Abstract
Metacognition refers to awareness of one’s own cognitive processes, including examining own biases and decision making. Metacognitive self (MCS), defined as accuracy in perception of own biases, is associated with pro-health behaviors and desire for feedback, including negative information.
Two studies investigated MCS in relation to emotion and hedonic capacity. First, in a longitudinal study of college students, MCS measure was stable over time, and correlated with feelings of love and joy. In the second study, MCS, mood, and hedonic capacity ratings were collected prior to evaluating stimuli for pleasure from engagement during an fMRI. Higher MCS was associated with greater hedonic capacity and increased signal in cortical areas involved in self-reflection and decision making.
Our findings implicate self-awareness of biases as a cognitive process supporting positive emotional state and hedonic capacity. Future studies should explore how MCS relates to changes in mood and vulnerability to mood disorders.
Keywords: metacognition, self-awareness, bias, emotion, anhedonia, temporoparietal junction
1. Introduction
Metacognition is usually described as knowledge about one’s own cognition and the use of this knowledge to regulate cognitive processes (Weinert & Kluwe, 1987). As interest in metacognitive concepts developed (Dewey, 1933; Flavell, 1979), measures of specific metacognitive abilities followed. Researchers developed questionnaires of metacognitive awareness in general (the Metacognitive Awareness Inventory (Schraw & Dennison, 1994)) and clinical psychologists focused on dysfunctional metacognition that fosters rumination. The Metacognitive Questionnaire (MCQ-30) was created (Cartwright-Hatton & Wells, 1997) to measure metacognitive beliefs and their relationship to emotional dysfunction. Wells and Matthews (Wells & Matthews, 1996) showed that dysfunctional beliefs are at the core of formation of psychic disturbances such as nervousness, anxiety attacks, obsessive-compulsive symptoms, hallucinations, anorexia, and psychosis. In contrast, social psychologists have focused on adaptive metacognition, which is important for self-regulatory functions (DeMarree et al., 2012). Beer and Moneta (2010) identified several adaptive metacognitive beliefs in highly self- regulated and resilient individuals that foster success amid a challenge. Adaptive individuals showed awareness of the need to free attention resources and experience positive emotions, confidence in interpreting emotions as cues that help solve the problem, and confidence in setting a sequence of goals to reach long-term success. A measure of adaptive metacognition, the Positive Metacognition and Positive Meta-Emotions Questionnaire (PMCEQ) (Beer & Moneta, 2010) was developed to assess the degree to which individuals manifest these behaviors. Another measure of adaptive metacognition, The Integrative Self-Knowledge Scale (ISK) (Ghorbani, Watson, & Hargis, 2008), has been shown to be associated with enhanced mood and well-being as well as decreases in anxiety, depression, and various stress indicators as the result of metacognitive beliefs, such as anchoring in present good mood (Ghorbani et al., 2008; Ghorbani, Watson, Salimian, & Chen, 2013). Another questionnaire that measures adaptive metacognition, specifically self -awareness of biased thinking, is the Metacognitive Self Questionnaire (MCSQ-21) (Brycz, Konarski, Kleka, Wright, 2019), the construct and measure used in this manuscript.
The recognition that humans have a tendency to make decisions based on biased cognitions is an important aspect of self-knowledge. Biases are frequent rules of thinking, mostly anchored in heuristics (Nisbett & Ross, 1980), and include fallacies such as illusory correlation or confirmation bias. The common tendency towards “confirmatory bias” (Heider, 1958) is understood as a tendency to process information in a way that confirms preexisting beliefs, resulting in incorrect statistical generalizations. Work by Kahneman and Tversky (Tversky & Kahneman, 1974), and the wealth of research that followed, demonstrated that all humans succumb to these biases. Numerous other biases have also been identified (e.g. positivity bias, reciprocity rule, etc.) and are considered common psychological tendencies (Taylor & Brown, 1988). Thus, the more an individual admits he/she is biased in his/her thinking, the more accurate is his/her metacognition. An individual who wants to assess whether a given bias impacts her/his behavior will look at the self reasonably and sincerely, focusing on internal states like analysis of own behavior, feelings, phenomenological experiences and the environmental forces that impact thoughts and feelings. Metacognitive awareness is enhanced by a piecemeal, reflective style of thinking as well as diagnostic information about self (Brycz, Wyszomirska- Góra, Konarski, & Wojciszke, 2018).
