Abstract
Alzheimer's disease (AD) is an age-specific neurodegenerative disease that compromises cognitive functioning and impacts the quality of life of an individual. Pathologically, AD is characterised by abnormal accumulation of beta-amyloid (Aβ) and hyperphosphorylated tau protein. Despite research advances over the last few decades, there is currently still no cure for AD. Although, medications are available to control some behavioural symptoms and slow the disease's progression, most prescribed medications are based on cholinesterase inhibitors. Over the last decade, there has been increased attention towards novel drugs, targeting alternative neurotransmitter pathways, particularly those targeting serotonergic (5-HT) system. In this review, we focused on 5-HT receptor (5-HTR) mediated signalling and drugs that target these receptors. These pathways regulate key proteins and kinases such as GSK-3 that are associated with abnormal levels of Aβ and tau in AD. We then review computational studies related to 5-HT signalling pathways with the potential for providing deeper understanding of AD pathologies. In particular, we suggest that multiscale and multilevel modelling approaches could potentially provide new insights into AD mechanisms, and towards discovering novel 5-HTR based therapeutic targets.
Keywords: Alzheimer's disease, Dementia, Beta-amyloid, Tau, Serotonin, Serotonin targeted drugs, GSK-3, Multiscale computational modelling, Mechanistic models, Boolean models, Data-driven models, Knowledge-driven models
Highlights
-
•
Alzheimer's disease (AD) drug treatment is limited, and alternatives are needed.
-
•
Serotonin (5-HT) mediated signalling pathways may regulate Aβ and tau levels.
-
•
5-HT based drugs have the potential to provide as novel therapeutics for AD.
-
•
Complex 5-HT signalling mechanisms for AD and related drugs hinder understanding.
-
•
Multiscale models may offer insights into mechanisms and therapeutic targets.
1. Introduction
Dementia is a clinical syndrome caused by a number of progressive illnesses that affect cognition, behaviour and the ability to perform daily activities (Weller and Budson, 2018). Dementia is one of the main causes of dependence and disability at older ages. Alzheimer's disease (AD) is the most common form of dementia affecting approximately 50 million people worldwide (Baldas et al., 2011). It has been estimated that by 2050, 115 million people worldwide will be living with AD (Wortmann, 2012). Other types of dementia include vascular dementia, frontotemporal dementia, Lewy body dementia, Huntington's disease, and Creutzfeldt-Jakob disease, and co-morbidity of AD with some of these are not uncommon (Gale et al., 2018). An intermediate stage between healthy and AD is labelled mild cognitive impairment (MCI) (Gale et al., 2018). However, MCI is a loosely defined and heterogenous group, consisting of non-neurodegenerative or non-AD converters and people with other illnesses e.g. psychiatric illness (Gale et al., 2018).
To a large extent, AD can be categorised as familial AD (family history of the disease) or sporadic (late-onset) AD, with the latter overwhelmingly the most common type (Dorszewska et al., 2016). Various genes are currently thought to be associated with these different AD types. Mutations in amyloid precursor protein (APP), presenilin-1 (PSEN1) and presenilin-2 (PSEN2) are associated with familial AD while apolipoprotein E (ApoE) gene has been linked to the sporadic type (Gale et al., 2018). More generally, AD neuropathology has been characterised by the accumulation of β-amyloid (Aβ) protein due to the aberrant processing of APP, neurofibrillary tangles (composing of hyperphosphorylated tau protein), oxidative stress, excitotoxicity, neuroinflammation, and impairment in neurotransmitter systems (Butzlaff and Ponimaskin, 2016; Francis et al., 1999; Haruhiko et al., 2000; Heneka et al., 2015; Hynd et al., 2004; Markesbery, 1997; Rajmohan and Reddy, 2017).
Within the brain, acetylcholine (ACh) is an important neurotransmitter and neuromodulator implicated in cognitive functions such as learning and memory, and abnormalities (e.g., reduction in presynaptic ACh receptors, diminished choline acetyltransferase activity) in cholinergic neurons (which produce ACh) are found in the brains of AD patients (Francis et al., 1999). One approach to reducing the rate of cognitive decline in AD is to inhibit the breakdown of ACh into inactive metabolites by blocking the enzyme acetylcholinesterase responsible for the process. For instance, donepezil (Aricept) is a second-generation cholinesterase inhibitor (AChEI) and is the most widely prescribed drug for the treatment of AD. A Cochrane review estimated a 1.37 (95% CI: (1.13,1.61)) Mini Mental State Examination (MMSE) score improvement at six months after AChEI initiation (Birks and Harvey, 2018). However, it should be emphasised that these drug treatments do not cure AD, but rather delay the rate of cognitive decline associated with AD (Douchamps and Mathis, 2017). Hence, identifying novel therapeutics, especially repurposed drugs, has become a priority research area in both academia and industry.
AD has also been reported to be linked to changes in non-cholinergic neuromodulators, especially the monoaminergic systems (Morgese and Trabace, 2019). In particular, reduced serotonin (5-HT) levels in the neocortex and altered serotonin receptor (5-HTR) density are reported with severe cognitive decline in AD patients (Lai et al., 2005, 2002). Also, reduced 5-HT transporter (5-HTT) levels are reported in cortical, limbic, sensory, motor, striatal and thalamic brain regions in MCI as compared to healthy controls. Such reduction in 5-HTT levels is also found in the AD patient's temporal cortex (Smith et al., 2017; Tsang et al., 2003). These pathological conditions are possible grounds of underlying depressive symptoms, commonly seen in AD patients, and can potentially play an important role in the pathophysiology of AD (Smith et al., 2017).
Depression seems to be common in 20–30% of patients with AD (Tsuno and Homma, 2009). In fact, AD and depression share a close relationship: depression can lead to higher risk of AD but AD may also contribute to depression (Galts et al., 2019; Ownby et al., 2006). For instance, progression of AD is often associated with stress due to the catastrophic decline in motor and cognitive functions which can trigger the neural circuits involved in mediating stress response (Justice, 2018). Such chronic exposure of stress disrupts the cascades of stress hormones (e.g., cortisol) and affects the brain areas involved in monoaminergic transmission (e.g., dorsal raphe), decision making (e.g., prefrontal cortex), anxiety, and hence, increase the risk of developing depression (Arnsten, 2009; Bocchio et al., 2016; Hannibal and Bishop, 2014; Sengupta et al., 2017; Tafet et al., 2001).
Further, administration of antidepressants such as selective serotonin reuptake inhibitors (SSRIs) is often prolonged in chronically depressed patients, and long-term usage of SSRIs is associated with many side effects including cognitive impairments (e.g., memory deficits) (Prado et al., 2018; Sayyah et al., 2016). This gives rise to the possibility that extended use of SSRIs can increase the risk of developing dementia or AD. Practically, it is very challenging to establish a link between depressed individuals and late-life AD, as there are many constraints for long-term studies. Only a handful of studies attempted to establish such a link. For instance, a meta-study by Wang and colleagues suggests that antidepressant usage is substantially linked with a greater risk of developing dementia (Wang et al., 2018). These findings are not consistent with a study by Kessings and colleagues that suggests sustaining long-term treatment of SSRIs does not affect the risk of having dementia (Kessing et al., 2011). In contrast, a study by Xie and colleagues shows a pacified effect of SSRIs (e.g., fluoxetine) on cognitive performance in AD (Xie et al., 2019). Another study shows SSRIs to be beneficial in delaying the onset of AD on patients with a history of depression, a known risk factor for AD (Elsworthy and Aldred, 2019). Thus, these mixed results suggest that further studies are needed.
Amidst these mixed behavioural results, at the biological level, the effects are clearer. For example, the administration of SSRIs in AD in human studies (Lyketsos et al., 2003) and preclinical studies (animal models) have demonstrated a commendable influence of SSRIs on pathological markers of AD including Aβ accumulation, tau deposits, and neurogenesis (Kim et al., 2013; Qiao et al., 2016; Sheline et al., 2014; Wang et al., 2014, 2016). As the administration of SSRIs, including in AD, can increase the level of 5-HT, which leads to the activation of several 5-HTRs (Tajeddinn et al., 2016; Tohgi et al., 1995), we shall next discuss in more detail the various effects of 5-HTR targeted drugs, with focus on 5-HT heteroreceptors, postsynaptic sites and AD.
2. Serotonin receptor targeted drugs
5-HT receptors (5-HTR) are abundant throughout the brain with 14 known receptor subtypes and categorised into 7 subfamilies, 5-HT1-7 (Stiedl et al., 2015). Apart from 5-HT3R, all other 5-HT receptors act via G-protein coupled receptors (GPCR) (Hannon and Hoyer, 2002; Masson et al., 2012). 5-HT3, the only 5-HT based ligand-gated ion channel, acts via changes in cation currents (e.g., Na+, Ca2+) (Masson et al., 2012). Numerous studies have associated 5-HT3 mediated drugs with the accumulation of specific proteins found in AD (Fakhfouri et al., 2019; Huang et al., 2020; Skovgård et al., 2018). For example, in a rat model of AD, a study showed a protective response of a drug, tropisetron, a 5-HT3 receptor antagonist, on Aβ-induced neurotoxicity on neurons in the hippocampus, a brain region associated with cognitive functions such as memory and spatial navigation (Rahimian et al., 2013). The hippocampus is known to be one of the regions of deterioration in early-stage AD (Gale et al., 2018). A follow-up study indicated that tropisetron protects rat pheochromocytoma cells (PC12) from oxidative-induced neurotoxicity via α7 nicotinic acetylcholine receptor (α7nAChR, which respond to ACh) and 5-HT3R antagonist (Khalifeh et al., 2015). However, when a 5-HT3 selective antagonist (ondansetron) is combined with acetylcholinesterase inhibitors (e.g., donepezil) in rats, they together potentiate and prolong the theta and gamma-band neural oscillations induced by donepezil alone. Such changes in hippocampal network oscillations and theta-gamma coupling are reported in a mouse model of AD, before the excessive production of Aβ (Goutagny et al., 2013; Skovgård et al., 2018).
