Abstract
Starting with the beginning of the last century, a multitude of scientific studies has documented that the lunar cycle times behaviors and physiology in many organisms. It is plausible that even the first life forms adapted to the different rhythms controlled by the moon. Consistently, many marine species exhibit lunar rhythms, and also the number of documented “lunar-rhythmic” terrestrial species is increasing. Organisms follow diverse lunar geophysical/astronomical rhythms, which differ significantly in terms of period length: from hours (circalunidian and circatidal rhythms) to days (circasemilunar and circalunar cycles). Evidence for internal circatital and circalunar oscillators exists for a range of species based on past behavioral studies, but those species with well-documented behaviorally free-running lunar rhythms are not typically used for molecular studies. Thus, the underlying molecular mechanisms are largely obscure: the dark side of the moon.
Here we review findings that start to connect molecular pathways with moon-controlled physiology and behaviors. The present data indicate connections between metabolic/endocrine pathways and moon-controlled rhythms, as well as interactions between circadian and circatidal/circalunar rhythms. Moreover, recent high-throughput analyses provide useful leads toward pathways, as well as molecular markers. However, for each interpretation, it is important to carefully consider the, partly substantially differing, conditions used in each experimental paradigm. In the future, it will be important to use lab experiments to delineate the specific mechanisms of the different solar- and lunar-controlled rhythms, but to also start integrating them together, as life has evolved equally long under rhythms of both sun and moon.
Keywords: lunar rhythms, transcriptome, proteome, physiology, hormones
Abbreviations: NM, new moon; FQM, first quarter moon; FM, full moon; LQM, last quarter moon; DD, dark/dark; FR-FM, free-running full moon; QTL, quantitative trait loci; PRX, peroxiredoxin; GnRH, gonadotropin-releasing hormone
Graphical abstract
Highlights
-
•
Historical and physiological/behavioral perspective on moon-controlled rhythms
-
•
Overview of the different types of lunar rhythms: possible mechanistic implications
-
•
Examples for inner oscillator-driven lunar rhythms
-
•
Overview of the interactions between known core circadian clocks and moon-controlled rhythms/clocks
-
•
Overview of neurohormonal systems in the context of lunar rhythms
-
•
Summary of several transcriptomics and proteomics analyses: possible commonalties and discrepancies
-
•
Physiological, behavioral, and molecular evidence for lunar influences on terrestrial organisms
Route to the Moon: From Popular Beliefs to Scientific Knowledge
“Houston, Tranquility Base here. The Eagle has landed.” With these words, American cosmonaut Neil Armstrong announced half a century ago the first human moon landing in 1969. Although this event probably represents the most iconic moment of lunar investigation, this journey started a long time before, and is still progressing, at pace with our technological advances, not only in an astronomic manner. Indeed, the moon has fascinated scientists and non-scientists as long as mankind reports, and possible connections between the regular lunar cycle and effects on living beings have been described over millennia, often surrounded by an aura of superstition and mythology. Classical authors, like Aristotle and Lucilius, allegedly reported on the fishermen's beliefs of a link between lunar rhythms and the apparent size of some marine animals, e.g., reflected in the quote: luna alit ostrea et implet echinos (the moon nourishes the mussels and inflates the urchins; Lucilius, ca. 150 B.C.) [1]. Since the beginning of the 20th century, detailed scientific studies have shown that the reproductive behavior and sexual maturation of animals as diverse as corals, polychaetes, echinoderms or fishes are synchronized by the lunar cycle, reviewed in [2,3]. As external fertilization is frequent among these animals, its synchronization with a reliable environmental cycle maximizes the probability that eggs and sperms encounter, and hence is critical for the survival of these species. In animals where the gonads contribute to a large proportion of the body mass, such as sea urchins or mussels, this effect is particularly prominent, leading indeed to significant changes in body mass, and suggesting that these ancient fishermen's beliefs were built on true observations of naturally occurring phenomena.
This connection between sexual maturation and the lunar cycle might also explain the “floating flames” reported in 1492 by Cristoforo Colombo and his crew on their way to El Salvador. Indeed, very plausibly, they were thousands of bioluminescent marine worms (e.g., of the genus Odontosyllis) during their swarming [4], a phenomenon unexplainable at that time, which certainly fueled the mystery surrounding the lunar cycle.
Today, it is the task of chronobiologists to “excavate” the true cores and to sort them from the overgeneralization and mystification due to a lack of scientific explanations at former times.
The Moon Controls Rhythms of Physiology and Behavior
Animals exploit reliable and steady environmental cues to synchronize crucial events of their life, like mating, or fundamental behaviors for their survival, as feeding and predator avoidance. As the moon formed about 4.51 billion years ago, life evolved equally long under both lunar and solar influences, and might have adapted multiple times to moon rhythms to coordinate complex processes. Indeed, several environmental factors have been shown to change directly or indirectly in connection with the lunar cycle, like moonlight intensity, water levels, temperature, food availability, oxygen levels, and magnetic field. In line with this, it is not surprising that lunar rhythms are widespread among marine species [3], especially those living in relatively shallow waters and coastal reef habitats.
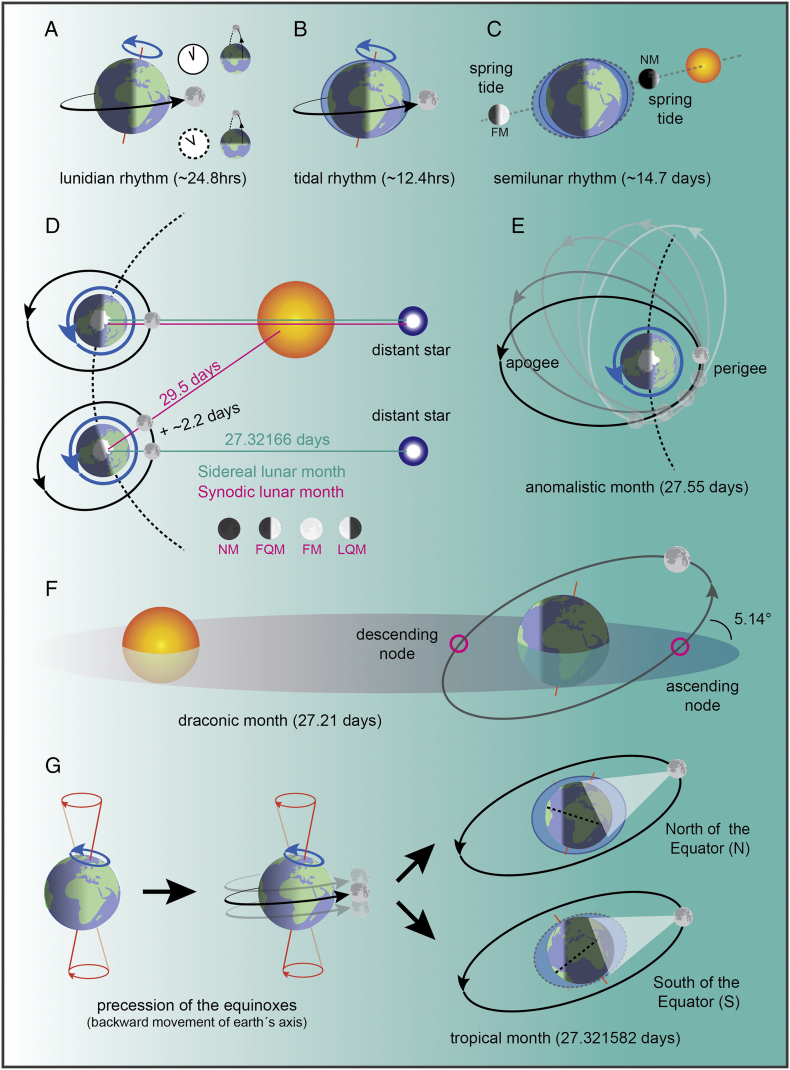
Among natural time-providing systems (zeitgebers) utilized by organisms, earth's rotation and revolution around the sun determine two different environmental cycles, a daily light/dark (~ 24 h) and an annual/seasonal (~ 365 days) cycle, respectively. Likewise, also the moon exhibits shorter and longer rhythmicity. For instance, the (average) time between two consecutive moonrises or, astronomically more accurately, two consecutive passes of the moon through its maximal height in the sky (meridian passes or southern position), is known as lunidian cycle (or lunar day) (Figure 1(a)). It is slightly longer than a solar day (24.8 h on average instead of 24 h) because the moon orbits the earth in the same direction of earth's rotation. This cycle shapes the tides' rhythmicity, with the levels of the oceans rising and falling each day twice in most coastal areas (semidiurnal tidal regime, from here onward we will refer only to this), or only once (diurnal tidal regime, in few locations). The overall complexity of tides rhythmicity is a combination of the earth's rotation, the moon orbiting around our planet, and the interaction between earth and moon's gravitational forces (Figure 1(b)).
Figure 1.
Schematized representations of the known astronomical and geophysical rhythms dictated by the moon. (A) Lunidian rhythm, the period between two consecutive maximal heights of the moon in the sky (meridian transits). (B) Tidal rhythm: water levels increase twice every day in two opposite sites on earth, with one being the closest to our satellite. (C) Semilunar rhythm: the alignment of moon, earth and sun potentiate tidal variations, creating spring (during FM and NM) and neap (during FQM and LQM) tides cycles. (D) Synodic and sidereal months. The first gives rise to the known lunar phases: NM, FQM, FM, and LQM or third quarter moon. (E) Anomalistic month. (F) Draconic month. The plane described by moon's orbit is inclined (5.14°) compared to the plane on which the sun and the earth lay, and the two planes cross in two points called nodes (purple circles). (G) Tropical month: the clockwise rotation of earth's axis affects moon's relative trajectory in the sky (declination). Thus, periodically, the moon faces a more northern or southern portion of earth relative to the Equator, and this affects both moonlight intensity and tides. Moonlight depicted in the figure is a reflection of sunlight. The sun was purposely left away from the scheme for the sake of clarity. For further details, see text. Sources: [5,6]; https://en.wikipedia.org/wiki/Lunar_month;https://www.youtube.com/watch?v=jWCBhVfeAQU;https://www.youtube.com/watch?v=Z6DpPQ8QdLg;https://www.youtube.com/watch?v=adzx547ptck;http://www.antikythera-mechanism.gr/faq/astronomical-questions/what-are-the-different-months;https://moonblink.info/Eclipse/why/months;https://www.slideshare.net/RAFIULALAM006/month-57977849).
In addition, the moon orbits around the earth, and its position relative to the sun or other celestial bodies creates different lunar cycles (or lunar months). The best-known is called synodic month, which represents the time the moon takes to complete one revolution around the earth relative to the sun (Figure 1(d)) and, because of this, determines changes in both moonlight intensity (lunar phases) and tidal force (spring–neap tides cycles). Lunar phases are often defined by the visible portion of our satellite illuminated by the sun: new moon (NM), first quarter moon (FQM), full moon (FM), and last quarter moon (LQM). The duration of this cycle is ~ 29.5 days, and corresponding endogenous biological rhythms approximating to it are called circalunar (Figure 1(d)). During this lunar cycle, earth, moon, and sun can align twice, at both FM and NM (see Figure 1(c)). Thus, during these lunar phases, the sun gravitational force strengthens the lunar one, creating high tides and low tides that are higher and lower than usual, respectively (called spring tides, opposed to neap tides during FQM and LQM) (Figure 1(c)). This cycle is defined as semilunar (~ 14.8 days, half a lunar synodic cycle) and is probably mostly related to tidal phenomena rather than moonlight intensity, which varies greatly between the two lunar phases taken into consideration (i.e. FM versus NM). In spite of this, it is possible that organisms can coordinate physiological processes also according to dimmer moonlight intensities matched during both FQM and LQM.
The synodic month is slightly longer than the sidereal month (27.32166 days, the time required by a moon revolution around our planet with respect to the background stars), as the earth is also moving around the sun in the same direction that moon orbits (Figure 1(d)).
Other lunar cycles can also periodically affect the synodic month features, both moonlight intensity as well as tidal periodicity and amplitude: anomalistic, draconic, and tropical months [5,7]. Here we summarize briefly their main characteristics and periodicity. The moon orbit is not perfectly round, but it approximates an ellipse, with the earth representing one of the two focal points. This implies that the distance between the moon and the earth is not constant, with the two extremes called perigee and apogee (see Figure 1(e)). This elliptical orbit (and thus perigee and apogee positions) is not fixed relative to the earth, but it rotates in the same direction of moon revolution around our planet (a full rotation requires 8.85 years). For this reason, passing twice from the perigee (or apogee) requires the moon slightly longer (27.55 days) compared to the sidereal cycle, and this period is called anomalistic month (Figure 1(e)). The anomalistic cycle, modulating the proximity of our satellite to earth, can impact for instance on tides amplitude during the synodic month (spring–neap tides cycle, 14.76 days), although slightly out of phase (13.78 days from perigee to apogee) [5,7].
Another lunar cycle, of particular interest for eclipses, is called draconic month. In short, the plane defined by moon's orbit is inclined (5.14°) compared to the plane intersecting sun and earth. The two points in which the moon crosses the latter plane are called nodes, and the period the moon takes to cross the same node twice represents the draconic month. As the plane of the moon's orbit gradually rotates in the opposite direction of the lunar revolution (a full rotation every 18.6 years), this periodicity is shorter than a sidereal month (27.212220 days) (Figure 1(f)).
