Abstract
Between March and May 2019, wildlife rehabilitation centers along coastal southern California admitted increased numbers of Brandt's cormorants (Phalacrocorax penicillatus) with neurological disease including head tilt, nystagmus, torticollis, tremors, paresis, paralysis, and ataxia. Seven cormorants from Los Angeles County and one cormorant from Orange County were submitted for postmortem examination. Gross findings included thin to fair body condition, generalized congestion/hyperemia, nematode parasites in the ventriculus, and diarrhea in the seven birds from Los Angeles County while the one bird from Orange County had icterus. Histologic examination revealed sarcocysts in the adductor muscles and meningoencephalitis characterized by coalescing infiltrations of macrophages, lymphocytes and plasma cells with severe perivascular cuffing and gliosis in all eight cormorants. Rare to few numbers of schizonts were seen in the cerebrum of the seven cormorants from Los Angeles County whereas the cormorant from Orange County had numerous schizonts in various stages of development in the cerebrum, cerebellum, and brainstem. All eight birds were positive for the generic Sarcocystis spp. 28S PCR. The seven cormorants from Los Angeles County tested positive for the S. calchasi-specific ITS1 and confirmed by sequencing, while the analysis of the 28S sequence in the cormorant from Orange County showed a 100% homology to S. falcatula. This bird also was positive by immunohistochemistry for Sarcocystis spp. using a polyclonal antibody that detects S. falcatula and S. neurona. This report demonstrates for the first time that seabirds such as Brandt's cormorants may be intermediate or dead-end hosts for S. calchasi and/or S. falcatula, and that S. calchasi can cause epizootic infection in a seabird.
Keywords: Avian, Seabird, Brandt's cormorant, Epizootic, Protozoal encephalitis, Sarcocystis
Graphical abstract
Highlights
-
•
Neurological disease in Brandt's cormorants along southern California coast.
-
•
Brandt's cormorants with protozoal encephalitis assocated with Sarcocystis spp.
-
•
Brandt's cormorants may be intermediate hosts for S. calchasi and S. falcatula.
-
•
Transmission of terrestrial protozoal pathogens to seabirds.
-
•
Lifecycles of S. calchasi and S. falcatula in marine environment needs research.
1. Introduction
Sarcocystis spp. are apicomplexan parasites that infect all classes of vertebrates; mammals, birds, reptiles, and less commonly fish and amphibians (Munday et al., 1979). These protozoans have an obligate two-host lifecycle. The definitive host, typically a predator species, becomes infected by digesting infected muscle tissues that contain mature sarcocysts whereas the intermediate host, typically a prey species, becomes infected through ingestion of infectious sporocysts via the fecal-oral route (Dubey, 1976; Mehlhorn and Heydorn, 1978). The complete lifecycles of Sarcocystis spp. infectious to birds are largely unknown (Olias et al., 2010a). Several Sarcocystis spp. have been detected in a broad spectrum of avian hosts, the majority involving species in terrestrial habitats (Box and Smith, 1982; Greiner, 2008). Only rarely have Sarcocystis spp. infections been described in seabirds and seldom associated with clinical disease (Spalding et al., 2002; Gjerde et al., 2018; Prakas et al., 2014, 2018).
Sarcocystis falcatula is the most well-documented Sarcocystis spp. occurring in birds (Greiner, 2008). The definitive host of S. falcatula is the Virginia opossum (Didelphis virginiana) and the intermediate hosts include avian species such as brown-headed cowbirds (Molothrus ater), house sparrows (Passer domesticus), rock pigeons (Columbia livia), canaries (Serinus canarius), and budgerigars (Melopsittacus undulatus) (Luznar et al., 2001; Olson et al., 2007). Infection with clinical signs has been reported in raptors including a free-ranging great horned owl (Bubo virginianus) and in bald (Haliaeetus leucocephalus) and golden (Aquila chryaetos) eagles in North America (Wünschmann et al., 2009, 2010). Sarcocystis falcatula is highly pathogenic for psittacines causing severe pulmonary lesions as well as affecting the liver, brain, kidneys, and skeletal muscles (Godoy et al., 2009).
Following the initial detection in domestic pigeons (Columba livia f. domestica) in Germany in 2009 (Olias et al., 2010a), S. calchasi has been associated with sporadic cases of encephalitis in domestic and feral rock pigeons (C. livia) from Minnesota and Missouri, USA (Wünschmann et al., 2011; Olias et al., 2014), and in a feral rock pigeon from Tokyo, Japan (Ushio et al., 2015). In Texas, USA, S. calchasi was detected in two Eurasian collared doves (Streptopelia decaocto) and suspected in a single white-winged dove (Zenaida asiatica) (Hodo et al., 2016). Sarcocystis calchasi was associated with encephalitis and mass mortality in two separate events in California, USA. The first involving captive psittacine species from a zoo aviary (Rimoldi et al., 2013) and the second an epizootic during the late winter and spring of 2017 involving feral rock pigeons from 10 different counties (Mete et al., 2019). The life cycle of Sarcocystis spp. is highly conducive to sporadic infection in individuals, while the relatively recent epizootic involving free-ranging birds infected with S. calchasi has been an exception. The definitive hosts for S. calchasi in Europe include the Northern goshawk (Accipiter genitalis) and the European sparrowhawk (A. nisus) (Maier et al., 2015) while the definitive hosts in North America remain unknown.
Here we describe protozoal encephalitis in eight Brandt's cormorants (Phalacrocorax penicillatus) during an apparent epizootic in late winter and spring 2019 along the southern California coast. Infection in Brandt's cormorants demonstrates for the first time that a seabird can be an intermediate host for S. calchasi and S. falcatula, and additionally S. calchasi can present as an outbreak in a near-shore marine bird.