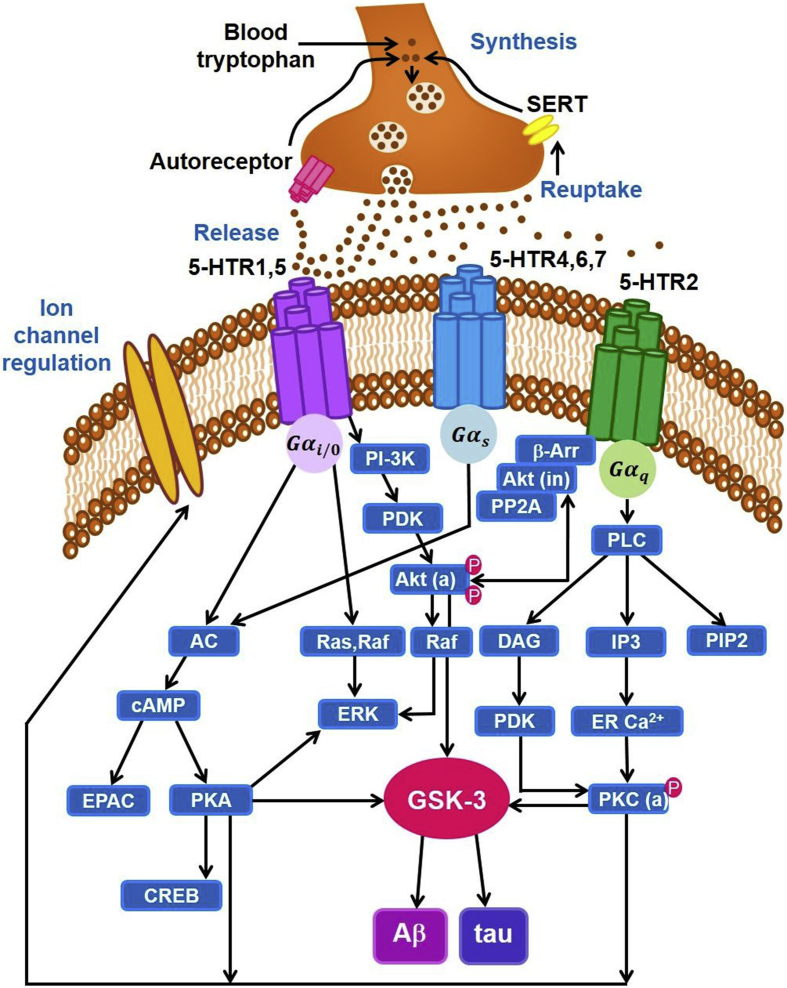
In contrast to 5-HT3R, the rest of the 5-HTR subtypes are associated with G protein-coupled receptors (GPCRs). In particular, the G-proteins for 5-HT1R and 5-HT5R act as members of the Gi/o family of receptors (Masson et al., 2012) (Fig. 1). Activation of these receptors regulates various signalling pathways including protein kinase A (PKA) pathways (Fig. 1) (Masson et al., 2012). 5-HT1R are expressed in large numbers in the hippocampus and are known to play a significant role in the regulation of memory processes (Ögren et al., 2008). Studies have also linked the level of 5-HT1AR with agitative/aggressive behaviour (symptoms of AD), and the various stages of AD (Lai et al., 2003). Specifically, for the latter, a higher density of 5-HT1AR was reported during the early stage of AD as compared to healthy controls (Truchot et al., 2008, 2007), whereas lower levels were detected during the advanced stage of AD (Becker et al., 2014; Kepe et al., 2006; Lanctôt et al., 2007; Vidal et al., 2016). In contrast, a post-mortem study indicated higher neocortical 5-HT1A density and relatively lower 5-HT levels in the brain tissue of AD patients as compared to healthy controls (Lai et al., 2002). There is also evidence that the drug Lecozotan, a 5-HT1AR antagonist, can be used to improve cognitive dysfunction associated with AD, as the drug potentially interacts and enhance the signalling pathways of other neurotransmitters (e.g. cholinergic, glutamatergic) systems (Schechter et al., 2005).
Fig. 1.
Schematic diagram of 5-HTR mediated signalling pathways: Activation of 5-HT1AR initiates several pathways including Gαi/o-adenylyl cyclase-cAMP-EPAC/PKA and Ras, Raf-ERK signalling pathways. When cAMP binds to the subunits of PKA, they phosphorylate downstream proteins to regulate key cellular processes. As a result, it increases the gene transcription involved in the development of long-term memory which is regulated by CREB. Furthermore, it alters the NMDA, AMPA and GABA receptor-mediated currents and also plays an active part in modulation of voltage-gated Na+, K+ and Ca+2 ion channels. Additionally, 5-HT1R activates Akt via PI-3K-PDK pathways. It is a key protein kinase and also regulates phosphor-Ser9-GSK3β. Similarly, activation of 5-HT2R initiates PLC-DAG-PDK-PKC/IP3-ERCa+2-PKC pathways. Importantly, the activated form of PKC also regulates phosphor-Ser9-GSK3β. Also, 5-HT2R affects β-Arrestin-Akt-PP2A signalling pathway. 5-HT: 5-hydroxytryptamine; SERT: serotonin reuptake transporter; Gαi/o, Gαs, Gαq: isoforms of the α subunits of G protein-coupled receptors (GPCR); AC: adenylyl cyclase; cAMP: cyclic adenosine monophosphate; EPAC: exchange proteins activated by cAMP; PKA: protein kinase A; CREB: cAMP response element-binding protein; Raf: rapidly accelerated fibrosarcoma kinase; ERK: extracellular signal regulated kinase; PI-3K: phosphoinositide 3-kinases; PDK1: phosphoinositide-dependent kinase1; Akt (a): protein kinase B (active); β-Arr: β-arrestin; Akt (in): Protein Kinase B (inactive); PP2A: protein phosphatase 2; PLC: phospholipase C; DAG: diacylglycerol; IP3: inositol 1,4,5-trisphosphate; PIP2: phospholipid phosphatidylinositol 4,5-bisphosphate; ER: endoplasmic reticulum; PKC: protein kinase C; GSK-3: glycogen synthase kinase-3, there are two isoforms GSK-3α, GSK-3β; tau: tau protein; Aβ: beta amyloid; P: Phosphorylated. Note: Signalling pathway specific to 5-HT metabotropic receptors are shown; for detailed signalling networks, see Masson et al. (2012) andWong-Lin et al. (2017), and for their association with GSK, see Polter and Li (2011).
Unlike 5-HT1R, 5-HT2R are coupled to Gq proteins and activate various signalling pathways including PKC and calcium/calmodulin-dependent kinase II (CaMKII), with the latter acting as a key protein kinase in neural plasticity and memory (Fig. 1). Several studies have linked the density of 5-HT2R with Aβ levels and cognitive impairment (Hasselbalch et al., 2008; Lai et al., 2005; Maroteaux et al., 2017; Nitsch et al., 1996; Štrac et al., 2016). In rats, intra-hippocampal injection of Aβ1-42 reduces 5-HT2ARs and impairs memory (Holm et al., 2010). Not surprisingly, 5-HT2AR expression levels were also reduced at the projection sites such as the medial prefrontal cortex (Christensen et al., 2008; Holm et al., 2010; Lorke et al., 2006). Additionally, the 5-HT2R gene (T102C) is also reported to be involved in hallucination, psychosis and aberrant motor behaviour associated with AD (Tang et al., 2017). Recently, a study by Afshar and colleagues reported that 5-HT2AR antagonist (NAD-299) individually, and in combination with 5-HT1AR antagonist (TCB-2), significantly reduces oxidative stress and neuronal loss in hippocampal neurons in a rat model of AD (Afshar et al., 2019). Moreover, 5-HT seems to directly regulate Aβ via amyloid precursor proteins (APPs) via 5-HT2AR and 5-HT2CR with different signalling pathways as they lead to the formation of APPs and ultimately the accumulation of Aβ (Fig. 1); this effect is blocked by 5-HT antagonists (ketanserin, mianserin, and ritanserin) (Nitsch et al., 1996).
The other classes of 5-HTRs, 5-HT4R, 5-HT6R, and 5-HT7R are coupled with Gs proteins and activate various signalling pathways including protein kinase A (PKA) and extracellular signal-regulated kinase (ERK) (Fig. 1). Compared to other 5-HT receptors, 5-HT4R targeted drugs have lately attracted considerable research interest, as many recent research studies have investigated its therapeutic potential in the treatment of AD (Bockaert et al., 2008). 5-HT4R has been associated with learning and memory (Bockaert et al., 2008; Hagena and Manahan-Vaughan, 2017). 5-HT4R expression has been found to be reduced in AD patients, while the activation of these receptors inhibits the (e.g., AC-cAMP-PKA) biochemical cascades that lead to AD (Fig. 1) (Hagena and Manahan-Vaughan, 2017; Reynolds et al., 1995).
Additionally, 5-HT4R agonists have been found to improve cognitive deficits in AD (e.g., Claeysen et al., 2015). For instance, Madsen and colleagues have reported that cerebral 5-HT4R binding is directly linked to abnormal accumulation of Aβ in AD patients (Madsen et al., 2011). Several other studies have also indicated that 5-HT4R agonists (e.g., RS6733, a partial agonist) inhibit the production of Aβ in the entorhinal cortex, a region of deterioration in early-stage AD (Gale et al., 2018), by promoting the production of the neurotropic soluble APP alpha (sAPPα) and helps in improving cognitive abilities (learning and memory) in animal models of AD (Baranger et al., 2017; Yahiaoui et al., 2016). Further, RS6733 is also used with nicotinic receptor allosteric modulator/cholinesterase inhibitor galantamine to compensate for the deficit associated with short- and long-term memory (Freret et al., 2017). Interestingly, RS67333 also acts as an acetylcholinesterase (AChE) inhibitor and helps in reviving the cholinergic functions which are typically altered in AD (Lecoutey et al., 2014). Moreover, chronic administration of this drug decreases the levels of Aβ in the hippocampus in 5XFAD mouse model (expressing human APP and PSEN1 transgenes) of AD (Giannoni et al., 2013). However, when another 5-HT4R agonist, SSP-002392, is applied to cultured human neuroblastoma cells, it increases sAPPα and cyclic adenosine monophosphate (cAMP) levels at a lower concentration than other well-known agonists (e.g., prucalopride), and suggesting the neuroprotective effect is mediated by EPAC (an exchange nucleotide protein directly activated by cAMP; Fig. 1) signalling (Cochet et al., 2013).
Apart from 5-HT4R, 5-HT6R has also attracted substantial research interest in the last few years. For instance, a 5-HT6R antagonist, SB271046 has been found to recover memory impairment by reducing the levels of Aβ via inhibiting the gamma-secretase activity (multi-subunit enzyme that produces Aβ) in a mouse model of AD (Yun et al., 2015). Stimulation of these receptors primarily modulates the extracellular concentration of glutamate and GABA in various neural circuits and contributes to the release of other neuromodulators (e.g., dopamine, norepinephrine (Ne), ACh) which are known to be impaired in AD (Khoury et al., 2018). Similarly, another 5-HT6R antagonist, idalopirdine (Lu AE58054), interacts with other neurotransmitter systems and increases the extracellular levels of dopamine, noradrenaline, and glutamate in the mPFC (de Jong and Mørk, 2017). Additionally, it has been suggested that these 5-HT6R antagonists may have synergistic effects when combined with acetylcholinesterase inhibitors (e.g., donepezil), but recent phase III trials have reported no additional benefit or significant improvements in cognitive functions (Andrews et al., 2018).
As with the other 5-HTRs, 5-HT7R is highly expressed in the hippocampus and plays an important role in memory formation, neuronal function and neurogenesis (Hashemi-Firouzi et al., 2017; Meneses, 2014; Shahidi et al., 2019). For example, activation of the 5-HT7R via AS19, a selective serotonin agonist, improves synaptic impairment in a rat model of AD by decreasing apoptosis (programmed cell death) in the hippocampus (Hashemi-Firouzi et al., 2017) and could potentially hinder the progression of AD.