The present study further explores this self-awareness of one’s own biases, which is at the core of the construct of metacognitive self (MCS). To measure a level of this construct, the Metacognitive Self Questionnaires MCSQ-40 and MCSQ-21 were developed and used to assess strength of self-knowledge about one’s own vulnerability to common cognitive biases (Brycz & Karasiewicz, 2011; Brycz, Konarski, Kleka, & Wright, 2019). To date, our research has demonstrated that high MCS is associated with more deliberate, reflective reasoning compared to low MCS individuals. Nevertheless, biased thinking persists despite self-awareness (Bar-Tal, Brycz, Dolinska, & Dolinski, 2017). Furthermore, high MCS individuals are more often motivated to use self-diagnostic information, which enhances their self-knowledge and desire for psychological self-improvement (Brycz, Wyszomirska-Góra, Bar-Tal & Wisniewski, 2014). These individuals are also willing to receive feedback about themselves even when the information is negative. Finally, high MCS individuals possess higher intrinsic motivation to work under conditions of overload, have a greater need for achievement, and endorse higher levels of values such as self-directedness and achievement compared to low MCS individuals (Brycz, Karasiewicz, & Klimaszewska, 2014). In a recent study, we demonstrated that high MCS individuals are more conscientious, agreeable, and emotionally stable compared to low MCS individuals (Brycz et al., 2019). In the same study, we tested the MCSQ-21 construct against the established maladaptive metacognition measure, the MCQ-30 (Wells & Cartwright-Hatton, 2004), using a nationwide Polish sample and observed a negative correlation. Two measures of adaptive metacognition were also included, and a positive correlation was found between the MCSQ21 and ISK (Ghorbani et al., 2008) as well as between the MCSQ21 and the PMCEQ (Beer & Moneta, 2010). Thus, we concluded that MCS is associated with indicators of psychological well-being and proactive behavior.
An important question for mental health researchers is understanding how cognitive factors may boost wellbeing and protect against prevalent mood disorders such as depression (Almeida, MacLeod et al. 2014). One of the two core symptoms of depression is anhedonia, broadly defined as loss of interest of pleasure. Anhedonia is associated with worse health outcomes, but treating anhedonia improves positive affect and wellbeing (Craske, Meuret et al. 2019). Anhedonia is also associated with lower positive bias when deciding about ambiguous stimuli (Pizzagalli, Jahn et al. 2005). Given the relevance of anhedonia to wellbeing and positive bias, we wanted to explore the relationship between anhedonia and MCS. We previously reported that decision making about one’s own preferences of pleasure from engagement is associated with less anhedonia and activates pleasure-related neural networks (Szczepanik, Reed et al. 2019). The goal of this study was to explore whether the metacognitive ability of recognizing own biases supports the process of selecting hedonic preferences.
Here, we present two studies examining the association between MCS levels and positive functioning. In the first study, we focused on MCS and emotional state to establish whether the two constructs are stable over time and examined the relationship between positive and negative mood and MCS. We assessed the emotional state of the participant via self-ratings of how they felt in the recent week along with measures of the metacognitive self-MCS. The ratings were obtained five times, six months apart. In the second study, we measured metacognitive strength via MCS and hedonic capacity (using anticipatory and consummatory anhedonia scales) and explored how high versus low MCS individuals engaged in an evaluation of potential pleasure from engagement in various common activities using an fMRI task. The task involved rating various stimuli presented as pictures or words of activities for potential pleasure, and the BOLD signal was collected for each individual decision-making interval when the decision was positive. The task was highly subjective as there were no pre-selected categories of what is pleasurable or not, thus eliciting idiosyncratic maps of hedonic process. We hypothesized that the individuals who have better insight into their own cognitive biases (high MCS scores) will show greater stability of positive emotions (in Study 1) as well as greater hedonic capacity (in Study 2). In the latter study, we wanted to explore how MCS impacts neural processes of hedonic decision making by possibly engaging the self-referential processing network identified within the regions comprising the default mode, especially the medial prefrontal cortex (Johnson et al., 2002) (Buckner, Andrews-Hanna et al. 2008) during decision-making of own preferences for pleasure.