2. Materials and methods
2.1. History and postmortem examination
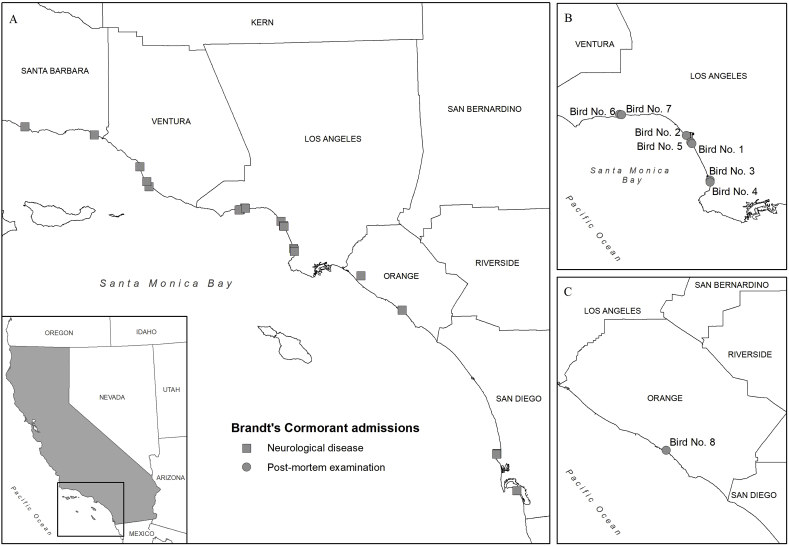
Between March and May 2019, five wildlife rehabilitation centers along the southern California coast admitted increased numbers of Brandt's cormorants with neurological disease from Santa Barbara, Ventura, Los Angeles, Orange, and San Diego counties (International Bird Rescue, unpublished data; WRMD, 2019). Additionally, several cormorants were admitted away from water such as in roads and near businesses or homes. In total, neurological signs were recorded for 22 of 69 (31.9%) Brandt's cormorants admitted during this period (Fig. 1A), with the majority of them coming from Los Angeles County (46.4%; 13/28). Seven carcasses (Nos. 1–7) from Los Angeles County (Fig. 1B) and one carcass (No. 8) from Orange County (Fig. 1C) were submitted to the California Department of Fish and Wildlife's Wildlife Investigations Laboratory (WIL; Rancho Cordova, CA) for mortality investigation. Six birds (Nos. 2–7) with neurologic disease were euthanized at intake due to poor prognosis and two birds (Nos. 1, 8) died shortly after intake. Seven birds (Nos. 1–7) were submitted frozen (−20 °C) and thawed at 4 °C for 4 days prior to necropsy at the WIL or the California Animal Health and Food Safety Laboratory (CAHFS; Davis, CA). One carcass (No. 8) was shipped overnight on ice packs the day it died, and the necropsy performed the following day.
Fig. 1.
Locations of 22 of 69 Brandt's cormorants admitted to wildlife rehabilitation centers along the coast of southern California, U.S.A., between March and May 2019 with neurological signs (square) (A). Locations of the eight (Nos. 1–8) Brandt's cormorants submitted to the California Department of Fish and Wildlife (Rancho Cordova, CA) for postmortem exam (circle) from wildlife rehabilitation centers in Los Angeles County (B) and Orange County (C).
At necropsy, carcasses were weighed, and gross findings were recorded including age, sex, adipose deposition, condition of the organs, and abnormalities (e.g. injuries). Birds were aged by plumage as subadult (second year) or adult (after second year) (Wallace and Wallace, 1998). Adipose deposition was rated as none (no subcutaneous or internal adipose), trace (no subcutaneous and minimal internal adipose), and moderate to heavy (adequate subcutaneous and internal adipose). Tissue samples from brain, liver, kidney, and skeletal muscle were collected and stored at −80 °C for molecular analysis. Tissue samples from skeletal muscle, brain, heart, lung, trachea, thyroid gland, liver, kidney, spleen, pancreas, esophagus, proventriculus, ventriculus, intestines, adrenals, gonads, peripheral nerves and eye were collected and fixed in 10% neutral buffered formalin, paraffin embedded, sectioned at 4 μm, and stained with hematoxylin and eosin for histological examination by light microscopy at CAHFS.
For suspect histological lesions, immunohistochemistry (IHC) for Sarcocystis spp., Toxoplasma spp., and West Nile virus were performed using standard operating procedures at CAHFS. A Sarcocystis spp. IHC was performed on selected tissues utilizing a polyclonal rabbit antibody directed against S. falcatula (isolate UCD-SF1), which cross-reacts with S. neurona. Briefly, 4 μm paraffin sections were deparaffinized using xylene and rehydrated through graded alcohols. Endogenous peroxidase was blocked by room temperature incubation for 10 min in 3% hydrogen peroxide in deionized water. Antigen unmasking was accomplished by placing the slides in acidulated pepsin solution for 15 min at 37 °C. Non-specific antibody binding was blocked by incubation in Background Punisher (Biocare Medical, Pacheco, CA) for 10 min at room temperature. The antibody (1:3000) was applied for 45 min at room temperature. Antibody binding was visualized by incubating the slides for 30 min at room temperature with the Envision + Anti-Rabbit HRP-Labelled Polymer (Agilent, Santa Clara, CA) followed by room temperature incubation with Dako Ready-to-Use AEC Substrate Chromogen (Agilent, Santa Clara, CA). The slides were counterstained with Mayer's hematoxylin and blue.
2.2. Molecular analysis
Frozen brain tissues from all eight birds (Nos. 1–8) and pectoral muscle tissues from six birds (Nos. 1, 4–8) were tested for Sarcocystis spp. by polymerase chain reaction (PCR). Approximately 50–100 mg of muscle and brain tissues from each bird were used to isolate DNA using the DNeasy Blood and Tissue extraction kit (Qiagen, Santa Clarita, CA), after an overnight proteinase K digestion at 56 °C following the manufacturers' instructions. DNA concentration was measured and approximately 100–250 ng of DNA were subsequently used for ITS1 and 28S amplification by PCR. For the S. calchasi-specific ITS1 amplification, the primer pair SCa1 (5′-CTCCTTGCTCGAGAATGAACATGAG-3′) and SCa2 (5′-GATCATCTTTTCGACGACAATATCG-3′) described previously by Olias et al. (2011) was used, with a predicted amplicon size of 225 bp. The amplification of a 349 bp fragment from the 28S rRNA region was achieved by using the Sarcocystis spp. conserved primers SAD2F (5′-GGAAGCGATTGGAACC-3′) and SAD2R (5′-CCTTGGTCCGTGTTTCA-3′) (Wünschmann et al., 2011). Both amplification reactions were performed in 50 μl mixtures containing 500 nM of each primer and 25 μl of Q5® Hot Start High-Fidelity 2 × Master Mix (New England BioLabs, Ipswich, MA), respectively. The PCR conditions described by Olias et al. (2011) were modified, following manufacturer recommendations, as follows: initial denaturation at 98 °C for 30 s was followed by 35 cycles of denaturation at 98 °C for 10 s, annealing at 63 °C (for ITS1) or 60 °C (for 28S) for 30 s and extension at 72 °C for 20 s and a final extension at 72 °C for 2 min. The PCR products were separated by electrophoresis on 1.8% agarose gel and visualized under UV light after staining with GelRed® Nucleic Acid Gel Stain (Biotium, Hayward, CA, USA) to check for 225 (ITS1) and 349 (28S) bp products. Sarcocystis calchasi and S. neurona positive DNA samples were used as positive controls, while nuclease-free water used as a template served as a negative control to ensure no cross-contamination occurred. It is expected that the S. neurona sample will not amplify in ITS1, as it is S. calchasi specific, but will amplify in 28S, since primers were Sarcocystis spp. conserved.