Additionally, glycogen synthase kinase (GSK-3) appears to be an important component in many 5-HT receptor-mediated signalling pathways (Fig. 1). Overactivity of GSK-3 is linked to familial and sporadic forms of AD in terms of increased levels of plaques and tangles (Proctor and Gray, 2010; Polter and Li, 2011). In general, GSK-3 is a ubiquitously present kinase that exists is two isoforms (GSK-3α and GSK-3β). Its activation largely depends upon the phosphorylation by upstream kinases including 5-HT mediated pathways (Fig. 1) (Lauretti et al., 2020; Polter and Li, 2011). It regulates key downstream biological pathways that are potentially involved in a range of diseases and disorders including cancer, diabetes, bipolar disorder, and neurodegeneration, and are often considered as a potential therapeutic target by many drug companies (Pandey and DeGrado, 2016; Saraswati et al., 2018).
For animal models of AD, several studies have reported that elevated GSK-3β activity is associated with increased levels of Aβ and tau hyperphosphorylation (Lauretti et al., 2020; Llorens-Martín et al., 2014). The functionality of GSK-3β can be regularised by phosphorylation/dephosphorylation that occurs at different sites (Lauretti et al., 2020). For example, phosphorylation on tyrosine-279/216 activates its activity while phosphorylation on serine 21/9 via different kinases (e.g., Akt, PKA) inhibits it (Sayas et al., 2006). Generally, GSK-3β activation is associated with generation and deposition of Aβ (Fig. 1). This is a multi-step process and involves modulation of the APP cleavage via different pathways (non-amyloidogenic, amyloidogenic) and includes synergic action of various enzymes. The non-amyloidogenic pathway of APP involves alpha-secretase (ADAM10, ADAM17) and gamma-secretase enzymes, and forms a degradable peptide (Lauretti et al., 2020). The amyloidogenic pathway includes the sequential action of beta-secretase (BACE-1) and gamma-secretase enzymes and constitutes intermediates: fibril and oligomers that eventually converts to Aβ (Lauretti et al., 2020; Llorens-Martín et al., 2014). In particular BACE-1 activity is elevated in AD patients (Decourt and Sabbagh, 2011). Further, studies have reported that inhibition of GSK-3β reduces BACE-1 mediated APP cleavage and ultimately reduces the Aβ levels (Lauretti et al., 2020; Ly et al., 2013). GSK-3 inhibitor (SAR502250) also provide neuroprotective effect in the animal model of AD (Griebel et al., 2019). Additionally, GSK-3β plays a key role in tau phosphorylation. GSK-3β phosphorylates at different sites including Thr231 and leads to the separation of microtubules that fosters the generation of tau oligomers and neurofibrillary tangles (NFTs) (Lauretti et al., 2020). Given the above results, GSK-3 inhibitors (e.g., Tideglusib) are currently undergoing phase II clinical trials (Griebel et al., 2019).
There is also evidence that 5-HT based drugs (e.g. SSRIs) interact with GSK pathways, and are effective in lowering some of the proteins that are impaired during AD. Specifically, the SSRI escitalopram was found to reduce Aβ1-42 induced hyperphosphorylation of tau via the 5-HT1AR mediated Akt/GSK-3β pathway in the hippocampal neurons (Wang et al., 2016) (Fig. 1) and this could be a key pathway for the potential treatment of AD, especially in its early stage. Furthermore, SSRI can also modulate GSK-3β signalling to regulate the neurogenesis in hippocampus neurons via activation of 5-HT1AR (Fig. 1) (Hui et al., 2015). Overall, these studies suggest that SSRIs and 5-HTR based drugs, and their combinations with other targeted neuromodulator (e.g. ACh) receptors, have the potential to perturb downward signalling pathways involve in the regulation of Aβ and tau. Currently, novel drug therapeutics for AD targeting the 5-HT system is the subject of intense research given that these drugs are already available and currently undergoing Phase II clinical trials (Šimić et al., 2017).
However, the system can be rather complex. For instance, it is known that any postsynaptic neuron can manifest a combination of two to three 5-HT receptor subtypes (Mengod et al., 2010). Thus, a pressing issue for the research community is to understand whether 5-HT induced signalling pathways crosstalk with other pathways which are impaired in AD. Given that 5-HT receptor subtypes can also respond with different affinities (Mengod et al., 2010), drug effects can lead to variable activation of intracellular signalling cascades. The complexity is compounded by the involvement of a multitude of proteins, enzymes, transporters, related genes, neuronal and synaptic properties, and ultimately cognition and behaviour – a multiscale and multilevel problem. Thus, the systems’ complexity has hindered a deeper understanding of the underlying pathophysiological mechanisms that give rise to the abnormal accumulation of Aβ and tau and cognitive decline in AD has yet to be achieved.
Computational modelling offers a platform to bridge such a gap and guide drug design and development, and treatment (Roy, 2018). One way this can be achieved is through the integration of data or information across several experiments and the development of biologically based computational models. Indeed, such models have the potential to allow systematic exploration across multiple scales of description, beyond current experimental capabilities (Wong-Lin et al, 2017). This shall be our next point of discussion.
3. Towards multiscale computational modelling of serotonergic system
Computational modelling or in silico investigation in preclinical and clinical research is an important research component towards facilitating understanding of brain functions and diseases such as AD, and for drug discovery and development (Cutsuridis and Moustafa, 2017a, 2016; 2017b; Geerts et al., 2017b; Roy, 2018). For years, mechanistic models have been developed to provide insights into the mechanism underlying the disease, to explore novel drug targets, and to gain a deeper understanding of drug actions (De Witte et al., 2016; Meier-Schellersheim et al., 2019; Schmidt et al., 2013). In particular, the key attractiveness of a quantitative model, captured by mathematical or statistical measures, is to provide a more integrated and quantitative understanding of mechanisms and patterns while eliminating ambiguities and adding rigour to “mental” models (for intuitive understanding) in experimental/clinical neurosciences. As in the field of physical sciences and engineering, experimental “what-if” scenarios can be tested in model simulations to evaluate hypotheses. Computational models also provide testable model predictions, which can guide future experiments (Mazein et al., 2018).
Several computational models of AD pathologies, symptoms and treatments have been proposed, and these are covered in recent comprehensive reviews (see e.g. Cutsuridis and Moustafa, 2017a, 2017b; Hassan et al., 2018, and references therein). Several earlier modelling papers in systems and theoretical biology were emphasised more towards understanding the dynamics of Aβ accumulation and its interactions with other proteins (Hassan et al. (2018)). Later modelling work encompassed the mapping of much larger number of biochemical interactions and other more data-driven, using either logic-based (Maude Petri net) models (Anastasio, 2011). For instance, such models could link Aβ effects on synaptic plasticity (Anastasio, 2014), and the search for potential combination of drugs to reduce microglial inflammation (Anastasio, 2015). More abstract probabilistic graphical (Bayesian) network models were also applied to understand key protein/drug interactions at the systems level (Rembach et al., 2015).
To date, there are only a small number of computational modelling studies on 5-HT mediated signalling pathways. Importantly, none of the computational studies are focused on understanding the role of 5-HT mediated intracellular signalling pathways in AD. Hence, these present opportunities for computational modellers to contribute. Thus, we shall henceforth first discuss a set of models on 5-HT mediated pathways, before discussing a separate set of models on aggregation of Aβ and hyperphosphorylated tau and tangles. Then, we argue the potential to integrate these two sets of models together.
In a highly detailed computational model of 5-HT receptor-mediated signalling, Chang et al. (2009) developed a model of 5-HT1AR and 5-HT2AR activated ERK(1/2) pathways using Michaelis–Menten formalism and the law of mass action. In the model, 5-HT1AR stimulated phosphoinositide 3-kinases (PI–3K) pathway while 5-HT2AR triggered mitogen-activated protein kinase (MAPK)/ERK pathway (also known as the Ras-Raf-MEK-ERK pathway) (Fig. 1). Their model's key results, in agreement with experimental data, showed the dominance of 5-HT2AR over 5-HT1AR in the MAPK signalling pathway, and the deleterious effects of regulator/enzymes affecting basal levels of ERK. In another modelling work, Zhang et al. (2012), building on the model by Pettigrew et al. (2005) (Pettigrew et al., 2005; Zhang et al., 2012), studied the effects of 5-HT on PKA-ERK interactions to enhance long-term facilitation of synapses. In a similar vein, Zhou et al. (2014) modelled both PKA and PKC signalling to show that PKC was sufficient for short-term facilitation of synapses, and that cooperation among the signalling cascades could potentially contribute to the enhancement of learning and memory, which had recently been validated experimentally (Liu et al., 2017; Zhou et al., 2014). These results may have implications in AD, given the latter's deterioration in learning and memory. Importantly, these models also laid the foundation for modelling 5-HT signalling pathways through PKA and PKC responses (Fig. 1).
As discussed earlier, and illustrated in Fig. 1, 5-HT signalling not only involves ERK and PKA pathways but also engages the GSK-3 enzymes via 5-HTRs. Also, GSK-3 can in turn modulate 5-HT1BR, which exists at presynaptic terminals of 5-HT neurons, leading to changes in 5-HT level. GSK-3 is inactivated by serine phospho-ser21/Ser9 (ps21/9), and the latter bridges between 5-HTRs and GSK-3 (Fig. 1). The intermediate transcription factor p53, which regulates the expression of cellular stress response genes, can interact with GSK-3 (Jazvinšćak Jembrek et al., 2018). Mild oxidative stress injury can lead to p53 ensuring antioxidative activities and promoting cell survival. But over antioxidative capacity can lead to cell death by p53 (apoptosis). Hence, this results in a possible link between 5-HT, GSK-3 and the fields of neuroinflammation and immunology.
Such biochemical reaction process involving GSK-3, p53, tau tangles and Aβ aggregation was first modelled by Proctor and Gray (2010) using Systems Biology Markup Language (SBML) and stochastic simulation, building on their previous model of p53 (Proctor and Gray, 2008). Their modelling results accounted for the overactivity of GSK-3 and p53 after a stress event, leading to increase in Aβ and tau tangles, providing a correlation between the latter two, but not causation. Later, Proctor et al. (2013) extended the model and showed that immunisation helped to clear plaques, but limited influence on soluble Aβ, phosphorylated tau and tangles, consistent with experimental observation (Proctor et al., 2013). Interestingly, the model results suggested interventions to be performed at a very early stage of AD. It should also be noted that GSK-3 is also associated with other neurodegeneration related to tauopathies, such as Pick's diseases, progressive supranuclear palsy and corticobasal degeneration (Ferrer et al., 2002). Hence, it may be worth investigating through computational modelling to further understand the 5-HT based drug (e.g., SSRI) effects and treatments on these other diseases.
Taken together, despite their small number, the abovementioned mechanistic models were used as examples which could potentially be integrated to provide a deeper understanding of the effects of 5-HT through GSK-3 to aggregation of plaques and tau tangles and subsequent changes in neuronal and synaptic properties, and potential novel therapeutics. For instance, 5-HT induced PKA and PKC pathways can modulate at least four membrane currents including 5-HT sensitive, voltage-dependent and calcium-activated potassium currents and L-type calcium currents (Baxter et al., 1999). Notably, modulation of voltage-dependent potassium currents by PKC plays a significant part in the broadening of spikes (Baxter et al., 1999). In contrast, simultaneous modulation of 5-HT sensitive potassium and calcium current increases the excitability within cells, potentially due to the indirect crosstalk among PKC and PKA pathways. Further, Aβ can interact with membrane ion channel currents and can affect their overall excitability. For example, computational studies by Zou and colleagues showed that Aβ blocked A-type currents and increased the excitability of pyramidal neurons, and subsequently the excitability of the (hippocampal septal) microcircuit (Zou et al., 2012, 2011). This could in turn lead to memory impairment and even symptoms of epileptic seizures, a common comorbidity in AD (Zou et al., 2012). Complementary to these studies, Abuhassan and colleagues used a large-scale model to understand the effects of synaptic loss, due to AD, on global oscillatory dynamics (Abuhassan et al., 2014). Thus, there is the potential for computational models to bridge from one level of description to another – multiscale and multilevel modelling. However, linking from one modelling scale/level to another requires its abstraction (Wong-Lin et al., 2017).