Finally, the tropical month represents the time required for the moon to return to the same celestial longitude (i.e. equinox, celestial meridian, maximum declination of the moon in the sky). This time is slightly shorter (27.321582 days) than the sidereal month, because of the precession of the equinoxes, which slowly move in the opposite direction (clockwise) relative to the moon (counterclockwise) because of earth's axis clockwise rotation (Figure 1(f)). As this affects the trajectory of the moon in the sky, this astronomical phenomenon can have effects on both moonlight intensity and tides [5,6,8] (Figure 1(g)).
It has to be said that, as visible from the period of these astronomical cycles, biological rhythms controlled by either of them might be difficult to discern from each other. However, it is interesting to report that potential effects of the anomalistic cycle on biological rhythmicity approximating the synodic month have been shown in Crassostrea gigas, both at the behavioral and reproductive levels [6,9].
In this review, we will refer to the synodic month when talking about “(circa)lunar rhythms,” but at present we might lack the resolution and detailed insight to correctly interpret if another of the above mentioned lunar rhythms is involved.
The lunar cycle has been shown to synchronize a plethora of different biological processes, such as reproduction in hundreds of studied species (reviewed in [2,3]), activity levels [10], valves gaping activity (linked to feeding and breathing [11]), photosensitivity (shielding pigment migration, [12]), zooplankton vertical migration [13], emergence/eclosion timing [[14], [15], [16]], and molting cycle/exuviation [17]. For instance, with the arrival of the rainy season, millions of terrestrial crabs (Gecarcoidea natalis) walk every year for hundreds of meters, like a horde of implacable and crawling red soldiers, from the inland forest to the northwest shore of the Christmas Island to breed [18]. In this case, the spawning occurred 17–18 days after mating, in a precise lunar phase, between LQM and NM [19]. As witnessed by the crew on Colombo's caravel, also in the Bermuda fireworm, Odontosyllis enopla, reproductive swarming occurs precisely during the first nights after FM in summer and early autumn [20]. Among all, probably the most spectacular and documented event orchestrated by animals according to the lunar cycle is certainly the mass spawning of corals. Like inside a shaken snow globe, once every year, the barrier reef explodes of eggs and sperms, few days after FM, during late spring/summer nights, a phenomenon even visible from the space [21,22]. Unfortunately, reef corals are losing this critical reproductive synchrony, likely due to anthropogenic impact [22,23]. This phenomenon threatens several species, not only corals, but entire reef communities.
While for short and long lunar period lengths marine examples are very well established, this is less clear for terrestrial organisms. We will briefly cover this point at the end of this review.
Finally, a brief notion on the exogenous versus endogenous nature of these oscillations. The strong scientific focus on diel rhythms and circadian clocks started in the 1970s, likely due to the possibility to dissect the molecular mechanisms with functionally amenable model systems like Drosophila melanogaster, Mus musculus, and Arabidopsis thaliana. Before this time, the scientific literature is rich in examples of other rhythms, including tidal and monthly. Those were mainly focused on behavioral observations and include many studies that provide evidence for free-running rhythms (for overviews, see [24,25]). For space constrains, this review focuses on more recent molecular advances in understanding moon-controlled rhythms and clocks. We provide much detail on the experimental settings, in order to allow for discriminations between experiments that tested possible endogenous oscillator effects, and those where environmental and endogenous contributions were not distinguished.
Core Circadian Clock Genes and Lunar-Regulated Processes: Are There Connections?
In spite of the increasing literature, the elucidation of the mechanisms underlying circalunar/circasemilunar and circatidal/circalunidian rhythms is still at an early stage. Given the depth of mechanistic knowledge, circadian clock genes have been particularly studied to investigate their potential interaction and contribution to both long and short lunar-controlled rhythms.
Circalunar rhythms and clocks
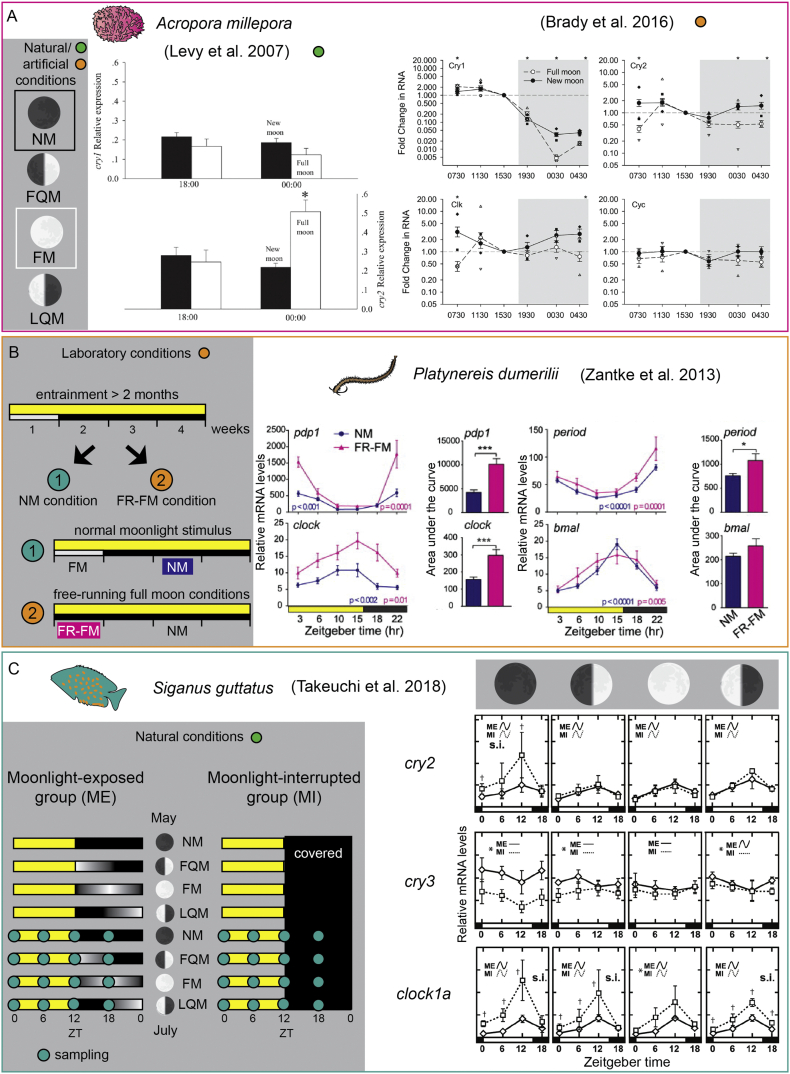
In the reef-building coral Acropora millepora, which reproduces once every year integrating both seasonal, lunar and daily cues, natural FM, as well as artificial night illumination mimicking FM, have been shown to impact on clock genes expression. In this species, four members of the Cryptochrome/Photolyase family have been identified (CRY1, CRY2, CRY3, and CRY-DASH), and interestingly, cry2 (but not the other investigated member, cry1) was found significantly upregulated during FM night compared to NM [26] (see Figure 2). Phylogenetic analysis revealed that CRY1 belongs to the vertebrate-type cryptochrome group, which counts protein acting as transcriptional regulators, but not sensing directly light. On the other hand, CRY2 showed a more enigmatic position [26,30]. Both cry1 and cry2 were expressed in the ectodermal layer of larvae and adults, and their mRNA levels peaked during daylight phases. Moreover, exposure to dark/dark (DD) flattened the expression of both genes also during the subjective light phase, with minor peaks occurring mostly during the subjective night phase [26]. However, lunar free-running experiments would be required to definitely address whether cry2 expression is under the control of a circalunar clock or represents a direct response to light cues (moonlight), as suggested by its behavior under DD. It has to be noted that further studies employing artificial illumination mimicking both light/dark cycles and moonlight provided conflicting results for some of the above-mentioned data collected under natural light conditions. For instance, only cry1 (but not cry2) was affected by exposure to DD, whereas, although not significantly, cry2 increased under natural moonlight compared to the artificial one during both LQM and FM [27,31]. Furthermore, cry2 upregulation under artificial FM conditions was not reproduced by Brady et al. [27], although the sampling was conducted at a similar nighttime (see Figure 2). These data suggest that the integration of multiple environmental features and/or the specific light spectrum of sunlight and moonlight might affect the expression of these genes. In line with this, also the expression of the circadian clock gene, timeless (tim), was found significantly different between natural conditions and artificial illumination during NM nights [27]. These and other data reported in this review suggest a high level of data variability, likely due to differences in experimental regimes, as well as strong environmental and possibly population variability.
Figure 2.
Circadian clock genes expression is affected by the lunar cycle or laboratory conditions mimicking moonlight cues. Gray areas on the left of each panel describe the environmental conditions or laboratory experimental settings employed. Green circles indicate experiments conducted under natural conditions, whereas orange circles refer to artificial (lab) experimental conditions. (A) The expression of A. millepora cry1, cry2, clk, and cyc is influenced by the lunar cycle. (B) Expression of a set of clock genes in P. dumerilii is regulated by an endogenous circalunar clock, as shown by experiments under lunar free-running conditions (absence of artificial moonlight cue at night). (C) Altered night illumination affect the expression of clock genes in a lunar phase-dependent manner in S. guttatus. For references, see [[26], [27], [28], [29]].
In the Caribbean reef coral Favia fragum, which in contrast to A. millipora reproduces monthly in precise lunar patterns, 4–5 days after FM [32], circadian clock transcripts for cry1, cry2, clk, and cyc showed differences between lunar phases, but without regular patterns [33].
The potential interaction between the circadian and the circalunar clocks has been well documented in the marine bristleworm Platynereis dumerilii [28]. This annelid reproduces every month in specific lunar phases also in laboratory conditions [28,34], similarly to the field [35]. This monthly rhythm has been shown to rely on an endogenous circalunar oscillator, as it free-runs in absence of lunar cues [28]. The exposure to continuous nocturnal light (“constant FM”) or never showing the worms nocturnal light (“constant NM”) throughout their entire growth (no entrainment) results in arrhythmic spawning patterns. In this species, the circalunar clock seems to also affect locomotor activity levels, with worms showing a weaker circadian rhythmicity during free-running FM (FR-FM) compared to NM. Gene expression analysis of circadian clock components under NM and FR-FM revealed interesting insights about the interaction of these two oscillators at the molecular level. Most of the genes analyzed showed similar circadian expression profiles in the two different lunar phases, but significantly higher mRNA levels during FR-FM were found for pdp1, period, clock, and tim (see Figure 2), suggesting that a subset of circadian clock transcripts is affected by the circalunar clock, independently of nocturnal light cues. To test whether the oscillation of circadian clock genes is essential for circalunar clock function, the worm's monthly spawning rhythm was monitored under circalunar free-running conditions for a month after the exposure to PF-670462, a CK1δ/ε inhibitor, which affects the circadian period in mammalian cells [36,37] and renders clock genes transcription as well as locomotor activity arrhythmic [28]. Interestingly, the spawning profile was found unaffected. While this experiment suggests the independence of the circalunar clock from classical circadian clock gene oscillations, their normal cycling could still be required for circalunar entrainment. Moreover, these individual genes could still play a functional role, maybe tissue-dependent, linked to developmental or reproductive processes, independently from their circadian oscillatory function [38,39].
The marine midge, Clunio marinus, provides an interesting example of combined circadian and circalunar timing, which precisely orchestrates its adult emergence [[40], [41], [42]]. The specific daily and lunar time point of adult emergence varies between strains from different geographic locations, presumably as an adaptation to the exact local lowest low tide occurrences. These differences are genetically encoded [[43], [44], [45]] and hence allow for population genetic analyses. Genetic linkage analysis identified two specific chromosomal regions responsible for diurnal emergence and two related to lunar timing. Interestingly, one of the two quantitative trait loci (QTLs) linked to circalunar timing overlaps largely with one of the two circadian QTLs [46], which might suggest a link between circadian and circalunar timing regulation. However, the mapping of all known genes with key roles in circadian timing or light input pathways revealed their complete absence from these QTLs [46], suggesting that, if there is a common denominator for circadian and circalunar timing adaptations in the midge, it is outside of the core circadian clock or conventional light input pathways.
The relationship between circadian clock genes and circalunar rhythms has been investigated in several studies focusing on fishes. In the golden rabbitfish, Siganus guttatus, which spawns around FQM [47,48], cry1 and cry3 transcripts in both brain and ovaries were analyzed, exhibiting an overall increase in the brain (mesencephalon and diencephalon) during NM and especially FQM, and lower levels at LQM. As cry1 and cry3 levels decrease following FQM, and increase again during NM, similarly to day/night fluctuations of cry1, the authors suggested a model for lunar phase recognition relying on a photo-repressive mechanism governed by moonlight. This system would involve both cry1 and cry3, and its functioning would be limited to the time window between ZT18 and ZT21 (the middle part of the night, corresponding to 24:00–3:00, a temporal range characterized by moonlight presence only during FM and LQM) [49]. However, the circalunar expression pattern of cry3 in partial or complete absence of moonlight cues (partial, restricting moonlight exposure only to the first or second half of the night, or complete, covering the tank for the whole night), was similar to the profile found in more natural condition (tank not covered) [50], indicating the presence of an endogenous oscillator. In contrast, per2 expression showed signs of phase shift when the moonlight was limited to the second part of the night, and an arrhythmic profile in the other altered nocturnal light regimes, more in line with a potential direct light effect. Moreover, per4 showed a robust oscillation over the lunar cycle under all the light regimes, but in antiphase compared to cry3. Thus, the data presented might actually suggest the presence of both a photo-repressive mechanism and an endogenous clock contributing together to the circalunar timing. The authors also reported CRY3 immunostaining in the subependymal cells in the mediobasal region of the diencephalon and proposed this brain area as the location of the putative circalunar clock. Interestingly, this region has been proposed as a key site for seasonal response to photoperiod in birds [51]. A deeper analysis of clock genes oscillations in diencephalon and pituitary gland over the lunar cycle revealed that moonlight interruption (by tank coverage) markedly impacted on the expression levels, cycle amplitude and daily pattern [29]. Curiously, the most remarkable effects of moonlight deprivation reported in the diencephalon occurred counterintuitively during NM (Figure 2). In the pituitary, cry1a and cry2 levels were higher in almost all lunar phases in moonlight-deprived specimens, as well as per2a and per2b. Interestingly, moonlight deprivation shifted the acrophase (the interval of expression peaks in two consecutive cycles) of several core circadian clock genes, in a lunar phase-dependent manner.