For sequence analysis, positive ITS1 samples were gel purified by using the Qiaquick gel extraction kit (Qiagen, Santa Clarita, CA) following manufacturer recommendations and sequenced by Sanger sequencing using an ABI 3730xl DNA Analyzer (Quintara Biosciences, San Francisco, CA) using the same set of primers described above. When the ITS1 PCR was negative but 28S was positive, the same procedure was carried out with the 28S PCR products. Sequences were analyzed using BioEdit sequence alignment editor v 7.0.5.3 (Hall, 1999) and compared to sequences available in GenBank(https://www.ncbi.nlm.nih.gov/genbank/) by BLASTn (Altschul et al., 1990) for either ITS1 or 28S.
3. Results
3.1. Postmortem examination
Eight Brandt's cormorants with neurological signs including head tilt, nystagmus, torticollis, tremors, paresis, paralysis, and ataxia were submitted for postmortem examination. Six (Nos. 1–6) of the birds were admitted to wildlife rehabilitation centers in March and two (Nos. 7–8) in May 2019. Four were aged as adults (Nos. 1, 3, 5, 8) and four (Nos. 2, 4, 6, 7) as subadults (second year); three were females (Nos. 2, 5, 7) and five were males (Nos. 1, 3, 4, 6, 8). Birds 1–3, 5, and 7–8 had an adipose deposition score of none (75.0%; 6/8), bird 4 had trace (12.5%; 1/8), and bird 6 had moderate to heavy (12.5%; 1/8). Body mass for birds with an adipose deposition score of none was 1494(153)g (mean(SD); range 1360–1685g; n = 4), trace 1600g (n = 1), and moderate to heavy 2250g (n = 1). Postmortem findings among the seven birds (Nos. 1–7) from Los Angeles County were similar and included diffusely red, congested or hyperemic muscles and organs (7/8), poor body condition (6/8), roundworms in the ventriculus (6/8), diarrhea (3/8), corneal abrasion (1/8), and serosanguineous fluid in the right orbit (1/8). The one bird (No. 8) from Orange County had poor body condition, blackened mucosal lining consistent with digested blood and roundworms in the ventriculus. The air sacs, connective tissues, and organs were diffusely yellow-tinged, compatible with icterus, the air sacs and connective tissues appeared thickened, the liver parenchyma of the right lobe had couple of, 1-1.5x1.5–2 cm firm, yellow-tan discolored, relatively well demarcated areas, and approximately 7 ml of yellowish-red fluid was present in the body cavity.
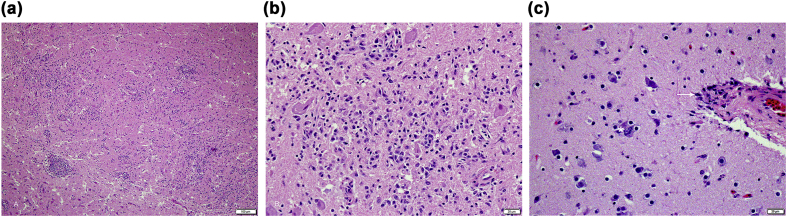
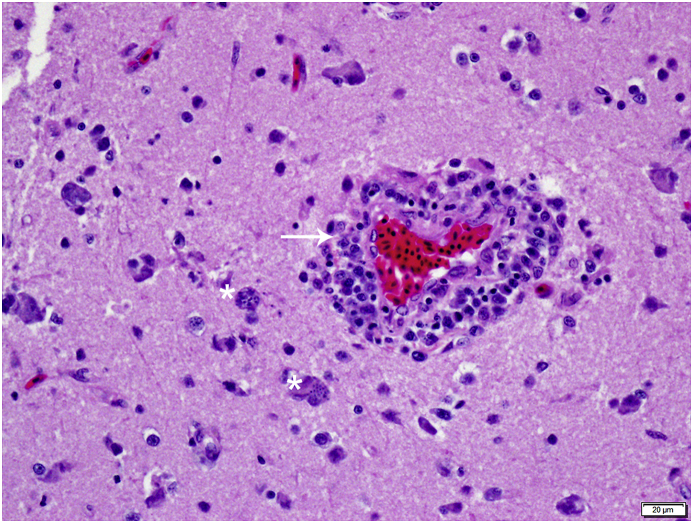
On histology, all eight cormorants exhibited multifocally extensive meningoencephalitis characterized by infiltrating macrophages and lymphocytes, few plasma cells and rare heterophils with frequent perivascular cuffing and gliosis. The brain lesions were severe (Fig. 2A and B) and more prominent in the cerebrum compared to the cerebellum and brainstem in the seven birds from Los Angeles County (Nos. 1–7). Rare to few schizonts adjacent to inflammatory regions were observed in the cerebrum (Fig. 2C). Distinct from the others, the bird (No. 8) from Orange County had large numbers of schizonts in various stages of development (Fig. 3) in all areas of the brain. Rare sarcocysts were noted in the heart and schizonts were observed in the pulmonary endothelium of this bird. Sarcocysts were not observed in the heart sections in Nos. 1–7.
Fig. 2.
Brain HE photomicrographs from the seven Brandt's cormorants from Los Angeles County with S. calchasi encephalitis. (A) Severe lymphohistiocytic encephalitis and (B) occasional schizonts (*) in the brain (C), with lymphohistiocytic vasculitis and perivascular encephalitis (arrow).
Fig. 3.