In fact, a challenge to mechanistic models lies in how they can be utilised when certain components of the network are not parameterised or missing, which can impact on model accuracy (Fröhlich et al., 2018). A practical way to estimate the optimal set of model parameter values is to fix certain known model parameters and manipulate others within a physiologically reasonable range until the model is able to mimic experimentally observed pattern(s) (Maex et al., 2009; Sterratt et al., 2011). However, as the number of unknown parameters increases, typically in the context of a larger signalling network, it becomes exponentially more difficult to systematically search the parameter space and unique solutions are not guaranteed, since there may exist many combinations of parameters that can furnish similar outputs. An alternative approach to dealing with such challenges is creating simpler, reduced computational or mathematical models that approximate the larger network (Albert and Thakar, 2014).
One approach is to reduce the complexity of the dynamic behaviour of the model that can be introduced when the model exhibits bi- or multi-modality is to use Boolean models. Such models can qualitatively recreate the temporal dynamics of a larger signalling network (Albert and Thakar, 2014). These models are particularly useful when limited kinetic details about the interaction of components are available (Albert and Thakar, 2014). For instance, when Boolean models are used in a gene regulatory network, they can describe the characteristics of circadian systems (Akman et al., 2012; Watterson and Ghazal, 2010). These models are also employed to analyse the influence of stress and SSRIs in complex networks of 5-HT, neurotrophin and cortisol mediated signalling pathways (Moreno-Ramos et al., 2013). Notably, one such model predicts the network dynamics, especially when specific genes are knocked out (Moreno-Ramos et al., 2013).
Another promising approach to minimise the number of unknown parameters in mechanistic models is to estimate the prior knowledge of the unknown components. This can be achieved by using Bayesian methods (Spiegelhalter et al., 2002). For example, Bayesian methods are used to deduce mechanistic parameters for amyloid formation kinetics (Nakatani-Webster and Nath, 2017). Other methods to reduce the parameter space in signalling pathways include perturbation techniques. For instance, Flower and Wong-Lin (2014) and Cullen and Wong-Lin (2015) used step perturbation technique to elucidate key model components (e.g. substrates) and their temporal dynamics, which lead to substantial reduction of model sizes for intracellular signalling in presynaptic terminals of 5-HT- and dopamine-producing neurons, respectively. Perhaps similar or more advanced techniques could be applied to models of postsynaptic 5-HT mediated signalling pathways.
4. Conclusion and future directions
AD is a complex neurodegenerative disorder characterised by cognitive impairment comorbid with behavioural changes that considerably affect day-to-day functioning. Neuropathologically, AD is marked by an excessive accumulation of Aβ and hyperphosphorylated tau protein. Currently available drugs for AD are primarily used to reduce symptoms or control behaviour, but not cure AD. Majority of them target neurotransmitter systems that include cholinergic, non-cholinergic, glutamatergic and their combinations.
The focus of our review is to highlight the role of 5-HT system in AD. We discussed the 5-HTR mediated signalling pathways. These pathways are targeted by drugs such as SSRIs. We then discussed the role of SSRIs and 5-HTR mediated drugs in AD. Newer generation of antidepressant drugs such as SNRIs (e.g., venlafaxine) can provide an alternative route for the treatment of AD, as there is emerging evidence that suggests that norepinephrine system is also involved in Aβ regulation (Liu et al., 2015; Mokhber et al., 2014; Ross et al., 2015). Hence, further modelling work on the interaction of neuromodulators will potentially be enlightening (Jalewa et al., 2014; Joshi et al., 2011, Joshi et al., 2015, Joshi et al., 2017; Wang and Wong-Lin, 2013).
We then highlighted the importance of the GSK-3 protein kinase, as a key kinase that sits in the downstream signalling pathway of most of the 5-HT receptors especially 5-HT1AR and 5HT2AR. We then discussed existing computational models that describe the mechanisms that link GSK-3 to aggregation of Aβ and tau hyperphosphorylation. Then, as an example, we suggested that both types of models could potentially be integrated to understand novel 5-HT based therapeutics for AD. Further, we highlighted the importance of reduced modelling approaches such as Boolean and Bayesian approaches, especially when there are many unknown parameters in a model or when the level of description in the data varies a lot. Abstraction of these models could be used as basis for models in adjacent scale, e.g. neuronal or neuronal network model.
Taken together, we have shown that there are currently not many multiscale computational models of 5-HT mediated signalling pathways and their links to AD. Hence, there are ample opportunities for computational scientists or mathematical modellers in this research area. Collating and unifying models will become essential (Lloret-Villas et al., 2017).
As hinted earlier, complementing mechanistic modelling approaches, we can also utilise knowledge- and data-driven approaches (Geerts et al., 2017a; Li et al., 2009). Compared to mechanistic models, non-mechanistic, data-driven computational models can forge relationships among the input and output datasets, without taking into account the underpinning biological processes (Zhang et al., 2018). These methods largely use probabilistic or statistical methods, including machine learning approaches, to solve complex problems, and a key advantage is that they could work well with highly heterogeneous datasets (Ding et al., 2018). Knowledge-driven approaches make use of available literature, clinical/medical records, and online resources to mine relevant information (Younesi and Hofmann-Apitius, 2013). Usually, these models operate in conjunction with data-driven models and can feed to other types of models (e.g., mechanistic), for example, to identify correlation or (probabilistic) causality for a better understanding of the relationship among the system's components (e.g., proteins, enzymes) present in the intracellular signalling network (Younesi and Hofmann-Apitius, 2013).
These types of models are highly relevant and can be used in conjunction with mechanistic models to understand disease mechanism(s) and potential novel therapeutic approaches. For instance, with such models, we could explore how drug(s) can activate 5-HTR mediated signalling pathways which involve GSK-3 protein kinase and regulates Aβ and tau. Not surprisingly, interaction and parameter values of these pathways may be different for different brain regions. Practically, it is impossible to estimate the activity of these signalling pathways for all brain regions. Thus, there remains considerable opportunity for computational studies to estimate the activities of key proteins for different brain areas and link outcomes with properties that can be mechanistically modelled e.g. synaptic currents. These modelling features can then be a key ingredient to study how changes in signalling pathways can affect the function of neural networks via current modulation which in turn could affect cognitive (dys)function (Cano-Colino et al., 2013; Eckhoff et al., 2009).To achieve this, it is of utmost importance that experimentalists and computational modellers work together more synergistically.
Acknowledgements
This work was supported by the Alzheimer's Research UK (ARUK) Pump Priming Award (ARUK-2017NC-NI) (A.J., D.-H.W., S.W., P.L.M., T.S. and K.W.-L.), BBSRC (BB/P003427/1) (A.J., T.S., C.K.B. and K.W.-L.), Ulster University Research Challenge Fund (C.K.B., P.L.M. and K.W.-L.), NSFC (31671077) (D.-H.W) and European Union’s INTERREG VA Programme, managed by the Special EU Programmes Body (SEUPB (Centre for Personalised Medicine, IVA 5036)) (P. L.M. and K.W.-L.). K.W.-L. and S.W. were supported by COST Action (CA15120) Open Multiscale Systems Medicine (OpenMultiMed) supported by COST (European Cooperation in Science and Technology). K.W.-L. was additionally supported by the Northern Ireland Functional Brain Mapping Project (1303/101154803) funded by Invest NI and Ulster University. The views and opinions expressed in this paper do not necessarily reflect those of the European Commission or the Special EU Programmes Body (SEUPB).
Contributor Information
Alok Joshi, Email: a.joshi@ulster.ac.uk.
KongFatt Wong-Lin, Email: k.wong-lin@ulster.ac.uk.