In general, in spite of existing discrepancies that likely originate from comparisons between studies adopting different experimental conditions, the evidence reviewed here strongly suggests an interaction of circalunar rhythms and clocks with at least sets of core circadian clock genes. The meaning and the nature of this interconnection remain to be determined by functional studies.
Circatidal rhythms and clocks
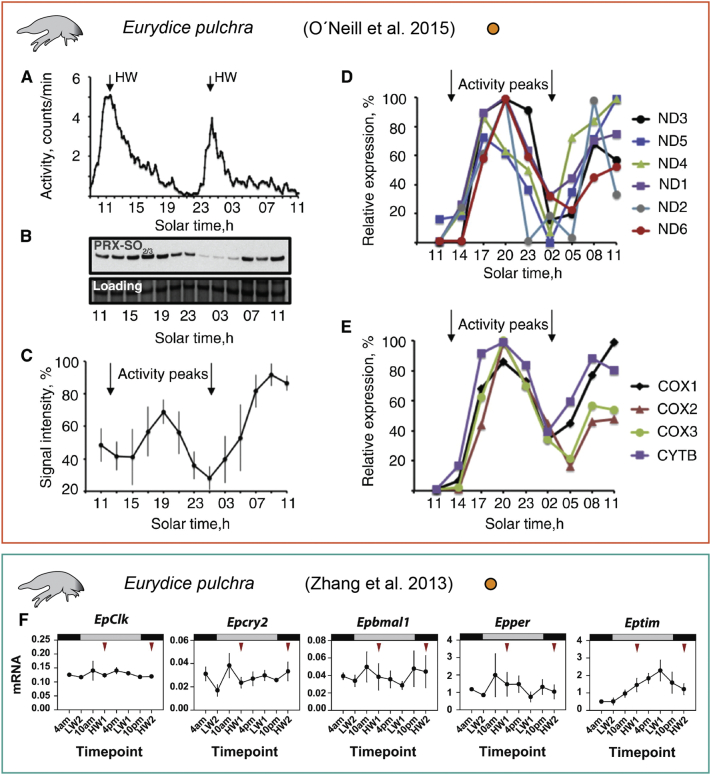
Circatidal clocks and their relation with circadian clocks are particularly interesting, because in essence a 12.4 h period is just half of 24.8 h, i.e. a period covered by the conventional core circadian clock network. The involvement of classical circadian clock oscillations in circatidal rhythmicity, also with a functional approach, has been studied in Eurydice pulchra (Figure 3). This intertidal isopod showed robust tidal rhythmicity after being caught in the wild and released in DD, suggesting the existence of a true circatidal endogenous oscillator. Moreover, vibrations mimicking water turbulence were effective in re-entraining animals that became arrhythmic after spending more than a month in DD [10].
Figure 3.
Tidal activity rhythm in E. pulchra generates antisense tidal cycles of metabolism but is independent from classical clock genes oscillation. (A) Swimming activity pattern of Eurydice specimens shows a period of 12.5 h in free-running conditions, with maxima during predicted high water (HW). (B) Representative western blot of overoxidized peroxiredoxin. (C) Relative intensity of overoxidized head peroxiredoxin. (D and E) Relative expression of mitochondrially-encoded genes (ND = NADH dehydrogenase subunits, COX = cytochrome oxidase subunits; CYTB = cytochrome B) under free-running laboratory conditions. (F) None of Eurydice circadian clock genes exhibit a tidal rhythm at the transcript level, and curiously, only tim shows a clear circadian profile (red arrows show the time of expected high water). For references, see [10,54]. Orange circles: artificial (lab) conditions.
In this species, the pharmacological inhibition of CK1δ/ε by PF-670462 altered both circadian cycles (pigment dispersion in chromatophores), as well as circatidal swimming rhythms [10]. In spite of the observed phase delay, the amplitude of tidal oscillations was robust and comparable to the untreated control. These evidences can in principle be interpreted in two different ways: a) the effects of CK1 δ/ε inhibition on core circadian clock genes impact also on the circatidal rhythm, or b) CK1 contributes to both circadian and circatidal functions, independently. RNAi experiments revealed that the circatidal timekeeping is at least independent from PER and TIM cycles. Furthermore, circadian rhythm disruption through LL exposure affected neither the tidal swimming period nor overall activity levels [10]. This indicates that these circadian clock genes are not involved in the circatidal oscillator. Furthermore, no circatidal oscillations have been found in Eurydice core clock genes. However, it should be noted that apart from timeless, Eurydice circadian clock genes also do not show a circadian expression pattern [10] (Figure 3F). This lack of both circatidal and circadian rhythmicity in clock gene expression is also conceivable with the idea that specific subsets of the core circadian clock might be differentially recruited in a tissue-dependent manner, and depending on cellular context form circadian, circatidal, or circalunidian clocks. In this scenario, depending on the diversity of rhythms in the different tissues, sampling whole heads could mask the rhythms in distinct neuronal clusters.
Principle evidence for tissue-dependent differences in the circadian clock core oscillator exists, for instance, for cryptochrome in D. melanogaster. Although this protein functions in the circadian clock entrainment pathway in the fly's brain without being part of the transcriptional oscillator itself, evidence for a transcriptional repressor role exists for peripheral cells [52]. Additional evidence for flexibility in “circadian” clock gene transcriptional periods comes from a recent study in the tidal oyster C. gigas. In this species “circadian” clock genes lose their 24-h periodic expression pattern under DD and exhibit a tidal frequency instead [53].
To better understand the role of circadian versus circatidal rhythmicity in physiological control, the activity of key metabolic actors like peroxiredoxin (PRX) has been investigated [54]. PRX, whose oxidation–reduction cycles constitute a marker for circadian rhythms from bacteria to animals [55], followed a circatidal pattern in E. pulchra (Figure 3). Moreover, also 10 out of the 11 detected mitochondrial genes (such as subunits of NADH dehydrogenase, cytochrome b, and cytochrome c oxidase, encoding components of the electron transport chain complexes I, III, and IV, respectively) were also expressed in a circatidal fashion. The period of these oscillations was consistent with swimming activity and respiratory rate tidal cycles, which peak around high tides [54,56]. It is interesting to note that activity peaks coincide with troughs in overoxidized PRX and mitochondrial genes expression. Such anti-timed correlation has also been observed in Drosophila [55]. Thus, the authors suggested that peaks of PRX activity and mitochondrially encoded transcript levels might anticipate changes in the energy demand and reactive oxygen species production originating from the prevailing metabolic program, following in this case a circatidal rest/activity rhythm.
Taking all above findings together might start to indicate that the circadian clockwork could be more plastic than previously thought and capable to adjust to the environmental cycle(s) most relevant for a given species [53,54,57].
Circalunar Rhythms and Clocks: An Endocrine Perspective
Melatonin
Given its role as an endocrine mediator of other light-dependent rhythms (daily/circadian, i.e. daily sleep–wake cycle) in vertebrates, also melatonin has been suggested as a potential component of the molecular mechanisms contributing to lunar-regulated rhythms. Moreover, melatonin synthesis is exquisitely light sensitive, inhibited by light exposure, and fueled in the dark [48,58,59] and therefore likely affected by moonlight.
Evidence of a possible involvement of melatonin in conveying lunar cues was provided in lunar and semilunar spawner fish species. In the golden (S. guttatus) and the seagrass rabbitfish (Siganus canaliculatus), for instance, plasma and ocular melatonin levels were reduced during FM compared to other lunar phases [48,58,59]. In S. canaliculatus, plasma melatonin levels during FQM and LQM were significantly different from FM, but not NM [58], whereas ocular melatonin under naturalistic conditions was higher in NM and LQM compared to both FM and FQM [59]. This suggests that moonlight intensity, moonlight exposure time, and tissue-specific melatonin levels might all contribute to enhance the specificity of melatonin signaling in discriminating different lunar phases. Further evidence for a relationship between melatonin and the lunar cycle was added recently by a study on the honeycomb grouper, Epinephelus merra, which spawns around FM [60]. Like for the Siganus species, melatonin content in the eye was higher in NM compared to FM at midnight. Moreover, melatonin administration markedly reduced the expression of gonadotropins subunits fshβ and lhβ. In the semilunar spawner, Takifugu niphobles (grass puffer), the expression levels of four melatonin receptors in the pineal gland showed ultradian oscillations with periods between 14.0 and 15.4 h in DD free-running experiments [61]. These data potentially indicate the existence of a circatidal clock in the grass puffer brain, which, together with the circadian clock, might contribute to set the proper daily and lunar-related reproductive rhythms.
Consistent with the lower melatonin signaling around FM described in other fish species, the expression levels of two melatonin receptors in the pineal gland and arylalkylamine N-acetyltransferase (aanat, enzyme involved in melatonin synthesis) in the retina were reduced during FM nights compared to NM in the grass puffer [62,63]. This decrease of melatonin receptors mRNA levels under natural FM was absent under “moonlight deprivation,” in line with a light-dependent inactivation of melatonin signaling. Similarly, two melatonin receptors showed a lunar phase-dependent expression pattern in the mudskipper, Boleophthalmus pectinirostris, a semilunar spawner, which reproduces once every year around FQM or LQM [64]. Indeed, mtnr1a1.4 and mtnr1a1.7 exhibited a circasemilunar expression pattern, with peaks around FQM and LQM in diencephalon and ovaries, respectively. Moreover, overall levels of melatonin receptors in the pituitary were lower around NM and FQM, with steady increase after this phase till the next NM [65]. Finally, melatonin injection was sufficient to increase plasma levels of 17a,20b-dihydroxy-4-pregnen-3-one (DHP), a sex steroid hormone (maturation-inducing hormone) both in vivo and in vitro (on ovarian fragments) [65]. In summary, the data provided in this section suggest that melatonin might contribute to the lunar phase-specific spawning timing, acting most likely in a light-dependent manner, and potentially modulating (either directly and indirectly) key endocrine reproductive pathways.
GnRH-like and other gonadotropic hormones
As mentioned above, most circalunar/semilunar rhythms reported refer to the synchronization of reproductive events. Likely for this reason, many efforts to understand the endocrine basis of circalunar rhythmicity focus on hormones essential for vertebrate reproduction. In these animals, sexual maturation is controlled by the hypothalamic–pituitary–gonadal axis. Integrating multiple environmental and metabolic cues through endocrine and neurotransmitter action [66], neurons in the hypothalamus secrete gonadotropin-releasing hormone (GnRH), which signals to the pituitary, regulating the release of gonadotropins such as follicular-stimulating hormone and luteinizing hormone. Once secreted into the bloodstream, these gonadotropins act directly on the gonads promoting the synthesis of steroid hormones, and in turn maturation of ovaries and testis. Several studies on birds and mammals provided clear evidence about the involvement of the GnRH system in the timing of reproductive events. In birds, a reduction in number and size of GnRH-immunoreactive cells has been found in non-photosensitive (photorefractory or reproductively inactive) individuals [67], a condition reversed in breeding photosensitive animals [68]. In mammals, GnRH release is reduced in the non-breeding (anestrous) season, and the reactivation of this signaling is triggered by a seasonal-dependent increase in kisspeptin expression and a concomitant reduction in gonadotropin inhibitory hormone production [[69], [70], [71]].
Over the last 15 years, fish species like grass puffers (Takifugu alboplumbeus, T. niphobles) have been extensively studied for their extremely precise reproductive timing and its possible interconnection with the GnRH-like system [[72], [73], [74], [75]]. However, although highly relevant for vertebrate reproductive endocrinology, those analyses are inconclusive concerning the link between the HPG axis and lunar rhythmicity, as the lunar rhythm read-out is a reproductive event (spawning) that per se requires the activation of this endocrine axis. For this reason, a recent study on premature individuals of the marine bristleworm P. dumerilii and its GnRH-like system is interesting. This analysis shows that homologs of the GnRH-like/Corazonin/AKH supergroup are differentially expressed throughout the worm's circalunar cycle with the most significant expression changes in the transition from the week prior to the FM stimulus to the FM week [76]. Functional data from one of the Platynereis GnRH-like genes, corazonin1/gnrh-like1, suggest a role in energy homeostasis and are possibly indicative of a circalunar regulation of the worm's metabolism, to enable the switch to reproduction when all intrinsic and extrinsic conditions are properly met.
Other neuropeptides
The tropical abalone, Haliotis asinina, spawns on NM and FM in the warmer half of the year. This spawning cycle was found still synchronous with the natural environment in individuals kept for 1 month without tidal or lunar cues [77], suggesting the existence of an endogenous rhythmicity. Transcripts of candidate neuropeptides, such as APGWamide peptide, myomodulin, FMRFamide, schistosomin-like peptide, whitnin, molluscan insulin-related peptide, and haliotid growth-associated peptide, were analyzed from the anterior ganglia during the 11 days encompassing the spawning event [78]. Although some genes showed sexually dimorphic patterns, the expression of most of the preprohormones peaked in both sexes at or in proximity of spawning, correlating with specific phases of the lunar cycle. No gene showed a tidal expression pattern. However, it remains to be functionally tested whether any of these neuropeptides are involved in the timing of a lunar reproductive cycle. It is noteworthy that particularly the preprohormone whitnin was found to be circalunarly regulated in a proteomics study of the functionally amenable bristle worm P. dumerilii [79].