Brain HE photomicrograph demonstrating multiple schizonts (*)easily detected in the Brandt's cormortant from Orange County (No. 8) infected with S. falcatula. Note the thick perivascular cuffing, severe lymhohistiocytic perivasculitis and encephalitis (arrow). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
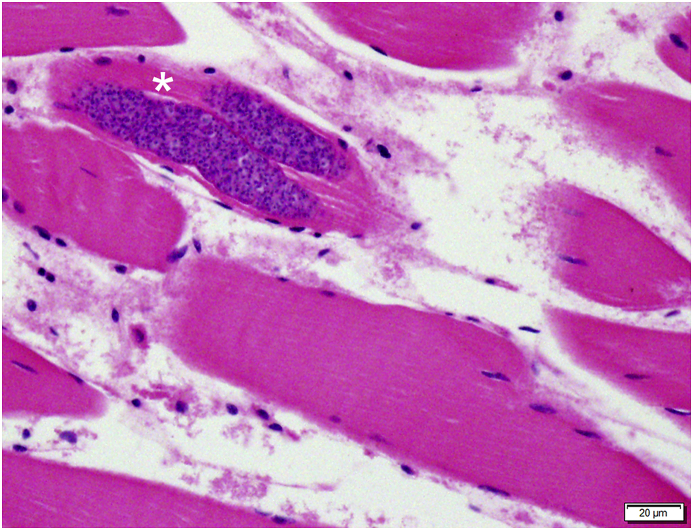
Skeletal muscle tissues from all eight birds had small numbers of protozoal cysts within muscle fibers, measuring 20–30 μm wide and up to 380 μm long filled with bradyzoites (Fig. 4). Mild focal myositis with infiltrating histiocytes and lymphocytes was present in one bird (1/8) and mild acute myodegeneration in two birds (2/8), not associated with the sarcocysts. Eye tissues were available from three birds (3/8) and all demonstrated protozoal cysts in the extraocular muscles without inflammation.
Fig. 4.
All eight Brandt's cormorants contained small numbers of protozoal cysts in the thigh skeletal muscles, sometimes in duplicate (*).
Additional histological lesions included mild lymphohistiocytic bronchopneumonia (1/8), focal minimal lymphocytic peripheral neuritis in sciatic nerves (2/8), renal acute tubular necrosis (3/8) and with mineral depositions 1/8), splenic hemosiderosis (1/8), caseonecrotic salpingitis with intralesional bacteria, hepatic hemosiderosis and lipogranulomas (1/8), and focal lymphocytic myocarditis (2/8). In five birds (5/8), there was granulomatous and lymphocytic ventriculitis with or without sections of nematode parasites present within the deep mucosa. Bird No. 8 also had mostly portal and occasionally random pleocellular hepatitis with necrosis, fibrin droplets, and marked generalized hemosiderosis in the liver, marked pulmonary congestion and edema, with scattered fibrin exudation and few vessels suspicious for fibrinoid change in the lung, and acute myonecrosis.
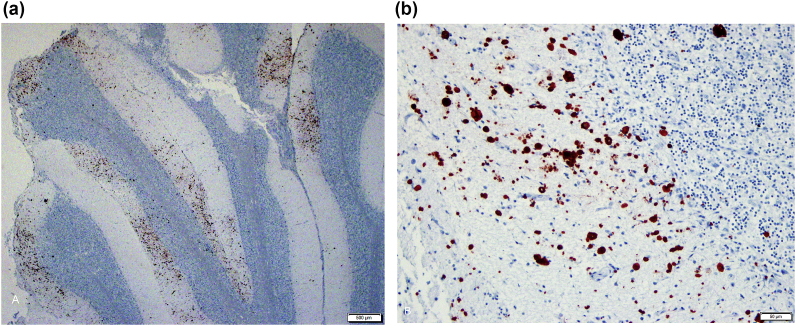
Immunohistochemistry using the polyclonal Sarcocystis spp. antibody that detects S. falcatula and S. neurona was performed on the brain sections of the eight cormorants; all birds were negative except for No. 8, in which IHC revealed numerous immunopositive schizonts (Fig. 5A and B). Bird 8 also had moderate numbers of schizonts throughout the lung and liver tissues. Brain sections from Nos. 1, 2 and 3 were negative by IHC for Sarcocystis spp., Toxoplasma spp., and West Nile virus. Heart sections from Nos. 7 and 8 were negative by IHC for West Nile virus.
Fig. 5.
Brain photomicrograph of cormorant No. 8, polyclonal IHC for S. falcatula and S. neurona. (A) Cerebellum exhibiting foci of dense protozoal immunopositivity, and (B) showing the large numbers of immunoreactive schizonts forming the foci. Sequencing revealed these are S. falcatula schizonts.
3.2. Molecular analysis
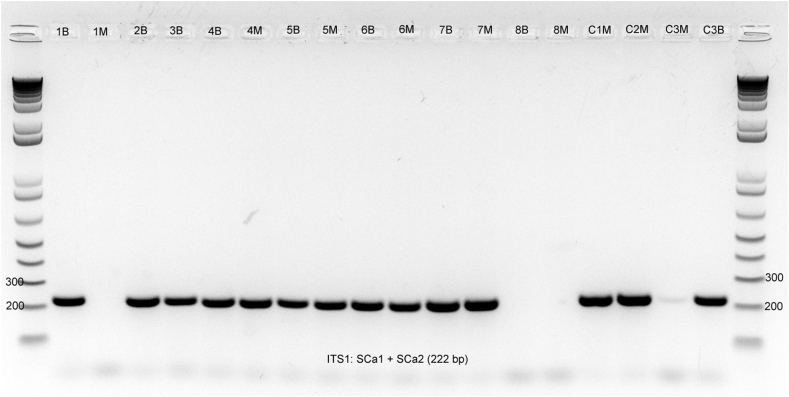
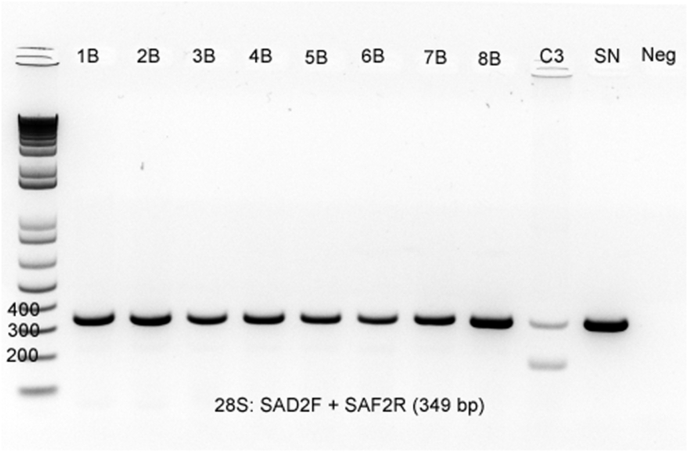
The ITS1 PCR and DNA sequencing from the brain tissues of the seven birds (Nos. 1–7) from Los Angeles County revealed sequences with 100% homology to S. calchasi (Table 1). Of the six birds with available muscle tissues (No. 1, Nos. 4–8), four birds (Nos. 4–7) had sequences with 100% homology to S. calchasi. The ITS1 PCR was negative for the muscle of one bird (No. 1), likely indicating the DNA was in too low of quantity for identification (Fig. 6). In the one cormorant (No. 8) from Orange County, the ITS1 PCR was negative for both the brain and muscle tissues; however, 28S PCR was positive in both tissues and the 345 bp segment sequence analysis showed a 100% match for S. falcatula (Fig. 7). The GenBank accession numbers for S. calchasi ITS1 (seven samples) are MT565291-97 and for S. falcatula 28S (one sample) is MT560214.