References
- Wang J., Zhang Y., Xu H., Zhu S., Wang H., He J., Zhang H., Guo H., Kong J., Huang Q., Li X.-M. Fluoxetine improves behavioral performance by suppressing the production of soluble β-amyloid in APP/PS1 mice. Curr. Alzheimer Res. 2014;11:672–680. doi: 10.2174/1567205011666140812114715. [DOI] [PubMed] [Google Scholar]
- Abuhassan K., Coyle D., Maguire L. Compensating for thalamocortical synaptic loss in Alzheimer's disease. Front. Comput. Neurosci. 2014;8 doi: 10.3389/fncom.2014.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afshar S., Shahidi S., Rohani A.H., Soleimani Asl S., Komaki A. Protective effects of 5-HT 1A receptor antagonist and 5-HT 2A receptor agonist on the biochemical and histological features in a rat model of Alzheimer's disease. J. Chem. Neuroanat. 2019;96:140–147. doi: 10.1016/j.jchemneu.2019.01.008. [DOI] [PubMed] [Google Scholar]
- Akman O.E., Watterson S., Parton A., Binns N., Millar A.J., Ghazal P. Digital clocks: simple Boolean models can quantitatively describe circadian systems. J. R. Soc. Interface. 2012;9:2365–2382. doi: 10.1098/rsif.2012.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert R., Thakar J. Boolean modeling: a logic-based dynamic approach for understanding signaling and regulatory networks and for making useful predictions. Wiley Interdiscip. Rev. Syst. Biol. Med. 2014;6:353–369. doi: 10.1002/wsbm.1273. [DOI] [PubMed] [Google Scholar]
- Anastasio T.J. Data-driven modeling of Alzheimer disease pathogenesis. J. Theor. Biol. 2011;290:60–72. doi: 10.1016/j.jtbi.2011.08.038. [DOI] [PubMed] [Google Scholar]
- Anastasio T.J. Computational identification of potential multitarget treatments for ameliorating the adverse effects of amyloid-ß on synaptic plasticity. Front. Pharmacol. 2014;5:85. doi: 10.3389/fphar.2014.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasio T.J. Computational identification of potential multi-drug combinations for reduction of microglial inflammation in Alzheimer disease. Front. Pharmacol. 2015;6:116. doi: 10.3389/fphar.2015.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews M., Tousi B., Sabbagh M.N. 5HT6 antagonists in the treatment of alzheimer's dementia: current progress. Neurol. Ther. 2018 doi: 10.1007/s40120-018-0095-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten A.F.T. Stress signalling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci. 2009 doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldas V., Lampiris C., Capsalis C., Koutsouris D. Lecture Notes of the Institute for Computer Sciences, Social-Informatics and Telecommunications Engineering. 2011. Early diagnosis of Alzheimer's type dementia using continuous speech recognition; pp. 105–110. [DOI] [Google Scholar]
- Baranger K., Giannoni P., Girard S.D., Girot S., Gaven F., Stephan D., Migliorati M., Khrestchatisky M., Bockaert J., Marchetti-Gauthier E., Rivera S., Claeysen S., Roman F.S. Chronic treatments with a 5-HT4 receptor agonist decrease amyloid pathology in the entorhinal cortex and learning and memory deficits in the 5xFAD mouse model of Alzheimer's disease. Neuropharmacology. 2017;126:128–141. doi: 10.1016/j.neuropharm.2017.08.031. [DOI] [PubMed] [Google Scholar]
- Baxter D.A., Canavier C.C., Clark J.W., Byrne J.H. Computational model of the serotonergic modulation of sensory neurons in Aplysia. J. Neurophysiol. 1999;82:2914–2935. doi: 10.1152/jn.1999.82.6.2914. [DOI] [PubMed] [Google Scholar]
- Becker G., Streichenberger N., Billard T., Newman-Tancredi A., Zimmer L. A postmortem study to compare agonist and antagonist 5-HT 1A receptor-binding sites in alzheimer's disease. CNS Neurosci. Ther. 2014;20:930–934. doi: 10.1111/cns.12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birks J.S., Harvey R.J. Donepezil for dementia due to Alzheimer's disease. Cochrane Database Syst. Rev. 2018 doi: 10.1002/14651858.CD001190.pub3. [DOI] [PubMed] [Google Scholar]
- Bocchio M., McHugh S.B., Bannerman D.M., Sharp T., Capogna M. Serotonin, amygdala and fear: assembling the puzzle. Front. Neural Circ. 2016 doi: 10.3389/fncir.2016.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockaert J., Claeysen S., Compan V., Dumuis A. 5-HT4 receptors: history, molecular pharmacology and brain functions. Neuropharmacology. 2008;55:922–931. doi: 10.1016/j.neuropharm.2008.05.013. [DOI] [PubMed] [Google Scholar]
- Butzlaff M., Ponimaskin E. The role of Serotonin Receptors in Alzheimer's disease. Opera Med Physiol. 2016;1:91–100. [Google Scholar]
- Cano-Colino M., Almeida R., Compte A. Serotonergic modulation of spatial working memory: predictions from a computational network model. Front. Integr. Neurosci. 2013;7 doi: 10.3389/fnint.2013.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. wen, Poteet E., Schetz J.A., Gümüş Z.H., Weinstein H. Towards a quantitative representation of the cell signaling mechanisms of hallucinogens: measurement and mathematical modeling of 5-HT1A and 5-HT2A receptor-mediated ERK1/2 activation. Neuropharmacology. 2009;56:213–225. doi: 10.1016/j.neuropharm.2008.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen R., Marcussen A.B., Wörtwein G., Knudsen G.M., Aznar S. Aβ(1-42) injection causes memory impairment, lowered cortical and serum BDNF levels, and decreased hippocampal 5-HT2A levels. Exp. Neurol. 2008;210:164–171. doi: 10.1016/j.expneurol.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Claeysen S., Bockaert J., Giannoni P. Serotonin: a new hope in alzheimer's disease? ACS Chem. Neurosci. 2015;6:940–943. doi: 10.1021/acschemneuro.5b00135. [DOI] [PubMed] [Google Scholar]
- Cochet M., Donneger R., Cassier E., Gaven F., Lichtenthaler S.F., Marin P., Bockaert J., Dumuis A., Claeysen S. 5-HT4 receptors constitutively promote the non-amyloidogenic pathway of APP cleavage and interact with ADAM10. ACS Chem. Neurosci. 2013;4:130–140. doi: 10.1021/cn300095t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen M., Wong-Lin K.F. Integrated dopaminergic neuronal model with reduced intracellular processes and inhibitory autoreceptors. IET Syst. Biol. 2015;9:245–258. doi: 10.1049/iet-syb.2015.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutsuridis V., Moustafa A.A. Multiscale models of pharmacological, immunological and neurostimulation treatments in Alzheimer's disease. Drug Discov. Today Dis. Model. 2016 doi: 10.1016/j.ddmod.2016.12.001. [DOI] [Google Scholar]
- Cutsuridis V., Moustafa A. Computational models of Alzheimer's disease. Scholarpedia. 2017;12:32144. doi: 10.4249/scholarpedia.32144. [DOI] [Google Scholar]
- Cutsuridis V., Moustafa A.A. Computational Models of Brain and Behavior. John Wiley & Sons, Ltd; Chichester, UK: 2017. Computational models of pharmacological and immunological treatment in alzheimer's disease; pp. 99–108. [DOI] [Google Scholar]
- de Jong I.E.M., Mørk A. Antagonism of the 5-HT6 receptor – preclinical rationale for the treatment of Alzheimer's disease. Neuropharmacology. 2017 doi: 10.1016/j.neuropharm.2017.07.010. [DOI] [PubMed] [Google Scholar]
- De Witte W.E.A., Wong Y.C., Nederpelt I., Heitman L.H., Danhof M., Van Der Graaf P.H., Gilissen R.A.H.J., De Lange E.C.M. Mechanistic models enable the rational use of in vitro drug-target binding kinetics for better drug effects in patients. Expet Opin. Drug Discov. 2016 doi: 10.1517/17460441.2016.1100163. [DOI] [PubMed] [Google Scholar]
- Decourt B., Sabbagh M.N. BACE1 as a potential biomarker for alzheimer's disease. J. Alzheim. Dis. 2011 doi: 10.3233/JAD-2011-110017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X., Bucholc M., Wang Haiying, Glass D.H., Wang Hui, Clarke D.H., Bjourson A.J., Dowey L.R.C., O'Kane M., Prasad G., Maguire L., Wong-Lin K. A hybrid computational approach for efficient Alzheimer's disease classification based on heterogeneous data. Sci. Rep. 2018;8:1–10. doi: 10.1038/s41598-018-27997-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorszewska J., Prendecki M., Oczkowska A., Dezor M., Kozubski W. Molecular basis of familial and sporadic alzheimer's disease. Curr. Alzheimer Res. 2016;13:952–963. doi: 10.2174/1567205013666160314150501. [DOI] [PubMed] [Google Scholar]
- Douchamps V., Mathis C. A second wind for the cholinergic system in Alzheimer's therapy. Behav. Pharmacol. 2017 doi: 10.1097/FBP.0000000000000300. [DOI] [PubMed] [Google Scholar]
- Eckhoff P., Wong-Lin K.F., Holmes P. Optimality and robustness of a biophysical decision-making model under norepinephrine modulation. J. Neurosci. 2009;29:4301–4311. doi: 10.1523/JNEUROSCI.5024-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsworthy R.J., Aldred S. Depression in alzheimer's disease: an alternative role for selective serotonin reuptake inhibitors? J. Alzheim. Dis. 2019 doi: 10.3233/JAD-180780. [DOI] [PubMed] [Google Scholar]
- Fakhfouri G., Rahimian R., Dyhrfjeld-Johnsen J., Zirak M.R., Beaulieu J.M. 5-HT3 receptor antagonists in neurologic and neuropsychiatric disorders: the iceberg still lies beneath the surface. Pharmacol. Rev. 2019;71:383–412. doi: 10.1124/pr.118.015487. [DOI] [PubMed] [Google Scholar]
- Ferrer I., Barrachina M., Puig B. Glycogen synthase kinase-3 is associated with neuronal and glial hyperphosphorylated tau deposits in Alzheimer's disease, Pick's disease, progressive supranuclear palsy and corticobasal degeneration. Acta Neuropathol. 2002;104:583–591. doi: 10.1007/s00401-002-0587-8. [DOI] [PubMed] [Google Scholar]
- Flower G., Wong-Lin K. Reduced computational models of serotonin synthesis, release, and reuptake. IEEE Trans. Biomed. Eng. 2014;61:1054–1061. doi: 10.1109/TBME.2013.2293538. [DOI] [PubMed] [Google Scholar]
- Francis P.T., Palmer A.M., Snape M., Wilcock G.K. The cholinergic hypothesis of Alzheimer's disease: a review of progress. J. Neurol. Neurosurg. Psychiatry. 1999 doi: 10.1136/jnnp.66.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freret T., Lelong-Boulouard V., Lecouflet P., Hamidouche K., Dauphin F., Boulouard M. Co-modulation of an allosteric modulator of nicotinic receptor-cholinesterase inhibitor (galantamine) and a 5-HT4 receptor agonist (RS-67333): effect on scopolamine-induced memory deficit in the mouse. Psychopharmacology (Berl) 2017;234:2365–2374. doi: 10.1007/s00213-017-4664-z. [DOI] [PubMed] [Google Scholar]
- Fröhlich F., Kessler T., Weindl D., Shadrin A., Schmiester L., Hache H., Muradyan A., Schütte M., Lim J.H., Heinig M., Theis F.J., Lehrach H., Wierling C., Lange B., Hasenauer J. Efficient parameter estimation enables the prediction of drug response using a mechanistic pan-cancer pathway model. Cell Syst. 2018;7:567–579. doi: 10.1016/j.cels.2018.10.013. e6. [DOI] [PubMed] [Google Scholar]