Steroid hormones
In vitro studies focused on more downstream reproductive processes, such as steroidogenesis, and its relationship with the lunar cycle. In S. guttatus, it has been shown that the abundance of steroid hormones within the plasma, as well as vitellogenin, shows a circalunar rhythm, with peaks during FQM, in concomitance with higher gonadosomatic index, sperm motility maxima, and spawning [47,80]. The incubation with human chorionic gonadotropin and steroid hormones was sufficient to induce maturation of both oocyte follicles and testis during FQM, but not NM. Moreover, the synthesis of estradiol-17β and 17a,20b-dihydroxy-4-pregnen-3-one seemed to be more effective during the phase preceding spawning (NM) and FQM, respectively [81], providing possible insights into how steroidogenesis might contribute to specific lunar-related reproductive rhythms.
Two studies used a similar approach to investigate steroidogenesis in testicular fragments and sperm preparations of S. guttatus and Siganus argenteus. The activity of steroidogenic pathways showed species-specific changes according to the lunar cycle, correlating with spawning in different lunar phases (FQM and LQM, respectively). In testicular fragments, 11-ketotestosterone (a potent androgen in fish species [82]) synthesis induced by hCG was higher in S. guttatus during the lunar phases where spawning occurred, and lower in S. argenteus. An increase in 17a,20b-dihydroxy-4-pregnen-3-one (which promotes spermatogenesis in zebrafish [83]), following 17a-hydroxyprogesterone stimulation, was detected in sperm preparations from both species during the lunar phases which coincided with spawning (FQM and LQM, respectively) [80,84]. Similarly, also in the semilunar spawner B. pectinirostris, major steroid hormones (estradiol-17b, testosterone, 17-α-hydroxyprogesterone, and 11-ketotestosterone) peaked in correspondence, or slightly after (3 days after) of FQM and LQM, when spawning occurs for about 5 days [64], while in the killifish (Fundulus heteroclitus), both estradiol-17b and vitellogenin were higher 2 and 6 days prior spawning, respectively [85].
-omics under the Moon: Transcriptional Signatures and Protein Dynamics from High-Throughput Data
High-throughput analyses have been employed to shed light on the molecular changes associated and possibly contributing to moon-controlled rhythms.
Corals
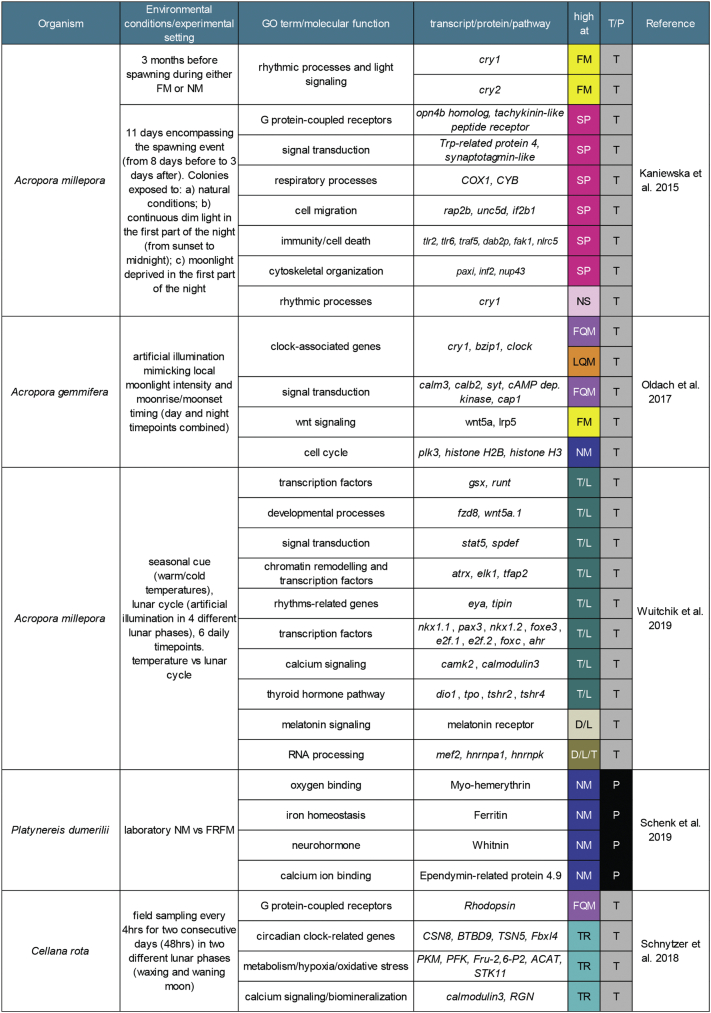
Gene expression dynamics in A. millepora colonies were analyzed during FM and NM 3 months before the predicted spawning month, revealing specific lunar cycle signatures. Interestingly, genes involved in the regulation of rhythmic processes and light signaling (i.e. cry1 and cry2) were found upregulated during FM nights compared to NM [86], confirming the results observed by Levy et al. [26] for cry2. As a further strategy to unveil lunar-dependent mechanisms underlying spawning, A. millepora transcriptome has been analyzed over the 8 days leading to the yearly spawning event in different nocturnal light conditions: ambient (natural condition), continuous light in the first part of the night (from 1815 to 2400 h), and continuous dark, mimicking NM [86]. Remarkable changes in transcriptional dynamics occurred especially around the spawning event in the ambient treatment, but the same regulation was not observed under continuous NM, suggesting the crucial role of moonlight in driving gene expression. Changes in gene expression have also been observed under the continuous light condition mimicking urban light pollution, especially for those genes associated with the spawning event, which anticipated their patterns during the day. However, colonies exposed to this treatment did not spawn, plausibly because of a disruption of a light-dependent process required for spawning. Gene ontology categories enriched during spawning were cell cycle, GTPase activity, and signal transduction. Genes highly upregulated belonged to G-protein-coupled receptors, signal transduction, and respiratory processes, whereas downregulation characterized genes involved in rhythmic processes, such as circadian clock related genes (for instance, cry1). Two of the genes upregulated during spawning were r-opsins, homologs of melanopsins, and trp-related protein 4, a gene encoding a transient receptor potential (TRP)-like protein. While melanopsins are interesting as they mediate light-dependent circadian phase shifts in mammals [87], the specific regulation of a trp channel suggests calcium signaling as a key pathway for triggering gametes release. Together with the overexpression of a synaptotagmin-like gene (often involved in the regulation of neurotransmitter and neurohormone release) and several GPCRs (i.e. tachykinin-like peptide receptor), these data delineate a potential molecular landscape for spawning induction, which spans from light perception to hormone signaling. In parallel, also cell migration, immunity/cell death, and cytoskeletal organization seem to be additional processes regulated at spawning. The observed transcriptional changes also included mitochondrial genes, such as COX1 and CYB, further implying deep metabolic and respiratory changes [86] (see Figure 4). A similar analysis conducted in Acropora digitifera focused on the transcriptional dynamics underlining spawning and post-spawning regulation in two different lunar phases (FM and NM). Interestingly, also in this species, several clock genes (clock, bmal, tim, cry1) were downregulated during the spawning event, as well as genes involved in immune response and inflammatory/apoptotic response, while cell cycle regulation, substrate adhesion, and GPCR signaling were the most represented functions in the upregulated genes. Finally, transcripts involved in immune response and JNK signaling were found downregulated during FM [88].
Transcriptional cycling of genes associated with biological clocks over the lunar month (during the day and/or during the night) was detected in a recent study on Acropora gemmifera [89]. Here, the authors sampled animals at midday and midnight, with nocturnal artificial illumination matching both the local moonlight intensity and the moonrise/moonset timing of the four main lunar phases (LQM, NM, FQM, FM). Combining noon and midnight time-points, 273 transcripts were identified as differentially expressed throughout the lunar cycle. Among them, the most enriched biological processes were linked to signal transduction, post-translational modification/protein turnover, intracellular trafficking, and secretion. Interestingly, different groups of genes showed peaks during different lunar phases: calcium signaling at FQM, wnt signaling at FM, and cell cycle at NM. Other well-represented clusters corresponded to the transport and metabolism of inorganic ions, amino acids, lipids, and carbohydrates, but also to energy production and chromatin/RNA dynamics [89]. A particular focus on the potential effect of light pollution on transcriptional dynamics has been adopted in A. digitifera, showing changes especially in transcripts involved in the regulation of cell cycle, stress response, circadian clock, as well as GPCR, ERK/MAPK, p53, and nuclear receptor signaling [90].
Little is known about the integration of multiple time keeping systems (circadian, seasonal, and lunar), although it is ultimately the biologically relevant condition. A deep RNAseq analysis on the reef-building coral A. millepora aimed to understand how the environmental cues, feeding these cycles, interact at the transcriptional level [91]. Animals were sampled at colder or warmer temperatures (treatments mimicking one of the major seasonal changes) after 18 days of acclimation, four different artificial moonlight conditions (FQM, FM, LQM, NM), and at six daily time points. The majority of differentially expressed genes (87.5% of DEGs) showed unique signatures, specific for each analysis (interaction daily/lunar cycles, daily cycle/temperature treatment, lunar cycle/temperature treatment, and daily/lunar cycles/temperature treatment). Genes involved in “biological rhythms” (such as bzip1 and bzip3, cnidarian homologs of clock and pdp1, respectively) formed the most represented group of genes regulated by both temperature and daily cues, whereas genes encoding transcription factors, and regulating developmental processes and amino acid metabolism were the most affected by the interaction between temperature and lunar phase. Focusing on the interaction between lunar phase and temperature, a set of genes exhibited shifts from a single peak in warm temperatures to semilunar dynamics in colder “winter-mimicking” conditions. Among these, the most regulated were stat5 and spdef, two genes that directly interact at the protein level in mammalian cells [92], and other genes involved in chromatin remodeling and transcriptional activity such as atrx, elk1, and tfap2. Striking antisense patterns over the lunar cycle between warm and cold temperatures were found for rhythms-related genes like eya and tipin (which product interacts with TIM [93]), transcription factors (gsx, runt), and members of wnt-pathway (fzd8, wnt5a). Other significant changes in transcriptional dynamics regulated by the interaction of both seasonal and lunar cues cover transcription factors (nkx1.1, pax3, nkx1.2, foxe3, e2f.1, e2f.2, foxc, ahr) and calcium signaling-related genes (camk2, calmodulin3). Ca2+-signaling had already been linked to lunar periodicity in corals [86]. Several differentially expressed genes involved in the thyroid hormone pathway (dio1, tpo, tshr2, tshr4) are of particular interest due to the established connection between thyroid hormones and photoperiod detection in mammals [94], although no insights have been provided about their role in lunar timing yet. On the other hand, the interaction between daily and lunar expression profiles revealed enrichment in genes associated with mRNA processing and translation, but also ion transport and a melatonin receptor. Finally, only 32 genes have been identified as regulated by the interaction of all the three cues, with mef2, hnrnpa1, and hnrnpk representing the most differentially expressed [91] (see Figure 4). Interestingly, these three genes have been shown to play a role in RNA processing/post-transcriptional regulation, and to interact with several components of the circadian clock machinery in other models [[95], [96], [97]].
…with and without symbionts
The presence of endosymbionts represents an evolutionary advantage for a multitude of organisms. The effects of this intimate relationship on lunar-regulated host rhythms were studied at the transcriptomic level in the sea anemone Aiptasia diaphana, which facultatively host the photosynthetic algae Symbiodinium. The comparison of the transcriptomes revealed that circatidal rhythmicity of gene expression predominates in the aposymbiotic anemones, whereas in Symbiodinium-associated morphs, ~ 24 h cycles in transcriptional dynamics and behavior are more common [98].
Worms
A different strategy to identify molecules involved in circalunar timekeeping systems was used in the bristleworm P. dumerilii [79]. Using head tissues as samples, the study combined transcriptomic and proteomic approaches and focused not only on the later phases of the life cycle that ultimately lead to spawning, but rather on the “entrainment phase” that likely occurs during most, if not the entire, pre-spawning development (immature and premature stages). Furthermore, both immature and premature worms (the latter separated by sex) were sampled during NM and free-running FM, in order to identify molecular signatures of the endogenous circalunar clock machinery rather than those resulting from direct light effects.
The overall number of circalunar-regulated transcripts was relatively low, likely because of the limited circalunar time-points (due to technical constrains). qPCR-studies, including more diel or lunar time-points, already indicate that more transcripts are regulated [28,76]. Molecular and functional clustering grouped circalunar-regulated differentially expressed transcript into four main categories: phosphorus-oxygen lyase activity, protein kinase activity, insulin receptor binding, and transposase activity.
In contrast to the transcriptomic analysis, about 25% of the analyzed head proteome was differentially regulated between NM and free-running FM phases from the identical samples used to generate the transcriptomes. As proteomic approaches do not quantify all proteins present in the tissue, but only those that are sufficiently abundant, this much higher (> 200 fold) circalunar regulation on protein than on transcript level can be interpreted in at least two directions. It suggests either a potential enrichment of circalunar-regulated proteins in head tissues or a generally stronger circalunar regulation on the protein rather than on transcript level. A vast array of distinct processes was found enriched in the circalunar differentially expressed proteins, with protein synthesis and activity, calcium signaling, and proton membrane transport being most represented.