Table 1.
Molecular analysis results of Sarcocystis spp. infecting Brandt's cormorants (Phalacrocorax penicillatus) submitted to the California Department of Fish and Wildlife (Rancho Cordova, CA) for postmortem exam from Los Angeles County (Nos. 1–7) and Orange County (No. 8), California, USA between March and May 2019.
|
Bird |
ITS1 PCR (S. calchasi) |
28S PCR (Sarcocystis spp.) |
|||
|---|---|---|---|---|---|
| SCa1+SCa2 |
SAD2F + SAD3R |
Sequence homology |
|||
| Brain | Muscle | Brain | Muscle | Brain | |
| 1 | POS | NEG | POS | POS | S. calchasi 100% |
| 2 | POS | N/A | POS | N/A | S. calchasi 100% |
| 3 | POS | N/A | POS | N/A | S. calchasi 100% |
| 4 | POS | POS | POS | POS | S. calchasi 100% |
| 5 | POS | POS | POS | POS | S. calchasi 100% |
| 6 | POS | POS | POS | POS | S. calchasi 100% |
| 7 | POS | POS | POS | POS | S. calchasi 100% |
| 8 | NEG | NEG | POS | POS | S. falcatula 100% |
POS: positive, NEG: negative, N/A: Not available.
Fig. 6.
Gel electrophoresis of the S. calchasi ITS1 PCR of the brain (B) and muscle (M) tissues from eight Brandt's cormorants with neurologic disease and protozoal encephalitis. Bird Nos. 1–7 from Los Angeles County are strongly positive (No. 1 muscle tissue is negative likely due to sampling and too low quantity) while No. 8 from Orange County is negative on all tissues. The encephalitis in bird 8 was demonstrated to be due to S. falcatula. Columns C1M, C2M, C3M and C3B are positive control tissues from pigeons with S. calchasi infection.
Fig. 7.
Gel electrophoresis of the 28S PCR on the brain (B) and muscle (M) tissues from eight Brandt's cormorants submitted from Coastal Southern California. All eight birds demonstrate a band positive for sarcocystis species. C3 is brain from a pigeon with S. calchasi used as control tissue and SN is the S. neurona positive control.
4. Discussion
Here we describe Sarcocystis spp. infections associated with fatal neurological disease in a seabird, the Brandt's cormorant, along the southern California coast during late winter and spring 2019. Sarcocystis calchasi was the predominant Sarcocystis spp. detected during the epizootic and identified in all seven cormorants from Los Angeles County, while S. falcatula was identified in the one cormorant from Orange County. Clinical signs of neurological disease in the infected cormorants included head tilt, nystagmus, torticollis, tremors, paresis, paralysis, and ataxia. Several cormorants were found away from water, similar to seabirds affected by domoic acid (Work et al., 1993).
All eight Brandt's cormorants had similar histological lesions in the brain, consistent with protozoal encephalitis. The S. calchasi-positive cormorants had severe meningoencephalitis with rare schizonts observed mostly in the cerebrum, comparable to pigeon protozoal encephalitis described in rock pigeons and other avian terrestrial species (Olias et al., 2010a; Maier et al., 2015; Hodo et al., 2016; Mete et al., 2019). In contrast, large numbers of schizonts were observed in the cerebrum, cerebellum, and brainstem in the S. falcatula-positive cormorant, as has been observed elsewhere (Wünschmann et al., 2009, 2010). The abundance of protozoa present in the brain of the one S. falcatula-positive cormorant evident on histology immediately differentiated it from the cormorants infected with S. calchasi. Numerous protozoal organisms with S. calchasi infection have been observed only in psittacine birds during an outbreak (Rimoldi et al., 2013), suggesting the presentation of S. calchasi may vary among bird species. The Sarcocystis spp. were identified and confirmed by PCR and sequencing. Only the S. falcatula-positive bird was positive by IHC for Sarcocystis spp. using a polyclonal antibody that detects S. falcatula.
Protozoal cysts were present in the skeletal muscles of all eight cormorants, and of the six cormorants with available muscle tissue, five tested positive for S. calchasi and one for S. falcatula by PCR, indicating that cormorants may be intermediate or dead end hosts for these Sarcocystis spp. Both parasites may cause occasional myositis associated with the muscle cysts during infection in other species (Hillyer et al., 1991; Villar et al., 2008; Olias et al., 2009). However, the variable numbers of muscle cysts present in all eight cormorants were not associated with inflammation.
None of the cormorants with S. calchasi infection had cysts in the heart muscle. Heart muscle cysts, and in some cases liver necrosis with numerous parasitic stages in hepatocytes, have been observed in pigeons with S. calchasi (Olias et al., 2010a, 2014; Hodo et al., 2016). Outbreaks reported previously in pigeons as well as in the Brandt's cormorants reported in the present study, had acute neurologic disease without involvement of other organs. Cardiac muscles and the lungs are the primary organs affected by S. falcatula in many avian species with death often resulting from severe pulmonary lesions (Smith et al., 1989; Luznar et al., 2001; Godoy et al., 2009). In the present study, the death of bird 8 was likely related to severe encephalitis although the myocardial, pulmonary and hepatic microscopic lesions with intralesional organisms are indicative of a systemic infection. The widespread pulmonary endothelial cell infection that is frequently seen in S. falcatula infections has not been reported in S. calchasi cases (Hillyer et al., 1991; Villar et al., 2008; Godoy et al., 2009; Wünschmann et al., 2009, 2010). Bird No. 8 also had icteric tissues attributed to the liver disease.