- Gale S.A., Acar D., Daffner K.R. Dementia. Am. J. Med. 2018 doi: 10.1016/j.amjmed.2018.01.022. [DOI] [PubMed] [Google Scholar]
- Galts C.P.C., Bettio L.E.B., Jewett D.C., Yang C.C., Brocardo P.S., Rodrigues A.L.S., Thacker J.S., Gil-Mohapel J. Depression in neurodegenerative diseases: common mechanisms and current treatment options. Neurosci. Biobehav. Rev. 2019 doi: 10.1016/j.neubiorev.2019.04.002. [DOI] [PubMed] [Google Scholar]
- Geerts H., Hofmann-Apitius M., Anastasio T.J. Brain Health Modeling Initiative, 2017a. Knowledge-driven computational modeling in Alzheimer's disease research: Current state and future trends. Alzheimers. Dement. 2017;13:1292–1302. doi: 10.1016/j.jalz.2017.08.011. [DOI] [PubMed] [Google Scholar]
- Geerts H., Spiros A., Roberts P., Carr R. Towards the virtual human patient. Quantitative Systems Pharmacology in Alzheimer's disease. Eur. J. Pharmacol. 2017;817:38–45. doi: 10.1016/j.ejphar.2017.05.062. [DOI] [PubMed] [Google Scholar]
- Giannoni P., Gaven F., De Bundel D., Baranger K., Marchetti-Gauthier E., Roman F.S., Valjent E., Marin P., Bockaert J., Rivera S., Claeysen S. Early administration of RS 67333, a specific 5-HT4 receptor agonist, prevents amyloidogenesis and behavioral deficits in the 5XFAD mouse model of Alzheimer's disease. Front. Aging Neurosci. 2013;5 doi: 10.3389/fnagi.2013.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goutagny R., Gu N., Cavanagh C., Jackson J., Chabot J.-G., Quirion R., Krantic S., Williams S. Alterations in hippocampal network oscillations and theta-gamma coupling arise before Aβ overproduction in a mouse model of Alzheimer's disease. Eur. J. Neurosci. 2013;38 doi: 10.1111/ejn.12446. 3527–3527. [DOI] [PubMed] [Google Scholar]
- Griebel G., Stemmelin J., Lopez-Grancha M., Boulay D., Boquet G., Slowinski F., Pichat P., Beeské S., Tanaka S., Mori A., Fujimura M., Eguchi J. The selective GSK3 inhibitor, SAR502250, displays neuroprotective activity and attenuates behavioral impairments in models of neuropsychiatric symptoms of Alzheimer's disease in rodents. Sci. Rep. 2019;9:1–15. doi: 10.1038/s41598-019-54557-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagena H., Manahan-Vaughan D. The serotonergic 5-HT4 receptor: a unique modulator of hippocampal synaptic information processing and cognition. Neurobiol. Learn. Mem. 2017;138:145–153. doi: 10.1016/j.nlm.2016.06.014. [DOI] [PubMed] [Google Scholar]
- Hannibal K.E., Bishop M.D. Chronic stress, cortisol dysfunction, and pain: a psychoneuroendocrine rationale for stress management in pain rehabilitation. Phys. Ther. 2014;94:1816–1825. doi: 10.2522/ptj.20130597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon J., Hoyer D. Serotonin receptors and systems: endless diversity? Acta Biol. Szeged. 2002;46:1–12. [Google Scholar]
- Haruhiko A., Barger S., Barnum S., Bradt B., Bauer J., Cole G.M., Cooper N.R., Eikelenboom P., Emmerling M., Fiebich B.L., Finch C.E., Frautschy S., Griffin W.S.T., Hampel H., Hull M., Landreth G., Lue L.F., Mrak R., Mackenzie I.R., Mcgeer P.L., Banion M.K.O., Pachter J., Pasinetti G., Salaman C.P., Rogers J., Rydel R., Shen Y., Streit W., Strohmeyer R., Tooyoma I., Muiswinkel F.L. Van, Veerhuis R., Walker D., Webster S., Wegrzyniak B., Wenk G., Coray T.W. 2000. Inflammation and Alzheimer's Disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemi-Firouzi N., Komaki A., Soleimani Asl S., Shahidi S. The effects of the 5-HT7 receptor on hippocampal long-term potentiation and apoptosis in a rat model of Alzheimer's disease. Brain Res. Bull. 2017;135:85–91. doi: 10.1016/j.brainresbull.2017.10.004. [DOI] [PubMed] [Google Scholar]
- Hassan M., Abbas Q., Seo S.Y., Shahzadi S., Al Ashwal H., Zaki N., Iqbal Z., Moustafa A.A. Computational modeling and biomarker studies of pharmacological treatment of Alzheimer's disease (Review) Mol. Med. Rep. 2018 doi: 10.3892/mmr.2018.9044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselbalch S.G., Madsen K., Svarer C., Pinborg L.H., Holm S., Paulson O.B., Waldemar G., Knudsen G.M. Reduced 5-HT2A receptor binding in patients with mild cognitive impairment. Neurobiol. Aging. 2008;29:1830–1838. doi: 10.1016/j.neurobiolaging.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Heneka M.T., Carson M.J., Khoury J. El, Landreth G.E., Brosseron F., Feinstein D.L., Jacobs A.H., Wyss-Coray T., Vitorica J., Ransohoff R.M., Herrup K., Frautschy S.A., Finsen B., Brown G.C., Verkhratsky A., Yamanaka K., Koistinaho J., Latz E., Halle A., Petzold G.C., Town T., Morgan D., Shinohara M.L., Perry V.H., Holmes C., Bazan N.G., Brooks D.J., Hunot S., Joseph B., Deigendesch N., Garaschuk O., Boddeke E., Dinarello C.A., Breitner J.C., Cole G.M., Golenbock D.T., Kummer M.P. Neuroinflammation in Alzheimer's disease. Lancet Neurol. 2015 doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm P., Ettrup A., Klein A.B., Santini M.A., El-Sayed M., Elvang A.B., Stensbøl T.B., Mikkelsen J.D., Knudsen G.M., Aznar S. Plaque deposition dependent decrease in 5-HT2A serotonin receptor in aβPPswe/PS1dE9 amyloid overexpressing mice. J. Alzheim. Dis. 2010;20:1201–1213. doi: 10.3233/JAD-2010-100117. [DOI] [PubMed] [Google Scholar]
- Huang L.K., Chao S.P., Hu C.J. Clinical trials of new drugs for Alzheimer disease. J. Biomed. Sci. 2020 doi: 10.1186/s12929-019-0609-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui J., Zhang J., Kim H., Tong C., Ying Q., Li Z., Mao X., Shi G., Yan J., Zhang Z., Xi G. Fluoxetine regulates neurogenesis in vitro through modulation of gsk-3b/b-catenin signaling. Int. J. Neuropsychopharmacol. 2015;18:1–12. doi: 10.1093/ijnp/pyu099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynd M.R., Scott H.L., Dodd P.R. Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer's disease. Neurochem. Int. 2004 doi: 10.1016/j.neuint.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Jalewa J., Joshi A., McGinnity T.M., Prasad G., Wong-Lin K., Hölscher C. Neural circuit interactions between the dorsal raphe nucleus and the lateral hypothalamus: an experimental and computational study. PloS One. 2014;9 doi: 10.1371/journal.pone.0088003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazvinšćak Jembrek M., Slade N., Hof P.R., Šimić G. The interactions of p53 with tau and Aß as potential therapeutic targets for Alzheimer's disease. Prog. Neurobiol. 2018;168:104–127. doi: 10.1016/j.pneurobio.2018.05.001. [DOI] [PubMed] [Google Scholar]
- Joshi A., Wong-Lin K., McGinnity T.M., Prasad G. Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society. EMBS; 2011. A mathematical model to explore the interdependence between the serotonin and orexin/hypocretin systems; pp. 7270–7273. [DOI] [PubMed] [Google Scholar]
- Joshi A., Belle M.D.C., Wong-Lin K.F., Piggins H.D. Orexin and Sleep: Molecular, Functional and Clinical Aspects. Springer International Publishing; 2015. Orexin and circadian influences in sleep and psychiatric disorders: a review of experimental and computational modelling studies; pp. 299–322. [DOI] [Google Scholar]
- Joshi A., Youssofzadeh V., Vemana V., McGinnity T.M., Prasad G., Wong-Lin K.F. An integrated modelling framework for neural circuits with multiple neuromodulators. J. R. Soc. Interface. 2017;14 doi: 10.1098/rsif.2016.0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice N.J. The relationship between stress and Alzheimer's disease. Neurobiol. Stress. 2018 doi: 10.1016/j.ynstr.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepe V., Barrio J.R., Huang S.C., Ercoli L., Siddarth P., Shoghi-Jadid K., Cole G.M., Satyamurthy N., Cummings J.L., Small G.W., Phelps M.E. Serotonin 1A receptors in the living brain of Alzheimer's disease patients. Proc. Natl. Acad. Sci. U.S.A. 2006;103:702–707. doi: 10.1073/pnas.0510237103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessing L.V., Forman J.L., Andersen P.K. Do continued antidepressants protect against dementia in patients with severe depressive disorder? Int. Clin. Psychopharmacol. 2011;26:316–322. doi: 10.1097/YIC.0b013e32834ace0f. [DOI] [PubMed] [Google Scholar]
- Khalifeh S., Fakhfouri G., Mehr S.E., Mousavizadeh K., Dehpour A.R., Khodagholi F., Kazmi S., Rahimian R. Beyond the 5-HT3 receptors: a role for α7nACh receptors in neuroprotective aspects of tropisetron. Hum. Exp. Toxicol. 2015;34:922–931. doi: 10.1177/0960327114562034. [DOI] [PubMed] [Google Scholar]
- Khoury R., Grysman N., Gold J., Patel K., Grossberg G.T. The role of 5 HT6-receptor antagonists in Alzheimer's disease: an update. Expet Opin. Invest. Drugs. 2018 doi: 10.1080/13543784.2018.1483334. [DOI] [PubMed] [Google Scholar]
- Kim H.J., Kim W., Kong S.Y. Antidepressants for neuro-regeneration: from depression to Alzheimer's disease. Arch Pharm. Res. (Seoul) 2013 doi: 10.1007/s12272-013-0238-8. [DOI] [PubMed] [Google Scholar]
- Lai M.K.P., Tsang S.W.Y., Francis P.T., Keene J., Hope T., Esiri M.M., Spence I., Chen C.P.L.H. Postmortem serotoninergic correlates of cognitive decline in Alzheimer's disease. Neuroreport. 2002;13:1175–1178. doi: 10.1097/00001756-200207020-00021. [DOI] [PubMed] [Google Scholar]
- Lai M.K.P., Tsang S.W.Y., Francis P.T., Esiri M.M., Keene J., Hope T., Chen C.P.L.H. Reduced serotonin 5-HT1A receptor binding in the temporal cortex correlates with aggressive behavior in Alzheimer disease. Brain Res. 2003;974:82–87. doi: 10.1016/S0006-8993(03)02554-X. [DOI] [PubMed] [Google Scholar]
- Lai M.K., Tsang S.W., Alder J.T., Keene J., Hope T., Esiri M.M., Francis P.T., Chen C.P. Loss of serotonin 5-HT2A receptors in the postmortem temporal cortex correlates with rate of cognitive decline in Alzheimer's disease. Psychopharmacology (Berl) 2005;179:673–677. doi: 10.1007/s00213-004-2077-2. [DOI] [PubMed] [Google Scholar]
- Lanctôt K.L., Hussey D.F., Herrmann N., Black S.E., Rusjan P.M., Wilson A.A., Houle S., Kozloff N., Verhoeff N.P.L.G., Kapur S. A positron emission tomography study of 5-hydroxytryptamine-1A receptors in alzheimer disease. Am. J. Geriatr. Psychiatr. 2007;15:888–898. doi: 10.1097/JGP.0b013e3180488325. [DOI] [PubMed] [Google Scholar]