Several candidates were further characterized for their transcript localization in the worms' heads [79] (see Figure 4). In general, although partially overlapping, the expression patterns of these circalunar candidates did not identify any specific “circalunar spatial hotspot“, suggesting that the worm's circalunar clock impacts on many brain regions.
Mollusks
The intertidal limpet Cellana rota exhibits circatidal locomotor activity [57] and oxygen consumption [99]. To identified circatidal signatures in the transcriptome, samples were collected in the field every 4 h for 2 different days and at FQM and LQM. At the transcript level, tidal rhythms were more abundant than diel ones [57]. Twelve transcripts regulating or regulated by the circadian clock have been found oscillating significantly with a tidal rhythm, as well as genes involved in metabolism, hypoxia/oxidative stress, biomineralization, and calcium signaling (see Figure 4). Rhodopsin peaked during the nights anticipating FM. Circadian clock genes showed moderate to none circadian rhythmicity, and no signs of circatidal oscillations at the transcript levels, consistent with a previous work in Eurydice [10].
Figure 4.
Summary table showing the experimental conditions, the main findings, and patterns of a sample of the high-throughput analysis reviewed. NM = new moon; FQM = first quarter moon; FM = full moon; LQM = last quarter moon; SP = spawning; NS = not spawning; T/L = regulated by the interaction between temperature and lunar cycle; D/L = regulated by the interaction between daily and lunar cycles; D/L/T = regulated by the interaction among daily, lunar, and temperature cues; TR = tidally regulated; T = transcript; P = protein. For references, see [57,79,86,89,91].
Were-Rodents, Were-Flies, and Humans: The Effect of the Moon on Terrestrial Animals
Most of the studies concerning lunar effects on animal behavior and physiology focused on marine species, where circalunar and circatidal periodicities have been most convincingly documented.
However, curious cases about lunar influence on animal behavior have also been reported in lake and terrestrial species [3], and the number of examples is constantly increasing. Below we briefly mention a selection.
Angling catch records of 341,959 muskellunge (Esox masquinongy) show a higher number of catches during FM and NM periods [100]. Several hypotheses have been taken into consideration, including “inland tides” in barometric pressure and gravitational forces [100]. Given that no effects of anglers´ experience were noted, the authors concluded that these findings could indicate feeding synchronization with the lunar cycle in those fish. Another recent example documents that the lunar cycle contributes to activity synchronization in the European nightjar, Caprimulgus europeaus [101]. These nocturnal birds increase their foraging activity during moonlit nights, and attempt migration flights mostly during the days following FM (with peaks 11 days after). In mammals, phases with moonlight often inhibit specific behaviors, possibly because the dim nocturnal light favors predators in spotting their prey, with consequences on energy allocation. Indeed, greater vigilance and lower food intake have been shown in gerbils (Gerbilus andersoni allenbyi) and wood mice (Apodemus sylvaticus) during brighter moon phases [102,103]. Similarly, the lunar cycle affects badgers' “modesty”. Indeed, in the species Meles meles, matings mostly occur during the darkest moon phases (from LQM to FQM). Moreover, other behaviors (such as squat marking and raised-leg urination) that usually increase in reproductively active individuals peak around NM [104].
The human female reproductive cycle represents another curious example. Are these cycle lengths a coincidence, or is there a connection to the lunar cycle? An overview on the literature of possible effects of the lunar cycle on human reproduction has recently been covered [3,105], but further studies are needed for conclusive answers.
Since the past, popular beliefs about exposure to moonlight and human behavior flourished, with some of them originating myths such as lycanthropy. Nowadays, several studies aimed to shed light on this interesting although debated biological aspect, with controversial results.
A disputed link between sleep–wake functions of melatonin and the lunar cycle has been suggested in a human study, providing evidence that sleep features change according to lunar phase [106]. Sleep quality generally decreased during FM with the proportion of deep sleep dropping by 30%, and impacts on time required to fall asleep and sleep duration. Interestingly, these changes in sleep quality correlated with a decrease in melatonin levels (measured in salivary samples) during FM.
Following this study, a lunar effect on sleep quality in humans was rejected by some further studies [107,108], whereas it was confirmed by others [109,110]. However, if lunar effects on sleep quality are real, then those effects on human sleep/wake patterns might be more evident in natural populations experiencing less artificial environmental signals. A comparison of two small-scale nonindustrial African populations showed an association between the lunar cycle and sleep, but opposite to the expected direction, with the higher activity around noon (and the lower during the night), the more exposed to moonlight people were [111].
Although the origin of the effect is puzzling and might even sound bizarre at first, further evidence linking the lunar cycle with human physiology and mental health was found in three recent analyses [115, 116, 144]. Specifically, bipolar patients switched their body temperature and sleep cycles from circadian to circalunidian every lunar cycle, fragments, or multiples thereof (notably, often at FM). This circadian to circalunidian switch coincided with a depressive-to-manic transition, but it was possible to stop the mood cycle treating patients with a rigid sleep schedule [115,144] or a combination of light treatments and thyroid-stimulating hormone pharmaceutical inhibition [116].
In addition to the observations mainly based on behaviors, what can be said about molecular impacts of moonlight or possible mechanisms underlying lunar rhythms in non-marine organisms?
In mammals, clock neurons have been thought to respond poorly to dim moonlight, as an adaptation to avoid night light-induced impairments in circadian entrainment [112,117], an hypothesis supported by lower light sensitivity thresholds found in rodents inhabiting moon-shaded and not open areas [113]. This view is challenged by multiple more recent studies. Indeed, nocturnal dim light comparable to moonlight (< 0.005 lx) altered circadian entrainment in hamsters [114,118]. Very dim light has been demonstrated to influence circadian photoentrainment also in mice [119,120], especially through rod photoreceptors and pupil constriction [121]. Moreover, activation of subsets of M1 intrinsically photosensitive retinal ganglion cells by a wide range of light irradiance, including light equivalent to moonlight, has been reported [122]. Interestingly, cells activated by light equivalent to moonlight were insensitive to irradiances higher than twilight, suggesting even the existence of specific set of cells specialized for dim light.
The effects of light equivalent to moonlight on melatonin secretion have been debated. Indeed, 0.04 and 0.3 lx nocturnal light showed no effects on melatonin levels, as well as on light-dependent phase shift in hamsters (see above) [112,123,124], while 8-h dim light pulses (~ 0.01 lx) were sufficient to reduce significantly melatonin serum concentrations, compared to dark nights, and also altered free-running rhythms [125]. Dim nocturnal light (< 0.2 lx) can also contribute to a more rapid re-synchronization of activity rhythms after light-induced phase shifts (49% and 38% faster in Siberian and Syrian hamsters, respectively) [126]. Taken together, the accumulated evidence strongly suggests how dim nocturnal light (at moonlight intensities) might affect circadian entrainment and melatonin secretion in rodents.
Also the elite model for circadian biology, D. melanogaster, has been employed to understand the impact of moonlight on circadian oscillators. Nighttime illumination intensity equivalent to quarter moonlight was shown to increase locomotor activity levels in flies. Moreover, typical morning and evening peaks, controlled by different clusters of clock neurons (morning and evening cells, [127,128]), were both shifted in the dark (M peak anticipated into the last part of the night and the E peak delayed into the first part of the night) [129]. Seemingly, flies interpreted moonlight conditions as longer photoperiods. In these animals, PER and TIM levels were attenuated in all clock neurons, and their peaks in M and E cells advanced and delayed, respectively. Mutants lacking compound eyes exhibited no changes in response to moonlight, suggesting that rhodopsins are likely the main photoreceptors mediating the nocturnal dim light effects on the timing of locomotor activity in D. melanogaster [129]. Further work showed that only mutants without all inner and/or all outer photoreceptor cells were insensitive to dim nocturnal light (no activity shift), implying the involvement of multiple rhodopsins (especially rh1 and rh6) [130]. Mutations in the fly's core circadian clock still shifted their activity during the night under artificial moonlight, suggesting both clock-dependent and clock-independent effects. At present, the meaning of these findings for flies in their natural environment is still unclear. Under conditions where both moonlight and dusk/dawn were simulated, twilight seemed to dominate over moonlight in setting activity patterns [131]. Moreover, under natural conditions, neither an increase in nocturnal activity nor a delay in PER expression in the brain has been detected around FM compared to NM [132]. Solutions to these still open questions will likely come only from more naturalistic studies, e.g. by further disentangling the contributions of light and temperature. Indeed, D. melanogaster's natural habitat are reportedly the tropics [133], which likely exhibit higher nighttime temperatures than the flies experienced under the tested natural conditions in Great Britain and northern Italy.
Although a direct connection with the lunar cycle is still elusive, it is interesting to note that a 12-h rhythm, reminiscent of circatidal oscillations, has been also found in transcriptional dynamics of mouse liver [134]. This rhythm was shown to be cell autonomous, independent from the circadian clock, and entrainable by metabolic and stress cues. Among the 3652 transcripts exhibiting 12 h cycling, genes related to mitochondrial and endoplasmic reticulum function, as well as quality control, were enriched. XBP1 (an unfolded protein response transcription factor) is required for the maintenance of this rhythmicity. Its loss caused hepatic steatosis and a condition reminiscent of human nonalcoholic fatty liver disease [134]. Interestingly, while impairing 12 h-transcript oscillations, the liver-specific deletion of XBP1 did not affect circadian rhythms [135]. This is the first functional genetic insight into a “circatidal” clock, and it will be exciting to test for the function of this gene in marine circatidal organisms like Eurydice.
Perspectives
Over the past 15 years, chronobiological studies on lunar effects have strongly increased. While we still lack a real mechanistic understanding, the accumulated data from different species underline the importance and likely complexity of the processes involved. For instance, several species do not only regulate their reproduction according to the lunar phase (circalunar/circasemilunar pattern), but also considering daily variation in tides and/or moonrise time (circatidal/circalunidian rhythms). It might be plausible to rather subdivide the molecular mechanisms behind biological cycles governed by sun and moon into short (~ 24, ~ 12.4, and ~ 24.8 h) and long (~ 29.5 and ~ 365 days) rhythms. Given that both the sun and the moon regulate rhythms of about 24 h periodicity, could this imply a recruitment of at least part of the same molecular machinery? The combined evidence from multiple non-dipteran insects [[136], [137], [138], [139], [140], [141]], bristleworms [28], crustaceans [10,142], and oysters [143] suggests that the ancestral machinery for ~ 24 h timing was more complex than what is present in Drosophila, mouse, and humans. Is it possible that, because of a partial redundancy, this originally higher complexity was used for timing ~ 24 h rhythms (or fractions thereof) set by the sun and/or moon? But wouldn't the recent finding of XBP1 in “circatidal” liver clocks argue against this view?
Our review also documents the many different schemes used for the study of lunar cycles. It might seem obvious, but dealing with the chronobiological complexity of re-adding lunar cycles to the classical paradigms makes experimental designs more difficult. However, technical limitations in mimicking environmental conditions represent an issue for circadian and seasonal studies as well. Which biological phenomena should be primarily investigated? Direct response to environmental stimuli? Presence of endogenous clocks? To what detail should and can circalunar or circatidal cues be mimicked in the laboratory? Which seasonal and daily cues should be used? What could and should be studied under laboratory conditions and what under more naturalistic field conditions? How to best reconcile studies done under both conditions? Indeed, but not unexpectedly, studies in corals and D. melanogaster show discrepancies in gene expression data between field and laboratory samples [26,27,132], and also P. dumerilii monthly spawning patterns are broader in the lab [28] compared to the field [35]. These ambiguities likely are derived from differences in natural versus artificial moonlight conditions, like spectrum and intensity, moonrise/moonset timing, duration over the lunar month, or absence of additional cues (such as mechanosensory, thermal, magnetic). Laboratory studies provide a better control of the multitude of environmental variables present in nature, allowing the investigation of the single contributions of a certain cue, but this should mirror as much as possible parameters measured in nature, in order to avoid potential artifacts.
Although pharmacological studies could provide interesting insights about the relevance of specific pathways and gene networks, the generation of knockouts and transgenic animals clearly allow for a detailed functional dissection that is difficult to meet with other methods. Therefore, the availability of new models that can be reared in captivity and are genetically accessible will feed further advances toward a more comprehensive understanding of the molecular nature of lunar-regulated rhythms and clocks.
The quest for the molecular mechanisms behind lunar-regulated rhythms and clocks does not only represent a challenging aim for basic research. It is also important for a complete understanding and counteraction of the complex chronobiological changes likely caused by anthropogenic impacts (i.e. light pollution, temperature changes, architectural barriers) that are endangering the world's ecosystems as we know them [22,23], and possibly human health. Understanding the complexity of both sun- and moon-driven biological rhythms is not a small step for scientists but worth the effort, as it represents a big, possibly giant leap for mankind.
Acknowledgment
We thank the members of the Tessmar-Raible and Raible groups for discussions, Franz Kerschbaum for highly useful comments on Figure 1, and all three reviewers for very valuable feedback.
K.T.-R. received funding for this research from the European Research Council under the European Community‘s Seventh Framework Programme (FP7/2007-2013; ERC Grant Agreement 337011), the Horizon 2020 Programme (ERC Grant Agreement 819952), the research platform “Rhythms of Life” of the University of Vienna, and the Austrian Science Fund (FWF, http://www.fwf.ac.at/en/) (Grant No. P28970 and SFB F78).
None of the funding bodies were involved in the design or writing of the manuscript.
Both authors declare no conflict of interest.