Sarcocystis spp. are predominately known to infect a range of terrestrial avian species (Smith et al., 1989; Luznar et al., 2001; Godoy et al., 2009; Greiner, 2008; Hodo et al., 2016; Mete et al., 2019), while infection in seabirds has only rarely been reported. Sarcocystis-associated encephalitis was identified in a Northern gannet (Morus bassanus) in Florida, USA, and proposed to be the first report in a seabird; however, the Sarcocystis spp. was not molecularly identified (Spalding et al., 2002). Sarcocystis halieti was detected in two hunter-killed great cormorants (P. carbo) in Lithuania with no overt signs of disease (Prakas et al., 2018). In Norway, S. halieti, as well as S. lari, were identified in the intestines of a white-tailed sea eagle (H. albicilla), a regular predator of the great cormorant and the presumed definitive host (Gjerde et al., 2018). How Brandt's cormorants became infected with S. calchasi and S. falcatula in the current study is unknown. Brandt's cormorants are endemic to near-shore marine habitats along the Pacific Coast of North America and are not known to be regularly consumed by avian predators (Wallace and Wallace, 1998).
Until this epizootic involving Brandt's cormorants, S. calchasi was primarily associated with encephalitis in captive and feral rock pigeons in Europe and the USA (Olias et al., 2010a; Wünschmann et al., 2011; Mete et al., 2019). Seemingly unique among the S. calchasi outbreak observed in the cormorants, as well as previous outbreaks involving rock pigeons, is that multiple individuals are affected during the same time period, which is unusual for Sarcocystis spp. given their two-host terrestrial lifecycle. During the initial detection of S. calchasi in racing pigeons in Germany, 47 of 244 pigeons in three different flocks were found to be infected between 2006 and 2008 (Olias et al., 2009). During the epizootic in feral rock pigeons in California in late winter and spring of 2017, infection was confirmed in 21 pigeons from 10 different counties (Mete et al., 2019). In Europe, Accipiter spp. were identified as definitive hosts for S. calchasi in rock pigeons (Olias et al., 2010a, Olias et al., 2010b). In California, the range of Cooper's hawks (A. cooperii) is more expansive than goshawks. As such, Cooper's hawks may be a reasonable definitive host for infection in rock pigeons; however, Cooper's hawks are unlikely to prey on a Brandt's cormorant given its large body size (1.5–2 kg). More likely potential predators of Brandt's cormorants may include peregrine falcons (Falco peregrinus) and bald or golden eagles, though none are known to be regular predators of Brandt's cormorants. Regardless, an avian definitive host alone, may not explain the epizootic-like nature of S. calchasi infection in rock pigeons or Brandt's cormorants.
The transmission of terrestrial pathogens to marine mammals has been demonstrated for protozoal parasites including S. neurona and Toxoplasma gondii. An epizootic of S. neurona impacting 40 southern sea otters (Enhydra lutris) occurred along the central California coast during April 2004, following a large rainstorm that may have concentrated sporocysts in creeks with outflow to the ocean (Miller et al., 2010; Shapiro et al., 2012). Likewise, sea otters sampled near areas of high freshwater runoff were roughly three times more likely to be seropositive for T. gondii, than otters sampled in areas of low runoff (Miller et al., 2002). Brandt's cormorants are a near-shore seabird, potentially making them more susceptible to land to sea transmission of protozoal parasites similar to what has been described for sea otters. Admissions of Brandt's cormorants to wildlife rehabilitation centers along the southern California coast nearly doubled between March and May 2019 (n = 69) compared to the previous two years (n = 31 in 2017 and n = 37 in 2018) (International Bird Rescue, unpublished data; WRMD, 2019) with the largest increase occurring in March 2019 (n = 23). Eleven of the 22 Brandt's cormorants admitted to rehabilitation centers with neurological disease between March and May 2019, including the seven with confirmed S. calchasi infections, were collected from the Santa Monica Bay in Los Angeles County, the outlet for Ballona Creek. Rainfall in Los Angeles County increased from 90.2 mm in December 2018 to 217.3 mm in January 2019, 392.1 mm in February, and 128.8 mm in March; dropping to 3.9 mm in April (PRISM, 2019). The heavy rainfall that took place between January and February, just prior to the admissions of cormorants with neurological disease, may have concentrated sporocysts during increased runoff from terrestrial surfaces to the ocean facilitating the land to sea transmission of S. calchasi and/or S. falcatula. Additionally, the transmission of S. calchasi and/or S. falcatula sporocysts to the cormorants may have been aided by a transport host, such as filter feeding fish or invertebrates, as has been described for S. neurona and T. gondii in marine mammals (Burgess et al., 2020; Massie et al., 2010).
This study provides another potential example of land to sea transmission of terrestrial pathogens to a marine species. Encephalitis associated with S. calchasi, and possibly S. falcatula, is described for the first time in a seabird, the Brandt's cormorant, following a period of increased coastal precipitation. Freshwater runoff from cities may provide new routes of exposure to terrestrial pathogens, such as Sarcocystis spp., to sensitive marine birds. This is of particular concern along the southern California coast as the Channel Islands, roughly 30 km offshore, are home to the largest breeding colonies of seabirds in southern California. In addition to Brandt's cormorants, California brown pelicans (Pelecanus occidentalis californicus), ashy storm petrels (Oceanodroma homochroa), Cassin's auklets (Ptychoramphus aleuticus), and state threatened Script's murrelets (Synthliboramphus scrippsi) breed and forage among the Channel Islands and may be similarly susceptible to infection under appropriate conditions and less likely detected. The molecular identification of S. calchasi and S. falcatula in Brandt's cormorants, demonstrates the previously unknown broad host range of these parasites beyond terrestrial bird species. However, further studies are needed to thoroughly identify the lifecycles of S. falcatula, and especially S. calchasi, in marine environments.
Declaration of competing interest
None.
Acknowledgements
We thank the staff and volunteers at the California Wildlife Care Center (Calabasas, CA), International Bird Rescue (San Pedro, CA), Project Wildlife (San Diego, CA), Santa Barbara Wildlife Care Network (Goleta, CA), and Wetlands and Wildlife Care Center (Huntington Beach, CA) for reporting Brandt's cormorant admissions during this period and for their continued dedication for caring for debilitated wildlife in California. We thank K. Rush and N. Shirkey with CDFW for assisting with the mortality investigation. This work was funded by the California Department of Fish and Wildlife. The authors declare no conflict of interest.
Contributor Information
Ozge Erdogan Bamac, Email: oerdogan@istanbul.edu.tr.
Krysta H. Rogers, Email: krysta.rogers@wildlife.ca.gov.
David Arranz-Solís, Email: darranz@ucdavis.edu.
Jeroen P.J. Saeij, Email: jsaeij@ucdavis.edu.