- Lauretti E., Dincer O., Praticò D. Glycogen synthase kinase-3 signaling in Alzheimer's disease. Biochim. Biophys. Acta Mol. Cell Res. 2020 doi: 10.1016/j.bbamcr.2020.118664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecoutey C., Hedou D., Freret T., Giannoni P., Gaven F., Since M., Bouet V., Ballandonne C., Corvaisier S., Fréon A.M., Mignani S., Cresteil T., Boulouard M., Claeysen S., Rochais C., Dallemagne P. Design of donecopride, a dual serotonin subtype 4 receptor agonist/acetylcholinesterase inhibitor with potential interest for Alzheimer's disease treatment. Proc. Natl. Acad. Sci. U.S.A. 2014;111:E3825–E3830. doi: 10.1073/pnas.1410315111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Zhu X., Chen J.Y. Building disease-specific drug-protein connectivity maps from molecular interaction networks and PubMed abstracts. PLoS Comput. Biol. 2009;5 doi: 10.1371/journal.pcbi.1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Ye K., Weinshenker D. Norepinephrine protects against amyloid-β toxicity via TrkB. J. Alzheim. Dis. 2015;44:251–260. doi: 10.3233/JAD-141062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R.-Y., Neveu C., Smolen P., Cleary L.J., Byrne J.H. Superior long-term synaptic memory induced by combining dual pharmacological activation of PKA and ERK with an enhanced training protocol. Learn. Mem. 2017;24:289–297. doi: 10.1101/lm.044834.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorens-Martín M., Jurado J., Hernández F., Ávila J. GSK-3β, a pivotal kinase in Alzheimer disease. Front. Mol. Neurosci. 2014 doi: 10.3389/fnmol.2014.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloret-Villas A., Varusai T.M., Juty N., Laibe C., Le NovÈre N., Hermjakob H., Chelliah V. The impact of mathematical modeling in understanding the mechanisms underlying neurodegeneration: evolving dimensions and future directions. CPT Pharmacometrics Syst. Pharmacol. 2017;6:73–86. doi: 10.1002/psp4.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorke D.E., Lu G., Cho E., Yew D.T. 2006. Serotonin 5-HT 2A and 5-HT 6 Receptors in the Prefrontal Cortex of Alzheimer and Normal Aging Patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly P.T.T., Wu Y., Zou H., Wang R., Zhou W., Kinoshita A., Zhang M., Yang Y., Cai F., Woodgett J., Song W. Inhibition of GSK3β-mediated BACE1 expression reduces Alzheimer-associated phenotypes. J. Clin. Invest. 2013;123:224–235. doi: 10.1172/JCI64516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyketsos C.G., DelCampo L., Steinberg M., Miles Q., Steele C.D., Munro C., Baker A.S., Sheppard J.M.E., Frangakis C., Brandt J., Rabins P.V. Treating depression in Alzheimer disease: efficacy and safety of sertraline therapy, and the benefits of depression reduction: the DIADS. Arch. Gen. Psychiatr. 2003;60:737–746. doi: 10.1001/archpsyc.60.7.737. [DOI] [PubMed] [Google Scholar]
- Madsen K., Neumann W.J., Holst K., Marner L., Haahr M.T., Lehel S., Knudsen G.M., Hasselbalch S.G. Cerebral serotonin 4 receptors and amyloid-β in early Alzheimer's disease. J. Alzheim. Dis. 2011;26:457–466. doi: 10.3233/JAD-2011-110056. [DOI] [PubMed] [Google Scholar]
- Maex R., Berends M., Cornelis H. 2009. Large-Scale Network Simulations in Systems Neuroscience. [Google Scholar]
- Markesbery W.R. Oxidative stress hypothesis in Alzheimer's disease. Free Radic. Biol. Med. 1997;23:134–147. doi: 10.1016/S0891-5849(96)00629-6. [DOI] [PubMed] [Google Scholar]
- Maroteaux L., Ayme-Dietrich E., Aubertin-Kirch G., Banas S., Quentin E., Lawson R., Monassier L. New therapeutic opportunities for 5-HT2 receptor ligands. Pharmacol. Ther. 2017 doi: 10.1016/j.pharmthera.2016.10.008. [DOI] [PubMed] [Google Scholar]
- Masson J., Emerit M.B., Hamon M., Darmon M. Serotonergic signaling: multiple effectors and pleiotropic effects. Wiley Interdiscip. Rev. Membr. Transp. Signal. 2012;1:685–713. doi: 10.1002/wmts.50. [DOI] [Google Scholar]
- Mazein A., Ostaszewski M., Kuperstein I., Watterson S., Le Novère N., Lefaudeux D., De Meulder B., Pellet J., Balaur I., Saqi M., Nogueira M.M., He F., Parton A., Lemonnier N., Gawron P., Gebel S., Hainaut P., Ollert M., Dogrusoz U., Barillot E., Zinovyev A., Schneider R., Balling R., Auffray C. Systems medicine disease maps: community-driven comprehensive representation of disease mechanisms. npj Syst. Biol. Appl. 2018;4 doi: 10.1038/s41540-018-0059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier-Schellersheim M., Varma R., Angermann B.R. Mechanistic models of cellular signaling, cytokine crosstalk, and cell-cell communication in immunology. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.02268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneses A. Memory formation and memory alterations: 5-HT6and 5-HT7receptors, novel alternative. Rev. Neurosci. 2014 doi: 10.1515/revneuro-2014-0001. [DOI] [PubMed] [Google Scholar]
- Mengod G., Cortés R., Vilaró M.T., Hoyer D. Handbook of Behavioral Neuroscience. 2010. Distribution of 5-HT receptors in the central nervous system; pp. 123–138. [DOI] [Google Scholar]
- Mokhber N., Abdollahian E., Soltanifar A., Samadi R., Saghebi A., Haghighi M.B., Azarpazhooh A. Comparison of sertraline, venlafaxine and desipramine effects on depression, cognition and the daily living activities in alzheimer patients. Pharmacopsychiatry. 2014 doi: 10.1055/s-0034-1377041. [DOI] [PubMed] [Google Scholar]
- Moreno-Ramos O.A., Lattig M.C., González Barrios A.F. Modeling of the hypothalamic-pituitary-adrenal axis-mediated interaction between the serotonin regulation pathway and the stress response using a Boolean approximation: a novel study of depression. Theor. Biol. Med. Model. 2013;10 doi: 10.1186/1742-4682-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgese M.G., Trabace L. Monoaminergic system modulation in depression and Alzheimer's disease: a new standpoint? Front. Pharmacol. 2019 doi: 10.3389/fphar.2019.00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani-Webster E., Nath A. Inferring mechanistic parameters from amyloid formation kinetics by approximate bayesian computation. Biophys. J. 2017;112:868–880. doi: 10.1016/j.bpj.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsch R.M., Deng M., Growdon J.H., Wurtman R.J. Serotonin 5-HT2a and 5-HT2c receptors stimulate amyloid precursor protein ectodomain secretion. J. Biol. Chem. 1996;271:4188–4194. doi: 10.1074/jbc.271.8.4188. [DOI] [PubMed] [Google Scholar]
- Ögren S.O., Eriksson T.M., Elvander-Tottie E., D'Addario C., Ekström J.C., Svenningsson P., Meister B., Kehr J., Stiedl O. The role of 5-HT1A receptors in learning and memory. Behav. Brain Res. 2008;195:54–77. doi: 10.1016/j.bbr.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Ownby R.L., Crocco E., Acevedo A., John V., Loewenstein D. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch. Gen. Psychiatr. 2006;63:530–538. doi: 10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey M.K., DeGrado T.R. Glycogen synthase kinase-3 (GSK-3)-targeted therapy and imaging. Theranostics. 2016 doi: 10.7150/thno.14334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew D.B., Smolen P., Baxter D.A., Byrne J.H. Dynamic properties of regulatory motifs associated with induction of three temporal domains of memory in Aplysia. J. Comput. Neurosci. 2005;18:163–181. doi: 10.1007/s10827-005-6557-0. [DOI] [PubMed] [Google Scholar]
- Polter A.M., Li X. Glycogen synthase kinase-3 is an intermediate modulator of serotonin neurotransmission. Front. Mol. Neurosci. 2011;4 doi: 10.3389/fnmol.2011.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado C.E., Watt S., Crowe S.F. A meta-analysis of the effects of antidepressants on cognitive functioning in depressed and non-depressed samples. Neuropsychol. Rev. 2018 doi: 10.1007/s11065-018-9369-5. [DOI] [PubMed] [Google Scholar]
- Proctor C.J., Gray D.A. Explaining oscillations and variability in the p53-Mdm2 system. BMC Syst. Biol. 2008;2:75. doi: 10.1186/1752-0509-2-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor C.J., Gray D.A. GSK3 and p53 - is there a link in Alzheimer's disease? Mol. Neurodegener. 2010;5:7. doi: 10.1186/1750-1326-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor C.J., Boche D., Gray D.A., Nicoll J.A.R. Investigating interventions in Alzheimer's disease with computer simulation models. PloS One. 2013;8 doi: 10.1371/journal.pone.0073631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao J., Wang J., Wang H., Zhang Y., Zhu S., Adilijiang A., Guo H., Zhang R., Guo W., Luo G., Qiu Y., Xu H., Kong J., Huang Q., Li X.M. Regulation of astrocyte pathology by fluoxetine prevents the deterioration of Alzheimer phenotypes in an APP/PS1 mouse model. Glia. 2016;64:240–254. doi: 10.1002/glia.22926. [DOI] [PubMed] [Google Scholar]
- Rahimian R., Fakhfouri G., Mehr S.E., Ghia J.E., Genazzani A.A., Payandemehr B., Dehpour A.R., Mousavizadeh K., Lim D. Tropisetron attenuates amyloid-beta-induced inflammatory and apoptotic responses in rats. Eur. J. Clin. Invest. 2013;43:1039–1051. doi: 10.1111/eci.12141. [DOI] [PubMed] [Google Scholar]
- Rajmohan R., Reddy P.H. Amyloid-beta and phosphorylated tau accumulations cause abnormalities at synapses of alzheimer's disease neurons. J. Alzheim. Dis. 2017 doi: 10.3233/JAD-160612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rembach A., Stingo F.C., Peterson C., Vannucci M., Do K.A., Wilson W.J., Macaulay S.L., Ryan T.M., Martins R.N., Ames D., Masters C.L., Doecke J.D. Bayesian graphical network analyses reveal complex biological interactions specific to Alzheimer's disease. J. Alzheim. Dis. 2015;44:917–925. doi: 10.3233/JAD-141497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds G.P., Mason S.L., Meldrum A., De Keczer S., Parties H., Eglen R.M., Wong E.H.F. 5‐Hydroxytryptamine (5‐HT)4 receptors in post mortem human brain tissue: distribution, pharmacology and effects of neurodegenerative diseases. Br. J. Pharmacol. 1995;114:993–998. doi: 10.1111/j.1476-5381.1995.tb13303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J.A., McGonigle P., Van Bockstaele E.J. Locus coeruleus, norepinephrine and Aβ peptides in Alzheimer's disease. Neurobiol. Stress. 2015 doi: 10.1016/j.ynstr.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy K. 2018. Computational Modeling of Drugs against Alzheimer's Disease. [Google Scholar]
- Saraswati A.P., Ali Hussaini S.M., Krishna N.H., Babu B.N., Kamal A. Glycogen synthase kinase-3 and its inhibitors: potential target for various therapeutic conditions. Eur. J. Med. Chem. 2018 doi: 10.1016/j.ejmech.2017.11.103. [DOI] [PubMed] [Google Scholar]