Edited by Eva Wolf
References
- 1.Fox H.M. Lunar periodicity in reproduction. Proc. Biol. Sci. 1924;95:523–550. doi: 10.1098/rspb.1924.0004. [DOI] [Google Scholar]
- 2.Tessmar-Raible K., Raible F., Arboleda E. Another place, another timer: marine species and the rhythms of life. BioEssays. 2011;33:165–172. doi: 10.1002/bies.201000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raible F., Takekata H., Tessmar-Raible K. An overview of monthly rhythms and clocks. Front. Neurol. 2017;8:1–14. doi: 10.3389/fneur.2017.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brugler M.R., Aguado M.T., Tessler M., Siddall M.E. The transcriptome of the Bermuda fireworm Odontosyllis enopla (Annelida: Syllidae): a unique luciferase gene family and putative epitoky-related genes. PLoS One. 2018;13 doi: 10.1371/journal.pone.0200944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coughenour C.L., Archer A.W., Lacovara K.J. Tides, tidalites, and secular changes in the earth–moon system. Earth-Science Rev. 2009;97:59–79. doi: 10.1016/j.earscirev.2009.09.002. [DOI] [Google Scholar]
- 6.Tran D., Nadau A., Durrieu G., Ciret P., Parisot J.P., Massabuau J.C. Field chronobiology of a molluscan bivalve: how the moon and sun cycles interact to drive oyster activity rhythms. Chronobiol. Int. 2011;28:307–317. doi: 10.3109/07420528.2011.565897. [DOI] [PubMed] [Google Scholar]
- 7.Kvale E.P. The origin of neap–spring tidal cycles. Mar. Geol. 2006;235:5–18. doi: 10.1016/j.margeo.2006.10.001. [DOI] [Google Scholar]
- 8.Archer A.W. Reliability of lunar orbital periods extracted from ancient cyclic tidal rhythmites. Earth Planet. Sci. Lett. 1996;141:1–10. doi: 10.1016/0012-821x(96)00063-5. [DOI] [Google Scholar]
- 9.Bernard I., Massabuau J.C., Ciret P., Sow M., Sottolichio A., Pouvreau S., Tran D. In situ spawning in a marine broadcast spawner, the Pacific oyster Crassostrea gigas: timing and environmental triggers. Limnol. Oceanogr. 2016;61:635–647. doi: 10.1002/lno.10240. [DOI] [Google Scholar]
- 10.Zhang L., Hastings M.H., Green E.W., Tauber E., Sladek M., Webster S.G., Kyriacou C.P., Wilcockson D.C. Dissociation of circadian and circatidal timekeeping in the marine crustacean Eurydice pulchra. Curr. Biol. 2013;23:1863–1873. doi: 10.1016/j.cub.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Payton L., Tran D. Moonlight cycles synchronize oyster behaviour. Biol. Lett. 2019;15:20180299. doi: 10.1098/rsbl.2018.0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleissner G., Schuchardt K., Neumann D., Bali G., Fleissner G., Fleissner G., Schuchardt K., Neumann D. A Lunar clock changes shielding pigment transparency in larval ocelli of Clunio marinus. 2009;25:17–30. doi: 10.1080/07420520801904008. [DOI] [PubMed] [Google Scholar]
- 13.Last K.S., Hobbs L., Berge J., Brierley A.S., Cottier F. Moonlight drives ocean-scale mass vertical migration of zooplankton during the Arctic winter. Curr. Biol. 2016;26:244–251. doi: 10.1016/j.cub.2015.11.038. [DOI] [PubMed] [Google Scholar]
- 14.Thakurdas P., Sharma S., Vanlalhriatpuia K., Sinam B., Chib M., Shivagaje A., Joshi D. Light at night alters the parameters of the eclosion rhythm in a tropical fruit fly, Drosophila jambulina. Chronobiol. Int. 2009;26:1575–1586. doi: 10.3109/07420520903529765. [DOI] [PubMed] [Google Scholar]
- 15.Corbet P.S. Lunar periodicity of aquatic insects in Lake Victoria. Nature. 1958;182:330–331. doi: 10.1038/182330a0. [DOI] [Google Scholar]
- 16.Neumann D. Physiologische Uhren von Insekten Zur Ökophysiologie lunarperiodisch kontrollierter Fortpflanzungszeiten. Naturwissenschaften. 1995;82:310–320. doi: 10.1007/BF01131527. [DOI] [Google Scholar]
- 17.Franke R., Hoerstgen-schwark G. Lunar-rhythmic molting in laboratory populations of the noble crayfish Astacus astacus (Crustacea, Astacidea): an experimental. Analysis. 2013;8:1–11. doi: 10.1371/journal.pone.0068653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gibson-Hill C.A. Field notes on the terrestrial crabs. Bull. Raffles Museum. 1947;18:43–52. https://papers2://publication/uuid/2EC42E05-C2A5-4C02-BD05-BD33EE047568 [Google Scholar]
- 19.A.M. Adamczewska, S. Morris, Ecology and behavior of Gecarcoidea natalis, the Christmas Island red crab, during the annual breeding migration, (2001) 305–320. [DOI] [PubMed]
- 20.Fischer A., Fischer U. On the life-style and life-cycle of the luminescent polychaete Odontosyllis enopla (Annelida: Polychaeta) Invert. Biol. 2019;114:236–247. doi: 10.2307/3226878. [DOI] [Google Scholar]
- 21.Babcock R.C., Bull G.D., Harrison P.L., Heyward A.J., Oliver J.K., Wallace C.C., Willis B.L. Synchronous spawnings of 105 scleractinian coral species on the Great Barrier Reef. Mar. Biol. 1986;90:379–394. doi: 10.1007/BF00428562. [DOI] [Google Scholar]
- 22.Fogarty N.D., Marhaver K.L. Coral spawning, unsynchronized. Science. 2019;365:987–988. doi: 10.1126/science.aay7457. [DOI] [PubMed] [Google Scholar]
- 23.Shlesinger T., Loya Y. Breakdown in spawning synchrony: a silent threat to coral persistence. Science. 2019;365:1002–1007. doi: 10.1126/science.aax0110. [DOI] [PubMed] [Google Scholar]
- 24.Fingerman M. Lunar rhythmicity in marine organisms. Am. Nat. 1957;91:167–178. [Google Scholar]
- 25.Numata H., Helm B. Springer; 2014. Annual, Lunar, and Tidal Clocks: Patterns and Mechanisms of Nature’s Enigmatic Rhythms. [Google Scholar]
- 26.Levy O., Appelbaum L., Leggat W., Gothlif Y., Hayward D.C., Miller D.J., Hoegh-Guldberg O. Light-responsive cryptochromes from a simple multicellular animal, the coral Acropora millepora. Science. 2007;318:467–470. doi: 10.1126/science.1145432. [DOI] [PubMed] [Google Scholar]
- 27.Brady A.K., Willis B.L., Harder L.D., Vize P.D. Lunar phase modulates circadian gene expression cycles in the broadcast spawning coral Acropora millepora. Biol. Bull. 2016;230:130–142. doi: 10.1086/BBLv230n2p130. [DOI] [PubMed] [Google Scholar]
- 28.Zantke J., Ishikawa-Fujiwara T., Arboleda E., Lohs C., Schipany K., Hallay N., Straw A.D., Todo T. Circadian and circalunar clock interactions in a marine annelid. Cell Rep. 2013;5:99–113. doi: 10.1016/j.celrep.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takeuchi Y., Kabutomori R., Yamauchi C., Miyagi H., Takemura A., Okano K., Okano T. Moonlight controls lunar-phase-dependency and regular oscillation of clock gene expressions in a lunar-synchronized spawner fish, Goldlined spinefoot. Sci. Rep. 2018;8:1–12. doi: 10.1038/s41598-018-24538-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oliveri P., Fortunato A.E., Petrone L., Ishikawa-Fujiwara T., Kobayashi Y., Todo T., Antonova O., Arboleda E. The Cryptochrome/Photolyase family in aquatic organisms. Mar. Genomics. 2014;14:23–37. doi: 10.1016/j.margen.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Brady A.K., Snyder K.A., Vize P.D. Circadian cycles of gene expression in the coral, Acropora millepora. PLoS One. 2011;6 doi: 10.1371/journal.pone.0025072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szmant-Froelich A., Reutter M., Riggs L. Sexual reproduction of Favia fragum (ESPER): lunar patterns of gametogenesis, embryogenesis and planulation in Puerto Rico. Bull. Mar. Sci. 1985;37:880–892. [Google Scholar]
- 33.Hoadley K.D., Szmant A.M., Pyott S.J. Circadian clock gene expression in the coral Favia fragum over diel and lunar reproductive cycles. PLoS One. 2011;6 doi: 10.1371/journal.pone.0019755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hauenschild C. Lunar periodicity. Cold Spring Harb. Symp. Quant. Biol. 1960;25:491–497. doi: 10.1101/sqb.1960.025.01.051. [DOI] [PubMed] [Google Scholar]
- 35.Ranzi S. Ricerche sulla biologia sessuale degli Anellidi. Pubbl. Del Stn. Zool. Napoli. 1931;11:271–292. [Google Scholar]
- 36.Eide E.J., Woolf M.F., Kang H., Woolf P., Hurst W., Camacho F., Vielhaber E.L., Giovanni A. Control of mammalian circadian rhythm by CKIepsilon-regulated proteasome-mediated PER2 degradation. Mol. Cell. Biol. 2005;25:2795–2807. doi: 10.1128/MCB.25.7.2795-2807.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walton K.M., Fisher K., Rubitski D., Marconi M., Meng Q.-J., Sládek M., Adams J., Bass M. Selective inhibition of casein kinase 1 epsilon minimally alters circadian clock period. J. Pharmacol. Exp. Ther. 2009;330:430–439. doi: 10.1124/jpet.109.151415. [DOI] [PubMed] [Google Scholar]
- 38.Di Cara F., King-Jones K. The circadian clock is a key driver of steroid hormone production in Drosophila. Curr. Biol. 2016;26:2469–2477. doi: 10.1016/j.cub.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 39.Rush B.L., Murad A., Emery P., Giebultowicz J.M. Ectopic cryptochrome renders Tim light sensitive in the Drosophila ovary. J. Biol. Rhythm. 2006;21:272–278. doi: 10.1177/0748730406290416. [DOI] [PubMed] [Google Scholar]
- 40.Neumann D. Die lunare und tägliche Schlüpfperiodik der Mücke Clunio—Steuerung und Abstimmung auf die Gezeitenperiodik. Z. Vgl. Physiol. 1966;53:1–61. doi: 10.1007/BF00343045. [DOI] [Google Scholar]
- 41.Neumann D. Behav Adapt. to Intertidal Life. Springer; USA: 1988. The timing of reproduction to distinct spring tide situations in the intertidal insect Clunio; pp. 45–54. [DOI] [Google Scholar]
- 42.Neumann D. Circadian components of semilunar and lunar timing mechanisms. J. Biol. Rhythm. 1989;4:173–182. doi: 10.1177/074873048900400213. [DOI] [PubMed] [Google Scholar]
- 43.Kaiser T.S., Neumann D., Heckel D.G. Timing the tides: genetic control of diurnal and lunar emergence times is correlated in the marine midge Clunio marinus. BMC Genet. 2011;12 doi: 10.1186/1471-2156-12-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaiser T.S., Heckel D.G. Genetic architecture of local adaptation in lunar and diurnal emergence times of the marine midge Clunio marinus (Chironomidae, Diptera) PLoS One. 2012;7 doi: 10.1371/journal.pone.0032092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neumann D. Genetic adaptation in emergence time of Clunio populations to different tidal conditions. Helgoländer Meeresun. 1967;15:163–171. doi: 10.1007/BF01618620. [DOI] [Google Scholar]
- 46.Kaiser T.S., Poehn B., Szkiba D., Preussner M., Sedlazeck F.J., Zrim A., Neumann T., Nguyen L.T. The genomic basis of circadian and circalunar timing adaptations in a midge. Nature. 2016;540:69–73. doi: 10.1038/nature20151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rahman M.S., Takemura A., Takano K. Correlation between plasma steroid hormones and vitellogenin profiles and lunar periodicity in the female golden rabbitfish, Siganus guttatus (Bloch) Comp. Biochem. Physiol. - B Biochem. Mol. Biol. 2000;127:113–122. doi: 10.1016/S0305-0491(00)00240-6. [DOI] [PubMed] [Google Scholar]
- 48.Takemura A., Susilo E.S., Rahman M.D.S., Morita M. Perception and possible utilization of moonlight intensity for reproductive activities in a lunar-synchronized spawner, the golden rabbitfish. J. Exp. Zool. Part A Comp. Exp. Biol. 2004;301:844–851. doi: 10.1002/jez.a.105. [DOI] [PubMed] [Google Scholar]
- 49.Fukushiro M., Takeuchi T., Takeuchi Y., Hur S.P., Sugama N., Takemura A., Kubo Y., Okano K. Lunar phase-dependent expression of cryptochrome and a photoperiodic mechanism for lunar phase-recognition in a reef fish, goldlined spinefoot. PLoS One. 2011;6 doi: 10.1371/journal.pone.0028643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Toda R., Okano K., Takeuchi Y., Yamauchi C., Fukushiro M., Takemura A., Okano T. Hypothalamic expression and moonlight-independent changes of Cry3 and Per4 implicate their roles in lunar clock oscillators of the lunar-responsive goldlined spinefoot. PLoS One. 2014;9 doi: 10.1371/journal.pone.0109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoshimura T. Molecular mechanism of the photoperiodic response of gonads in birds and mammals. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2006;144:345–350. doi: 10.1016/j.cbpa.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 52.Collins B., Mazzoni E.O., Stanewsky R., Blau J. Drosophila cryptochrome is a circadian transcriptional repressor. Curr. Biol. 2006;16:441–449. doi: 10.1016/j.cub.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 53.Tran D., Perrigault M., Ciret P., Payton L. Bivalve mollusc circadian clock genes can run at tidal frequency. Proc. Biol. Sci. 2020;287:20192440. doi: 10.1098/rspb.2019.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Neill J.S., Lee K.D., Zhang L., Feeney K., Webster S.G., Blades M.J., Kyriacou C.P., Hastings M.H. Metabolic molecular markers of the tidal clock in the marine crustacean Eurydice pulchra. Curr. Biol. 2015;25:R326–R327. doi: 10.1016/j.cub.2015.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Edgar R.S., Green E.W., Zhao Y., Van Ooijen G., Olmedo M., Qin X., Xu Y., Pan M. Peroxiredoxins are conserved markers of circadian rhythms. Nature. 2012;485:459–464. doi: 10.1038/nature11088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hastings M.H. Semi-lunar variations of endogenous circa-tidal rhythms of activity and respiration in the isopod Eurydice pulchra. Mar. Ecol Prog Ser. 1981;4:85–90. doi: 10.3354/meps004085. [DOI] [Google Scholar]