Stephany Lewis, Email: stephany@cawildlife.org.
Rebecca Duerr, Email: rebecca.duerr@bird-rescue.org.
Julie Skoglund, Email: julie.skoglund@bird-rescue.org.
Lisa Peronne, Email: lisa.bpoagent@gmail.com.
Aslı Mete, Email: amete@ucdavis.edu.
References
- Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Box E.D., Smith J.H. The intermediate host spectrum in a Sarcocystis species of birds. J. Parasitol. 1982:668–673. [PubMed] [Google Scholar]
- Burgess T.L., Tinker M.T., Miller M.A., Smith W.A., Bodkin J.L., Murray M.J., Nichol L.M., Saarinen J.A., Larson S., Tomoleoni J.A., Conrad P.A., Johnson C.K. Spatial epidemiological patterns suggest mechanisms of land-sea transmission for Sarcocystis neurona in a coastal marine mammal. Sci. Rep. 2020;10(1):1–9. doi: 10.1038/s41598-020-60254-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey J.T. A review of Sarcocystis of domestic animals and of other coccidia of cats and dogs. J. Am. Vet. Med. Assoc. 1976;169:1061–1078. [PubMed] [Google Scholar]
- Greiner E.C. Isospora, atoxoplasma, and sarcocystis. In: Atkinson C.T., Thomas J.J., Hunter D.B., editors. Parasitic Diseases of Wild Birds. Wiley-Blackwell; Ames, IA, USA: 2008. pp. 108–119. [Google Scholar]
- Gjerde B., Vikøren T., Hamnes I.S. Molecular identification of Sarcocystis halieti n. sp., Sarcocystis lari and Sarcocystis truncata in the intestine of a white-tailed sea eagle (Haliaeetus albicilla) in Norway. Int. J. Parasitol. Parasites Wildl. 2018;7:1–11. doi: 10.1016/j.ijppaw.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godoy S.N., De Paula C.D., Cubas Z.S., Matushima E.R., Catão-Dias J. Occurrence of Sarcocystis falcatula in captive psittacine birds in Brazil. J. Avian Med. Surg. 2009;23:18–23. doi: 10.1647/2008-006R.1. [DOI] [PubMed] [Google Scholar]
- Hall T.A. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999;41:95–98. [Google Scholar]
- Hillyer E.V., Anderson M.P., Greiner E.C., Atkinson C.T., Frenkel J.K. An outbreak of Sarcocystis in a collection of psittacines. J. Zoo Wildl. Med. 1991:434–445. [Google Scholar]
- Hodo C.L., Whitley D.B., Hamer S.A., Corapi W.V., Snowden K., Heatley J.J., Hoffmann A.R. Histopathologic and molecular characterization of Sarcocystis calchasi encephalitis in white-winged doves (Zenaida asiatica) and Eurasian collared doves (Streptopelia decaocto), East-central Texas, USA, 2010–13. J. Wildl. Dis. 2016;52:395–399. doi: 10.7589/2015-10-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luznar S.L., Avery M.L., Dame J.B., MacKay R.J., Greiner E.C. Development of Sarcocystis falcatula in its intermediate host, the brown-headed cowbird (Molothrus ater) Vet. Parasitol. 2001;95:327–334. doi: 10.1016/s0304-4017(00)00399-x. [DOI] [PubMed] [Google Scholar]
- Maier K., Olias P., Enderlein D., Klopfleisch R., Mayr S.L., Gruber A.D., Lierz M. Parasite distribution and early-stage encephalitis in Sarcocystis calchasi infections in domestic pigeons (Columba livia f. domestica) Avian Pathol. 2015;44:5–12. doi: 10.1080/03079457.2014.978263. [DOI] [PubMed] [Google Scholar]
- Massie G.N., Ware M.W., Villegas E.N., Black M.W. Uptake and transmission of Toxoplasma gondii oocysts by migratory, filter-feeding fish. Vet. Parasitol. 2010;169:296–303. doi: 10.1016/j.vetpar.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Mehlhorn H., Heydorn A.O. Advances in Parasitology. Elsevier; 1978. The sarcosporidia (Protozoa, Sporozoa): life cycle and fine structure; pp. 43–91. [DOI] [PubMed] [Google Scholar]
- Mete A., Rogers K.H., Wolking R., Bradway D.S., Kelly T., Piazza M., Crossley B. Sarcocystis calchasi outbreak in feral rock pigeons (Columba livia) in California. Vet. Pathol. 2019;56:317–321. doi: 10.1177/0300985818794262. [DOI] [PubMed] [Google Scholar]
- Miller M.A., Conrad P.A., Harris M., Hatfield B., Langlois G., Jessup D.A., Magargal S.L., Packham A.E., Toy-Choutka S., Melli A.C. A protozoal-associated epizootic impacting marine wildlife: mass-mortality of southern sea otters (Enhydra lutris nereis) due to Sarcocystis neurona infection. Vet. Parasitol. 2010;172:183–194. doi: 10.1016/j.vetpar.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M.A., Gardner I.A., Kreuder C., Paradies D.M., Worcester K.R., Jessup D.A., Dodd E., Harris M., Ames J.A., Packham A.E., Conrad P.A. Coastal freshwater runoff is a risk factor for Toxoplasma gondii infection of southern sea otters (Enhydra lutris nereis) Int. J. Parasitol. 2002;32:997–1006. doi: 10.1016/s0020-7519(02)00069-3. [DOI] [PubMed] [Google Scholar]
- Munday B.L., Hartley W.J., Harrigan K.E., Presidente P.J.A., Obendorf D.L. Sarcocystis and related organisms in Australian wildlife: II. Survey findings in birds, reptiles, amphibians and fish. J. Wildl. Dis. 1979;15:57–73. doi: 10.7589/0090-3558-15.1.57. [DOI] [PubMed] [Google Scholar]
- Olias P., Gruber A.D., Hafez H.M., Heydorn A.O., Mehlhorn H., Lierz M. Sarcocystis calchasi sp. nov. of the domestic pigeon (Columba livia f. domestica) and the Northern goshawk (Accipiter gentilis): light and electron microscopical characteristics. Parasitol. Res. 2010;106:577–585. doi: 10.1007/s00436-009-1701-9. [DOI] [PubMed] [Google Scholar]
