-19 in children: the link in the transmission chain. Lancet Infect Dis. 2020;20(6):633–634. doi: 10.1016/S1473-3099(20)30236-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Supinda Bunyavanich, Anh Do, Alfin Vicencio. Nasal Gene Expression of Angiotensin-Converting Enzyme 2 in Children and Adults. JAMA. 2020 doi: 10.1001/jama.2020.8707. [DOI] [PMC free article] [PubMed] [Google Scholar]