- 57.Schnytzer Y., Simon-Blecher N., Li J., Ben-Asher H.W., Salmon-Divon M., Achituv Y., Hughes M.E., Levy O. Tidal and diel orchestration of behaviour and gene expression in an intertidal mollusc. Sci. Rep. 2018;8:1–13. doi: 10.1038/s41598-018-23167-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rahman M.S., Kim B.H., Takemura A., Park C.B., Lee Y.D. Effects of moonlight exposure on plasma melatonin rhythms in the seagrass rabbitfish, Siganus canaliculatus. J. Biol. Rhythm. 2004;19:325–334. doi: 10.1177/0748730404266712. [DOI] [PubMed] [Google Scholar]
- 59.Rahman M.S., Kim B.H., Takemura A., Park C.B., Lee Y.D. Influence of light-dark and lunar cycles on the ocular melatonin rhythms in the seagrass rabbitfish, a lunar-synchronized spawner. J. Pineal Res. 2004;37:122–128. doi: 10.1111/j.1600-079X.2004.00147.x. [DOI] [PubMed] [Google Scholar]
- 60.Fukunaga K., Yamashina F., Ohta N., Mizuno H., Takeuchi Y., Yamauchi C., Takemura A. Involvement of melatonin in transducing moon-related signals into the reproductive network of the female honeycomb grouper Epinephelus merra. Gen. Comp. Endocrinol. 2019;282:113211. doi: 10.1016/j.ygcen.2019.113211. [DOI] [PubMed] [Google Scholar]
- 61.Ikegami T., Maruyama Y., Doi H., Hattori A., Ando H. Ultaradian oscillation in expression of four melatonin receptor subtype genes in the pineal gland of the grass puffer, a semilunar-synchronized spawner, under constant darkness. Front. Neurosci. 2015;9:1–10. doi: 10.3389/fnins.2015.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kashiwagi T., Park Y.J., Park J.G., Imamura S., Takeuchi Y., Hur S.P., Takemura A. Moonlight affects mRNA abundance of arylalkylamine N-acetyltransferase in the retina of a lunar-synchronized spawner, the goldlined spinefoot. J. Exp. Zool. Part A Ecol. Genet. Physiol. 2013;319:505–516. doi: 10.1002/jez.1814. [DOI] [PubMed] [Google Scholar]
- 63.Park Y.J., Park J.G., Takeuchi Y., Hur S.P., Lee Y.D., Kim S.J., Takemura A. Influence of moonlight on mRNA expression patterns of melatonin receptor subtypes in the pineal organ of a tropical fish. Mar. Genomics. 2014;14:67–70. doi: 10.1016/j.margen.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 64.Wang Q., Hong W., Chen S., Zhang Q. Variation with semilunar periodicity of plasma steroid hormone production in the mudskipper Boleophthalmus pectinirostris. Gen. Comp. Endocrinol. 2008;155:821–826. doi: 10.1016/j.ygcen.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 65.Hong L.Y., Hong W.S., Zhu W.B., Shi Q., You X.X., Chen S.X. Cloning and expression of melatonin receptors in the mudskipper Boleophthalmus pectinirostris: their role in synchronizing its semilunar spawning rhythm. Gen. Comp. Endocrinol. 2014;195:138–150. doi: 10.1016/j.ygcen.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 66.Roa J., Tena-Sempere M. Energy balance and puberty onset: emerging role of central mTOR signaling. Trends Endocrinol. Metab. 2010;21:519–528. doi: 10.1016/j.tem.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 67.Hahn T.P., Ball G.F. Changes in brain GnRH associated with photorefractoriness in house sparrows (Passer domesticus) Gen. Comp. Endocrinol. 1995;99:349–363. doi: 10.1006/gcen.1995.1119. [DOI] [PubMed] [Google Scholar]
- 68.Deviche P., Saldanha C.J., Silver R. Changes in brain gonadotropin-releasing hormone- and vasoactive intestinal polypeptide-like immunoreactivity accompanying reestablishment of photosensitivity in male dark-eyed juncos (Junco hyemalis) Gen. Comp. Endocrinol. 2000;117:8–19. doi: 10.1006/gcen.1999.7361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smith J.T., Coolen L.M., Kriegsfeld L.J., Sari I.P., Jaafarzadehshirazi M.R., Maltby M., Bateman K., Goodman R.L. Variation in kisspeptin and RFamide-related peptide (RFRP) expression and terminal connections to gonadotropin-releasing hormone neurons in the brain: a novel medium for seasonal breeding in the sheep. Endocrinology. 2008;149:5770–5782. doi: 10.1210/en.2008-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Clarke I.J., Smith J.T., Caraty A., Goodman R.L., Lehman M.N. Kisspeptin and seasonality in sheep. Peptides. 2009;30:154–163. doi: 10.1016/j.peptides.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smith J.T. The role of kisspeptin and gonadotropin inhibitory hormone in the seasonal regulation of reproduction in sheep. Domest. Anim. Endocrinol. 2012;43:75–84. doi: 10.1016/j.domaniend.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 72.Ando H., Shahjahan M., Hattori A. Molecular neuroendocrine basis of lunar-related spawning in grass puffer. Gen. Comp. Endocrinol. 2013;181:211–214. doi: 10.1016/j.ygcen.2012.07.027. [DOI] [PubMed] [Google Scholar]
- 73.Ando H., Ogawa S., Shahjahan M., Ikegami T., Doi H., Hattori A., Parhar I. Diurnal and circadian oscillations in expression of kisspeptin, kisspeptin receptor and gonadotrophin-releasing hormone 2 genes in the grass puffer, a semilunar-synchronised spawner. J. Neuroendocrinol. 2014;26:459–467. doi: 10.1111/jne.12165. [DOI] [PubMed] [Google Scholar]
- 74.Ando H., Shahjahan M., Kitahashi T. Periodic regulation of expression of genes for kisspeptin, gonadotropin-inhibitory hormone and their receptors in the grass puffer: implications in seasonal, daily and lunar rhythms of reproduction. Gen. Comp. Endocrinol. 2018;265:149–153. doi: 10.1016/j.ygcen.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 75.Shahjahan M., Ikegami T., Osugi T., Ukena K., Doi H., Hattori A., Tsutsui K., Ando H. Synchronised expressions of LPXRFamide peptide and its receptor genes: seasonal, diurnal and circadian changes during spawning period in grass puffer. J. Neuroendocrinol. 2011;23:39–51. doi: 10.1111/j.1365-2826.2010.02081.x. [DOI] [PubMed] [Google Scholar]
- 76.Andreatta G., Broyart C., Borghgraef C., Vadiwala K., Kozin V., Polo A., Bileck A., Beets I. Corazonin signaling integrates energy homeostasis and lunar phase to regulate aspects of growth and sexual maturation in Platynereis. Proc. Natl. Acad. Sci. 2020;117:1097–1106. doi: 10.1073/pnas.1910262116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Counihan R., McNamara D., Souter D., Jebreen E., Preston N., Johnson C., Degnan B. Pattern, synchrony and predictability of spawning of the tropical abalone Haliotis asinina from Heron Reef, Australia. Mar. Ecol. Prog. Ser. 2001;213:193–202. doi: 10.3354/meps213193. [DOI] [Google Scholar]
- 78.York P.S., Cummins S.F., Degnan S.M., Woodcroft B.J., Degnan B.M. Marked changes in neuropeptide expression accompany broadcast spawnings in the gastropod Haliotis asinina. Front. Zool. 2012;9:9. doi: 10.1186/1742-9994-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schenk S., Bannister S.C., Sedlazeck F.J., Anrather D., Minh B.Q., Bileck A., Hartl M., von Haeseler A. Combined transcriptome and proteome profiling reveals specific molecular brain signatures for sex, maturation and circalunar clock phase. Elife. 2019;8 doi: 10.7554/eLife.41556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rahman M.S., Takemura A., Takano K. Lunar synchronization of testicular development and steroidogenesis in rabbitfish. Comp. Biochem. Physiol. - B Biochem. Mol. Biol. 2001;129:367–373. doi: 10.1016/S1096-4959(01)00323-2. [DOI] [PubMed] [Google Scholar]
- 81.Rahman M.S., Takemura A., Takano K. Lunar synchronization of in vitro steroidogenesis in ovaries of the golden rabbitfish, Siganus guttatus (Bloch) Gen. Comp. Endocrinol. 2002;125:1–8. doi: 10.1006/gcen.2001.7708. [DOI] [PubMed] [Google Scholar]
- 82.Nagahama Y., Miura T., Kobayashi T. The onset of spermatogenesis in fish. Ciba Found. Symp. 1994;182 doi: 10.1002/9780470514573.ch14. [DOI] [PubMed] [Google Scholar]
- 83.Chen S.X., Bogerd J., Schoonen N.E., Martijn J., de Waal P.P., Schulz R.W. A progestin (17α,20β-dihydroxy-4-pregnen-3-one) stimulates early stages of spermatogenesis in zebrafish. Gen. Comp. Endocrinol. 2013;185:1–9. doi: 10.1016/j.ygcen.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 84.Rahman M.S., Morita M., Takemura A., Takano K. Hormonal changes in relation to lunar periodicity in the testis of the forktail rabbitfish, Siganus argenteus. Gen. Comp. Endocrinol. 2003;131:302–309. doi: 10.1016/S0016-6480(03)00025-X. [DOI] [PubMed] [Google Scholar]
- 85.Cerdá J., Calman B.G., LaFleur G.J., Limesand S. Pattern of vitellogenesis and follicle maturational competence during the ovarian follicular cycle of Fundulus heteroclitus. Gen. Comp. Endocrinol. 1996;103:24–35. doi: 10.1006/gcen.1996.0090. [DOI] [PubMed] [Google Scholar]
- 86.Kaniewska P., Alon S., Karako-Lampert S., Hoegh-Guldberg O., Levy O. Signaling cascades and the importance of moonlight in coral broadcast mass spawning. Elife. 2015;4:1–14. doi: 10.7554/elife.09991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Panda S., Sato T.K., Castrucci A.M., Rollag M.D., DeGrip W.J., Hogenesch J.B., Provencio I., Kay S.A. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science. 2002;298:2213–2216. doi: 10.1126/science.1076848. [DOI] [PubMed] [Google Scholar]
- 88.Rosenberg Y., Doniger T., Harii S., Sinniger F., Levy O. Canonical and cellular pathways timing gamete release in Acropora digitifera, Okinawa, Japan. Mol. Ecol. 2017;26:2698–2710. doi: 10.1111/mec.14062. [DOI] [PubMed] [Google Scholar]
- 89.Oldach M.J., Workentine M., Matz M.V., Fan T.Y., Vize P.D. Transcriptome dynamics over a lunar month in a broadcast spawning acroporid coral. Mol. Ecol. 2017;26:2514–2526. doi: 10.1111/mec.14043. [DOI] [PubMed] [Google Scholar]
- 90.Rosenberg Y., Doniger T., Harii S., Sinniger F., Levy O. Demystifying circalunar and diel rhythmicity in Acropora digitifera under constant dim light. IScience. 2019;22:477–488. doi: 10.1016/j.isci.2019.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wuitchik D.M., Wang D., Pells T.J., Karimi K., Ward S., Vize P.D. Seasonal temperature, the lunar cycle and diurnal rhythms interact in a combinatorial manner to modulate genomic responses to the environment in a reef-building coral. Mol. Ecol. 2019;28:3629–3641. doi: 10.1111/mec.15173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Park K.-S., Korfhagen T.R., Bruno M.D., Kitzmiller J.A., Wan H., Wert S.E., Khurana Hershey G.K., Chen G. SPDEF regulates goblet cell hyperplasia in the airway epithelium. J. Clin. Invest. 2007;117:978–988. doi: 10.1172/JCI29176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gotter A.L. Tipin, a novel timeless-interacting protein, is developmentally co-expressed with timeless and disrupts its self-association. J. Mol. Biol. 2003;331:167–176. doi: 10.1016/s0022-2836(03)00633-8. [DOI] [PubMed] [Google Scholar]
- 94.Hazlerigg D., Loudon A. New insights into ancient seasonal life timers. Curr. Biol. 2008;18:795–804. doi: 10.1016/j.cub.2008.07.040. [DOI] [PubMed] [Google Scholar]