- Olias P., Gruber A.D., Heydorn A.O., Kohls A., Hafez H.M., Lierz M. Unusual biphasic disease in domestic pigeons (Columba livia f. domestica) following experimental infection with Sarcocystis calchasi. Avian Dis. 2010;54:1032–1037. doi: 10.1637/9303-031110-Reg.1. [DOI] [PubMed] [Google Scholar]
- Olias P., Gruber A.D., Heydorn A.O., Kohls A., Mehlhorn H., Hafez H.M., Lierz M. A novel Sarcocystis-associated encephalitis and myositis in racing pigeons. Avian Pathol. 2009;38:121–128. doi: 10.1080/03079450902737847. [DOI] [PubMed] [Google Scholar]
- Olias P., Maier K., Wuenschmann A., Reed L., Armién A.G., Shaw D.P., Gruber A.D., Lierz M. Sarcocystis calchasi has an expanded host range and induces neurological disease in cockatiels (Nymphicus hollandicus) and North American rock pigeons (Columbia livia f. dom.) Vet. Parasitol. 2014;200:59–65. doi: 10.1016/j.vetpar.2013.11.012. [DOI] [PubMed] [Google Scholar]
- Olias P., Olias L., Krücken J., Lierz M., Gruber A.D. High prevalence of Sarcocystis calchasi sporocysts in European Accipiter hawks. Vet. Parasitol. 2011;175:230–236. doi: 10.1016/j.vetpar.2010.10.025. [DOI] [PubMed] [Google Scholar]
- Olson E.J., Wünschmann A., Dubey J.P. Sarcocystis sp.-associated meningoencephalitis in a bald eagle (Haliaeetus leucocephalus) J. Vet. Diagn. Invest. 2007;19:564–568. doi: 10.1177/104063870701900519. [DOI] [PubMed] [Google Scholar]
- Prakas P., Butkauskas D., Švažas S., Stanevičius V. Morphological and genetic characterisation of Sarcocystis halieti from the great cormorant (Phalacrocorax carbo) Parasitol. Res. 2018;117:3663–3667. doi: 10.1007/s00436-018-6083-4. [DOI] [PubMed] [Google Scholar]
- Prakas P., Kutkiene L., Butkauskas D., Sruoga A., Zalakevicius M. Description of Sarcocystis lari sp. n. (Apicomplexa: sarcocystidae) from the great black-backed gull, Larus marinus (Charadriiformes: laridae), on the basis of cyst morphology and molecular data. Folia Parasitol. 2014;61:11. [PubMed] [Google Scholar]
- PRISM . Oregon State University; Corvallis, OR, USA: 2019. PRISM Climate Group.http://www.prism.oregonstate.edu available: accessed August 22, 2019. [Google Scholar]
- Rimoldi G., Speer B., Wellehan J.F., Jr., Bradway D.S., Wright L., Reavill D., Barr B.C., Childress A., Shivaprasad H.L., Chin R.P. An outbreak of Sarcocystis calchasi encephalitis in multiple psittacine species within an enclosed zoological aviary. J. Vet. Diagn. Invest. 2013;25:775–781. doi: 10.1177/1040638713502981. [DOI] [PubMed] [Google Scholar]
- Shapiro K., Miller M., Mazet J. Temporal association between land-based runoff events and California sea otter (Enhydra lutris nereis) protozoal mortalities. J. Wildl. Dis. 2012;48:394–404. doi: 10.7589/0090-3558-48.2.394. [DOI] [PubMed] [Google Scholar]
- Smith J.H., Neill P.J.G., Box E.D. Pathogenesis of Sarcocystis falcatula (Apicomplexa: sarcocystidae) in the budgerigar (Melopsittacus undulatus) III. Pathologic and quantitative parasitologic analysis of extrapulmonary disease. J. Parasitol. 1989:270–287. [PubMed] [Google Scholar]
- Spalding M.G., Yowell C.A., Lindsay D.S., Greiner E.C., Dame J.B. Sarcocystis meningoencephalitis in a northern gannet (Morus bassanus) J. Wildl. Dis. 2002;38:432–437. doi: 10.7589/0090-3558-38.2.432. [DOI] [PubMed] [Google Scholar]
- Ushio N., Watanabe K., Chambers J.K., Shibato T., Nakayama H., Uchida K. Sarcocystis calchasi encephalitis in a rock pigeon. J. Vet. Med. Sci. 2015;77:1523–1526. doi: 10.1292/jvms.15-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar D., Kramer M., Howard L., Hammond E., Cray C., Latimer K. Clinical presentation and pathology of sarcocystosis in psittaciform birds: 11 cases. Avian Dis. 2008;52:187–194. doi: 10.1637/8104-090207-Case. [DOI] [PubMed] [Google Scholar]
- Wallace E.A., Wallace G.E. Brandt's Cormorant (Phalacrocorax penicillatus), version 2.p. In: Poole A.F., Gills F.B., editors. The Birds of North America. 1998. https://birdsna.org/species-account/bna/species/bracor/ Cornell Lab of Ornithology, Ithaca, NY, USA. Available: accessed August 28, 2019. [Google Scholar]
- Work T.M., Barr B., Beale A.M., Fritz L., Quilliam M.A., Wright J.L. Epidemiology of domoic acid poisoning in brown pelicans (Pelecanus occidentalis) and Brandt's cormorants (Phalacrocorax penicillatus) in California. J. Zoo Wildl. Med. 1993:54–62. [Google Scholar]
- WRMD . Wild Neighbors Database Project; Middletown, CA USA: 2019. Wildlife Rehabilitation Medical Database.https://www.wrmd.org/ available. accessed August 22, 2019. [Google Scholar]
- Wünschmann A., Armien A.G., Reed L., Gruber A.D., Olias P. Sarcocystis calchasi‐associated neurologic disease in a domestic pigeon in North America. Transbound. Emerg. Dis. 2011;58:526–530. doi: 10.1111/j.1865-1682.2011.01254.x. [DOI] [PubMed] [Google Scholar]
- Wünschmann A., Rejmanek D., Conrad P.A., Hall N., Cruz-Martinez L., Vaughn S.B., Barr B.C. Natural fatal Sarcocystis falcatula infections in free-ranging eagles in North America. J. Vet. Diagn. Invest. 2010;22:282–289. doi: 10.1177/104063871002200222. [DOI] [PubMed] [Google Scholar]
- Wünschmann A., Rejmanek D., Cruz-Martinez L., Barr B.C. Sarcocystis falcatula-associated encephalitis in a free-ranging great horned owl (Bubo virginianus) J. Vet. Diagn. Invest. 2009;21:283–287. doi: 10.1177/104063870902100223. [DOI] [PubMed] [Google Scholar]