- Sayas C.L., Ariaens A., Ponsioen B., Moolenaar W.H. GSK-3 is activated by the tyrosine kinase Pyk2 during LPA 1-mediated neurite retraction. Mol. Biol. Cell. 2006;17:1834–1844. doi: 10.1091/mbc.E05-07-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayyah M., Eslami K., AlaiShehni S., Kouti L. Cognitive function before and during treatment with selective serotonin reuptake inhibitors in patients with depression or obsessive-compulsive disorder. Psychiatry J. 2016 doi: 10.1155/2016/5480391. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter L.E., Smith D.L., Rosenzweig-Lipson S., Sukoff S.J., Dawson L.A., Marquis K., Jones D., Piesla M., Andree T., Nawoschik S., Harder J.A., Womack M.D., Buccafusco J., Terry A.V., Hoebel B., Rada P., Kelly M., Abou-Gharbia M., Barrett J.E., Childers W. Lecozotan (SRA-333): a selective serotonin 1A receptor antagonist that enhances the stimulated release of glutamate and acetylcholine in the hippocampus and possesses cognitive-enhancing properties. J. Pharmacol. Exp. Therapeut. 2005;314:1274–1289. doi: 10.1124/jpet.105.086363. [DOI] [PubMed] [Google Scholar]
- Schmidt B.J., Papin J.A., Musante C.J. Mechanistic systems modeling to guide drug discovery and development. Drug Discov. Today. 2013 doi: 10.1016/j.drudis.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta A., Bocchio M., Bannerman D.M., Sharp T., Capogna M. Control of amygdala circuits by 5-HT neurons via 5-HT and glutamate cotransmission. J. Neurosci. 2017;37:1785–1796. doi: 10.1523/JNEUROSCI.2238-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahidi S., Mahmoodi M., Sadeghimehr N. Involvement of serotonin 5-HT7 receptors in learning and memory in mice. Neurophysiology. 2019;51:77–82. doi: 10.1007/s11062-019-09796-7. [DOI] [Google Scholar]
- Sheline Y.I., West T., Yarasheski K., Swarm R., Jasielec M.S., Fisher J.R., Ficker W.D., Yan P., Xiong C., Frederiksen C., Grzelak M.V., Chott R., Bateman R.J., Morris J.C., Mintun M.A., Lee J.M., Cirrito J.R. An antidepressant decreases CSF Aβ production in healthy individuals and in transgenic AD mice. Sci. Transl. Med. 2014;6 doi: 10.1126/scitranslmed.3008169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šimić G., Babić Leko M., Wray S., Harrington C.R., Delalle I., Jovanov-Milošević N., Bažadona D., Buée L., de Silva R., Di Giovanni G., Wischik C.M., Hof P.R. Monoaminergic neuropathology in Alzheimer's disease. Prog. Neurobiol. 2017 doi: 10.1016/j.pneurobio.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skovgård K., Agerskov C., Kohlmeier K.A., Herrik K.F. The 5-HT3 receptor antagonist ondansetron potentiates the effects of the acetylcholinesterase inhibitor donepezil on neuronal network oscillations in the rat dorsal hippocampus. Neuropharmacology. 2018;143:130–142. doi: 10.1016/j.neuropharm.2018.09.017. [DOI] [PubMed] [Google Scholar]
- Smith G.S., Barrett F.S., Joo J.H., Nassery N., Savonenko A., Sodums D.J., Marano C.M., Munro C.A., Brandt J., Kraut M.A., Zhou Y., Wong D.F., Workman C.I. Molecular imaging of serotonin degeneration in mild cognitive impairment. Neurobiol. Dis. 2017;105:33–41. doi: 10.1016/j.nbd.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelhalter D.J., Best N.G., Carlin B.P., van der Linde A. Bayesian measures of model complexity and fit. J. R. Stat. Soc. Ser. B (Statistical Methodol. 2002;64:583–639. doi: 10.1111/1467-9868.00353. [DOI] [Google Scholar]
- Sterratt D., Graham B., Gillies A., Willshaw D. Cambridge University Press; 2011. Principles of Computational Modelling in Neuroscience. [DOI] [Google Scholar]
- Stiedl O., Pappa E., Konradsson-Geuken Å., Ögren S.O. The role of the serotonin receptor subtypes 5-HT1A and 5-HT7 and its interaction in emotional learning and memory. Front. Pharmacol. 2015 doi: 10.3389/fphar.2015.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Štrac D.Š., Pivac N., Mück-Šeler D. The serotonergic system and cognitive function. Transl. Neurosci. 2016 doi: 10.1515/tnsci-2016-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tafet G.E., Idoyaga-Vargas V.P., Abulafia D.P., Calandria J.M., Roffman S.S., Chiovetta A., Shinitzky M. Correlation between cortisol level and serotonin uptake in patients with chronic stress and depression. Cognit. Affect Behav. Neurosci. 2001;1:388–393. doi: 10.3758/cabn.1.4.388. [DOI] [PubMed] [Google Scholar]
- Tajeddinn W., Persson T., Calvo-Garrido J., Seed Ahmed M., Maioli S., Vijayaraghavan S., Kazokoglu M.S., Parrado-Fernández C., Yoshitake T., Kehr J., Francis P., Winblad B., Höglund K., Cedazo-Minguez A., Aarsland D. Pharmacological modulations of the serotonergic system in a cell-model of familial alzheimer's disease. J. Alzheim. Dis. 2016;53:349–361. doi: 10.3233/JAD-160046. [DOI] [PubMed] [Google Scholar]
- Tang L., Wang Y., Chen Y., Chen L., Zheng S., Bao M., Xiang J., Luo H., Li J., Li Y. The association between 5HT2A T102C and behavioral and psychological symptoms of dementia in alzheimer's disease: a meta-analysis. BioMed Res. Int. 2017 doi: 10.1155/2017/5320135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohgi H., Abe T., Takahashi S., Saheki M., Kimura M. Indoleamine concentrations in cerebrospinal fluid from patients with Alzheimer type and Binswanger type dementias before and after administration of citalopram, a synthetic serotonin uptake inhibitor. J. Neural Transm. Park. Dis. Dement. Sect. 1995;9:121–131. doi: 10.1007/bf02259654. [DOI] [PubMed] [Google Scholar]
- Truchot L., Costes S.N., Zimmer L., Laurent B., Le Bars D., Thomas-Antérion C., Croisile B., Mercier B., Hermier M., Vighetto A., Krolak-Salmon P. Up-regulation of hippocampal serotonin metabolism in mild cognitive impairment. Neurology. 2007;69:1012–1017. doi: 10.1212/01.wnl.0000271377.52421.4a. [DOI] [PubMed] [Google Scholar]
- Truchot L., Costes N., Zimmer L., Laurent B., Le Bars D., Thomas-Antérion C., Mercier B., Hermier M., Vighetto A., Krolak-Salmon P. A distinct [18F]MPPF PET profile in amnestic mild cognitive impairment compared to mild Alzheimer's disease. Neuroimage. 2008;40:1251–1256. doi: 10.1016/j.neuroimage.2008.01.030. [DOI] [PubMed] [Google Scholar]
- Tsang S.W.Y., Lai M.K.P., Francis P.T., Wong P.T.-H., Spence I., Esiri M.M., Keene J., Hope T., Chen C.P.L.-H. Serotonin transporters are preserved in the neocortex of anxious Alzheimerʼs disease patients. Neuroreport. 2003;14:1297–1300. doi: 10.1097/01.wnr.0000077546.91466.a8. [DOI] [PubMed] [Google Scholar]
- Tsuno N., Homma A. What is the association between depression and Alzheimer's disease? Expert Rev. Neurother. 2009 doi: 10.1586/ern.09.106. [DOI] [PubMed] [Google Scholar]
- Vidal B., Sebti J., Verdurand M., Fieux S., Billard T., Streichenberger N., Troakes C., Newman-Tancredi A., Zimmer L. Agonist and antagonist bind differently to 5-HT1A receptors during Alzheimer's disease: a post-mortem study with PET radiopharmaceuticals. Neuropharmacology. 2016;109:88–95. doi: 10.1016/j.neuropharm.2016.05.009. [DOI] [PubMed] [Google Scholar]
- Wang D.H., Wong-Lin K.F. Comodulation of dopamine and serotonin on prefrontal cortical rhythms: a theoretical study. Front. Integr. Neurosci. 2013;7:54. doi: 10.3389/fnint.2013.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.J., Ren Q.G., Gong W.G., Wu D., Tang X., Li X.L., Wu F.F., Bai F., Xu L., Zhang Z.J. Escitalopram attenuates β-amyloid-induced tau hyperphosphorylation in primary hippocampal neurons through the 5-HT1A receptor mediated Akt/GSK-3β pathway. Oncotarget. 2016;7:13328–13339. doi: 10.18632/oncotarget.7798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.-C., Tai P.-A., Poly T.N., Islam M.M., Yang H.-C., Wu C.-C., Li Y.-C.J. Increased risk of dementia in patients with antidepressants: a meta-analysis of observational studies. Behav. Neurol. 2018:5315098. doi: 10.1155/2018/5315098. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson S., Ghazal P. Use of logic theory in understanding regulatory pathway signaling in response to infection. Future Microbiol. 2010;5:163–176. doi: 10.2217/fmb.10.8. [DOI] [PubMed] [Google Scholar]
- Weller J., Budson A. 2018. Current understanding of Alzheimer's disease diagnosis and treatment. F1000Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Lin K., Wang D.H., Moustafa A.A., Cohen J.Y., Nakamura K. Toward a multiscale modeling framework for understanding serotonergic function. J. Psychopharmacol. 2017 doi: 10.1177/0269881117699612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wortmann M. Dementia: a global health priority - highlights from an ADI and world health organization report. Alzheimer's Res. Ther. 2012 doi: 10.1186/alzrt143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y., Liu P.-P., Lian Y.-J., Liu H.-B., Kang J.-S. The effect of selective serotonin reuptake inhibitors on cognitive function in patients with Alzheimer's disease and vascular dementia: focusing on fluoxetine with long follow-up periods. Signal Transduct. Target. Ther. 2019;4:1–3. doi: 10.1038/s41392-019-0064-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahiaoui S., Hamidouche K., Ballandonne C., Davis A., de Oliveira Santos J.S., Freret T., Boulouard M., Rochais C., Dallemagne P. Design, synthesis, and pharmacological evaluation of multitarget-directed ligands with both serotonergic subtype 4 receptor (5-HT4R) partial agonist and 5-HT6R antagonist activities, as potential treatment of Alzheimer's disease. Eur. J. Med. Chem. 2016;121:283–293. doi: 10.1016/j.ejmech.2016.05.048. [DOI] [PubMed] [Google Scholar]
- Younesi E., Hofmann-Apitius M. From integrative disease modeling to predictive, preventive, personalized and participatory (P4) medicine. EPMA J. 2013 doi: 10.1186/1878-5085-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun H.M., Park K.R., Kim E.C., Kim S., Hong J.T. Serotonin 6 receptor controls alzheimer's disease and depression. Oncotarget. 2015;6:26716–26728. doi: 10.18632/oncotarget.5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Liu R.Y., Heberton G.A., Smolen P., Baxter D.A., Cleary L.J., Byrne J.H. Computational design of enhanced learning protocols. Nat. Neurosci. 2012;15:294–297. doi: 10.1038/nn.2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Spurgeon S., Xu L., Yu D. Computational intelligence in data-driven modelling and its engineering applications. Math. Probl Eng. 2018 doi: 10.1155/2018/2576239. [DOI] [Google Scholar]
- Zhou L., Baxter D.A., Byrne J.H. Contribution of PKC to the maintenance of 5-HT-induced short-term facilitation at sensorimotor synapses of aplysia. J. Neurophysiol. 2014;112:1936–1949. doi: 10.1152/jn.00577.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X., Coyle D., Wong-Lin K., Maguire L. Computational study of hippocampal-septal theta rhythm changes due to beta-amyloid-altered ionic channels. PloS One. 2011;6 doi: 10.1371/journal.pone.0021579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X., Coyle D., Wong-Lin K.F., Maguire L. Beta-amyloid induced changes in A-type K + current can alter hippocampo-septal network dynamics. J. Comput. Neurosci. 2012;32:465–477. doi: 10.1007/s10827-011-0363-7. [DOI] [PubMed] [Google Scholar]