- 95.Sivachenko A., Li Y., Abruzzi K.C., Rosbash M. The transcription factor Mef2 links the Drosophila core clock to Fas2, neuronal morphology, and circadian behavior. Neuron. 2013;79:281–292. doi: 10.1016/j.neuron.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim H.J., Lee H.R., Seo J.Y., Ryu H.G., Lee K.H., Kim D.Y., Kim K.T. Heterogeneous nuclear ribonucleoprotein A1 regulates rhythmic synthesis of mouse Nfil3 protein via IRES-mediated translation. Sci. Rep. 2017;7:42882. doi: 10.1038/srep42882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nolte C., Staiger D. RNA around the clock—regulation at the RNA level in biological timing. Front. Plant Sci. 2015;6:1–15. doi: 10.3389/fpls.2015.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sorek M., Schnytzer Y., Waldman Ben-Asher H., Caspi V.C., Chen C.S., Miller D.J., Levy O. Setting the pace: host rhythmic behaviour and gene expression patterns in the facultatively symbiotic cnidarian Aiptasia are determined largely by Symbiodinium. Microbiome. 2018;6:1–12. doi: 10.1186/s40168-018-0465-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Balaparameswara Rao M. Studies on the oxygen consumption of a tropical intertidal limpet Cellana radiata (Born): effect of body size and tidal rhythm. Hydrobiologia. 1980;71:175–179. doi: 10.1007/BF00005842. [DOI] [Google Scholar]
- 100.Vinson M.R., Angradi T.R. Muskie lunacy: does the lunar cycle influence angler catch of muskellunge (Esox masquinongy)? PLoS One. 2014;9 doi: 10.1371/journal.pone.0098046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Norevik G., Åkesson S., Andersson A., Bäckman J., Hedenström A. The lunar cycle drives migration of a nocturnal bird. PLoS Biol. 2019;17 doi: 10.1371/journal.pbio.3000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Navarro-castilla Á., Barja I. Does predation risk, through moon phase and predator cues, modulate food intake, antipredatory and physiological responses in wood mice (Apodemus sylvaticus)? Behav. Ecol. Sociobiol. 2014;68:1505–1512. doi: 10.1007/s00265-014-1759-y. [DOI] [Google Scholar]
- 103.Kotler B.P., Brown J., Mukherjee S., Berger-Tal O., Bouskila A. Moonlight avoidance in gerbils reveals a sophisticated interplay among time allocation, vigilance and state-dependent foraging. Proc. Biol. Sci. 2010;277:1469–1474. doi: 10.1098/rspb.2009.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dixon D.R., Dixon L.R.J., Bishop J.D., Pettifor R.A. Lunar-related reproductive behaviour in the badger (Meles meles) Acta Ethol. 2006;9:59–63. doi: 10.1007/s10211-006-0016-4. [DOI] [Google Scholar]
- 105.Häfker N.S., Tessmar-Raible K. Rhythms of behavior: are the times changin’? Curr. Opin. Neurobiol. 2020;60:55–66. doi: 10.1016/j.conb.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 106.Cajochen C., Altanay-Ekici S., Münch M., Frey S., Knoblauch V., Wirz-Justice A. Evidence that the lunar cycle influences human sleep. Curr. Biol. 2013;23:1485–1488. doi: 10.1016/j.cub.2013.06.029. [DOI] [PubMed] [Google Scholar]
- 107.Cordi M., Ackermann S., Bes F.W., Hartmann F., Konrad B.N., Genzel L., Pawlowski M., Steiger A. Lunar cycle effects on sleep and the file drawer problem. Curr. Biol. 2014;24:R549–R550. doi: 10.1016/j.cub.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 108.Sjödin A., Hjorth M.F., Damsgaard C.T., Ritz C., Astrup A., Michaelsen K.F. Physical activity, sleep duration and metabolic health in children fluctuate with the lunar cycle: science behind the myth. Clin. Obes. 2015;5:60–66. doi: 10.1111/cob.12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Smith M., Croy I., Persson Waye K. Human sleep and cortical reactivity are influenced by lunar phase. Curr. Biol. 2014;24:R551–R552. doi: 10.1016/j.cub.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 110.Chaput J.P., Weippert M., LeBlanc A.G., Hjorth M.F., Michaelsen K.F., Katzmarzyk P.T., Tremblay M.S., Barreira T.V. Are children like werewolves? Full moon and its association with sleep and activity behaviors in an international sample of children. Front. Pediatr. 2016;4:24. doi: 10.3389/fped.2016.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Samson D.R., Crittenden A.N., Mabulla I.A., Mabulla A.Z.P., Nunn C.L. Does the moon in fl uence sleep in small-scale societies? Sleep Heal. J. Natl. Sleep Found. 2018;4:509–514. doi: 10.1016/j.sleh.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 112.Nelson D.E., Takahashi J.S. Sensitivity and integration in a visual pathway for circadian entrainment in the hamster (Mesocricetus auratus) J. Physiol. 1991;439:115–145. doi: 10.1113/jphysiol.1991.sp018660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Decoursey P.J. Circadian photoentrainment in nocturnal mammals—ecological overtones. Biol. Behav. 1990;15:213–238. [Google Scholar]
- 114.Frank D.W., Evans J.A., Gorman M.R. Time-dependent effects of dim light at night on re-entrainment and masking of hamster activity rhythms. J. Biol. Rhythm. 2010;25:103–112. doi: 10.1177/0748730409360890. [DOI] [PubMed] [Google Scholar]
- 115.Wehr T.A. Bipolar mood cycles associated with lunar entrainment of a circadian rhythm. Transl. Psychiatry. 2018:4–9. doi: 10.1038/s41398-018-0203-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Avery D.H., Alexander E.M., Wehr T.A. Synchrony between bipolar mood cycles and lunar tidal cycles ended after initiation of light treatment and treatment of hypothyroidism. J. Psychiatr. Pract. 2019;25:475–480. doi: 10.1097/PRA.0000000000000423. [DOI] [PubMed] [Google Scholar]
- 117.Brainard G.C., Richardson B.A., Hurlbut E.C., Steinlechner S., Matthews S.A., Reiter R.J. The influence of various irradiances of artificial light, twilight, and moonlight on the suppression of pineal melatonin content in the Syrian hamster. J. Pineal Res. 1984;1:105–119. doi: 10.1111/j.1600-079x.1984.tb00202.x. [DOI] [PubMed] [Google Scholar]
- 118.Gorman M.R., Evans J.A., Elliott J.A. Potent circadian effects of dim illumination at night. Chronobiol. Int. 2006;23:245–250. doi: 10.1080/07420520500521905. [DOI] [PubMed] [Google Scholar]
- 119.Lall G.S., Revell V.L., Momiji H., Al Enezi J., Altimus C.M., Gu A.D., Aguilar C., Cameron M.A. Distinct contributions of rod, cone, and melanopsin photoreceptors to encoding irradiance. Neuron. 2010;66:417–428. doi: 10.1016/j.neuron.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Altimus C.M., Güler A.D., Alam N.M., Arman A.C., Prusky G.T., Sampath A.P., Hattar S. Rod photoreceptors drive circadian photoentrainment across a wide range of light intensities. Nat. Publ. Gr. 2010;13:1107–1112. doi: 10.1038/nn.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pennesi M.E., Lyubarsky A.L., Pugh E.N. Extreme responsiveness of the pupil of the dark-adapted mouse to steady retinal illumination. Invest. Ophthalmol. Vis. Sci. 1998;39:2148–2156. [PubMed] [Google Scholar]
- 122.Milner E.S., Do M.T.H. A population representation of absolute light Intensity in the mammalian retina. Cell. 2017;171:865–876. doi: 10.1016/j.cell.2017.09.005. e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Brainard G.C., Richardson B.A., Petterborg L.J., Reiter R.J. The effect of different light intensities on pineal melatonin content. Brain Res. 1982;233:75–81. doi: 10.1016/0006-8993(82)90931-3. [DOI] [PubMed] [Google Scholar]
- 124.Nelson D.E., Takahashi J.S. Comparison of visual sensitivity for suppression of pineal melatonin and circadian phase-shifting in the golden hamster. Brain Res. 1991;554:272–277. doi: 10.1016/0006-8993(91)90200-f. [DOI] [PubMed] [Google Scholar]
- 125.Evans J.A., Elliott J.A., Gorman M.R. Circadian effects of light no brighter than moonlight. J. Biol. Rhythm. 2007;22:356–367. doi: 10.1177/0748730407301988. [DOI] [PubMed] [Google Scholar]
- 126.Evans J.A., Elliott J.A., Gorman M.R. Dim nighttime illumination accelerates adjustment to timezone travel in an animal model. Curr. Biol. 2009;19:156–157. doi: 10.1016/j.cub.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 127.Grima B., Chélot E., Xia R., Rouyer F. Morning and evening peaks of activity rely on different clock neurons of the Drosophila brain. Nature. 2004;431:869–873. doi: 10.1038/nature02935. [DOI] [PubMed] [Google Scholar]
- 128.Picot M., Cusumano P., Klarsfeld A., Ueda R., Rouyer F. Light activates output from evening neurons and inhibits output from morning neurons in the Drosophila circadian clock. PLoS Biol. 2007;5 doi: 10.1371/journal.pbio.0050315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bachleitner W., Kempinger L., Wulbeck C., Rieger D., Helfrich-Forster C. Moonlight shifts the endogenous clock of Drosophila melanogaster. Proc. Natl. Acad. Sci. 2007;104:3538–3543. doi: 10.1073/pnas.0606870104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kempinger L., Dittmann R., Rieger D., Helfrich-Förster C. The nocturnal activity of fruit flies exposed to artificial moonlight is partly caused by direct light effects on the activity level that bypass the endogenous clock. Chronobiol. Int. 2009;26:151–166. doi: 10.1080/07420520902747124. [DOI] [PubMed] [Google Scholar]
- 131.Schlichting M., Grebler R., Menegazzi P., Helfrich-Förster C. Twilight dominates over moonlight in adjusting Drosophila’s activity pattern. J. Biol. Rhythm. 2015;30:117–128. doi: 10.1177/0748730415575245. [DOI] [PubMed] [Google Scholar]
- 132.Vanin S., Bhutani S., Montelli S., Menegazzi P., Green E.W., Pegoraro M., Sandrelli F., Costa R. Unexpected features of Drosophila circadian behavioural rhythms under natural conditions. Nature. 2012;484:371–375. doi: 10.1038/nature10991. [DOI] [PubMed] [Google Scholar]
- 133.Lachaise D., Silvain J.-F. How two Afrotropical endemics made two cosmopolitan human commensals: the Drosophila melanogaster–D. simulans palaeogeographic riddle. Genetica. 2004;120:17–39. doi: 10.1007/978-94-007-0965-2_2. [DOI] [PubMed] [Google Scholar]
- 134.Zhu B., Zhang Q., Pan Y., Mace E.M., York B., Antoulas A.C., Dacso C.C., O’Malley B.W. A cell-autonomous mammalian 12 hr clock coordinates metabolic and stress rhythms. Cell Metab. 2017;25:1305–1319. doi: 10.1016/j.cmet.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pan Y., Ballance H., Meng H., Gonzalez N., Kim S.-M., Abdurehman L., York B., Chen X. 12-h clock regulation of genetic information flow by XBP1s. PLoS Biol. 2020;18 doi: 10.1371/journal.pbio.3000580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tomioka K., Matsumoto A. Circadian molecular clockworks in non-model insects. Curr. Opin. Insect Sci. 2015;7:58–64. doi: 10.1016/j.cois.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 137.Nose M., Tokuoka A., Bando T., Tomioka K. timeless2 plays an important role in reproduction and circadian rhythms in the cricket Gryllus bimaculatus. J. Insect Physiol. 2018;105:9–17. doi: 10.1016/j.jinsphys.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 138.Tokuoka A., Itoh T.Q., Hori S., Uryu O., Danbara Y., Nose M., Bando T., Tanimura T. Cryptochrome genes form an oscillatory loop independent of the per/tim loop in the circadian clockwork of the cricket Gryllus bimaculatus. Zool. Lett. 2017;3:1–14. doi: 10.1186/s40851-017-0066-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhu H., Sauman I., Yuan Q., Casselman A., Emery-Le M., Emery P., Reppert S.M. Cryptochromes define a novel circadian clock mechanism in monarch butterflies that may underlie sun compass navigation. PLoS Biol. 2008;6:138–155. doi: 10.1371/journal.pbio.0060004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang Y., Markert M.J., Groves S.C., Hardin P.E., Merlin C. Vertebrate-like cryptochrome 2 from monarch regulates circadian transcription via independent repression of CLOCK and BMAL1 activity. Proc. Natl. Acad. Sci. 2017;114:E7516–E7525. doi: 10.1073/pnas.1702014114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ikeno T., Numata H., Goto S.G. Molecular characterization of the circadian clock genes in the bean bug, Riptortus pedestris, and their expression patterns under long- and short-day conditions. Gene. 2008;419:56–61. doi: 10.1016/j.gene.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 142.Biscontin A., Wallach T., Sales G., Grudziecki A., Janke L., Sartori E., Bertolucci C., Mazzotta G. Functional characterization of the circadian clock in the Antarctic krill, Euphausia superba. Sci. Rep. 2017;7:1–13. doi: 10.1038/s41598-017-18009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Perrigault M., Tran D. Identification of the molecular clockwork of the oyster Crassostrea gigas. PLoS One. 2017;12:1–21. doi: 10.1371/journal.pone.0169790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wehr T.A. Bipolar mood cycles and lunar tidal cycles. Nat. Publ. Gr. 2017;23:923–931. doi: 10.1038/mp.2016.263. [DOI] [PMC free article] [PubMed] [Google Scholar]