2. Methods: Study 1 - Longitudinal assessment of metacognitive self and emotional state
2.1. Participants
The participants were undergraduate students recruited from the Humanities and Social Sciences departments of the University of Gdansk, Poland. All participants provided written informed consent to take part in research, which was approved by the National Classified Board and its division, the Personal Data Protection Council at the University of Gdansk. Assessments were carried out every six months over the three-year study period, for a total of five study waves. Four hundred fifteen students were initially enrolled, and 349 completed all five assessments. One participant who only completed one assessment was removed from the analysis, but all participants with two or more assessments were retained. The age of the students varied across all waves of the study, ranging from 19 to 25 (M = 20.95, Md = 21, SD = 1.15).
2.2. Study Procedures and Materials
Assessments took place at the end of academic semesters, before end-of-semester examinations. Students participated individually or in groups of up to 30 people. Metacognitive strength was measured using MCSQ-21, and the Emotion Scale (Wojciszke, Baryła, 2005) was used to assess emotional experience. The metacognitive strength construct was measured using the MCSQ-21. Each item of the MCSQ-21 describes a situation reflecting one of the common biases, such as positivity bias (Weinstein, 1980) ‘I tend to judge other people positively rather than negatively’. Participants used a six-point Likert scale (from 1 ‘totally disagree’ to 6 ‘totally agree’), to indicate the extent to which they believed each behavior applied to them. While there were no explicit questions about how much one was aware of biased thinking, previous research confirmed that asking about agreement with a first-person perspective description of bias does indeed measure bias awareness (Beer and Moneta 2010). The Emotion Scale measures two positive emotions (love, joy) and four negative emotions (anger, sadness, shame, and hate). Participants assessed experiencing each emotion over the last week, described by five adjectives, on a seven-point Likert scale from 1 (never) to 7 (always). Demographic data were collected at each time point. Students were thanked for their participation at each wave of the study and did not receive financial compensation for participation.
2.3. Statistical Analysis
In order to investigate the relationship between MCS and emotion at five time points, a Bayesian analysis of correlation between two variables was carried out (Ly, Verhagen, & Wagenmakers, 2016). We chose this method because it allowed us to calculate a single value for the correlation coefficient for repeated measurements. The classic method of determining the value of the correlation coefficient would require the calculation of separate values for each measurement time point. Bayesian analysis allows the correlation value from measurement "i-1" to be used as a prior when calculating the coefficient from measurement “i” (Cocks et al., 2016). This provides a single piece of information about the relationship between MCS level and the intensity of the emotions, which simplifies the interpretation of results. An additional advantage of Bayesian analysis is the ability to estimate the magnitude of the effect expressed as probability, which facilitates the interpretation of results, especially in large samples where trivial correlations can be statistically significant only due to the sample size.
2.4. Results
A high degree of stability was found between emotion measurements. The average measure ICC3k for love was .881 with a 95% confidence interval from .864 to .897 (F(507, 2028) = 8.41, p < .001); for joy it was .860 with a 95% confidence interval from .840 to .879 (F(507, 2028) = 7.166, p < .001); for sadness it was .891 with a 95% confidence interval from .875 to .905 (F(507, 2028) = 9.139, p < .001); for shame it was .888 with a 95% confidence interval from .872 to .902 (F(507, 2028) = 8.91, p < .001); for fear it was .873 with a 95% confidence interval from .855 to .890 (F(507, 2028) = 7.90, p < .001); and for anger it was .857 with a 95% confidence interval from .837 to .876 (F(507, 2028) = 7.0, p < .001).
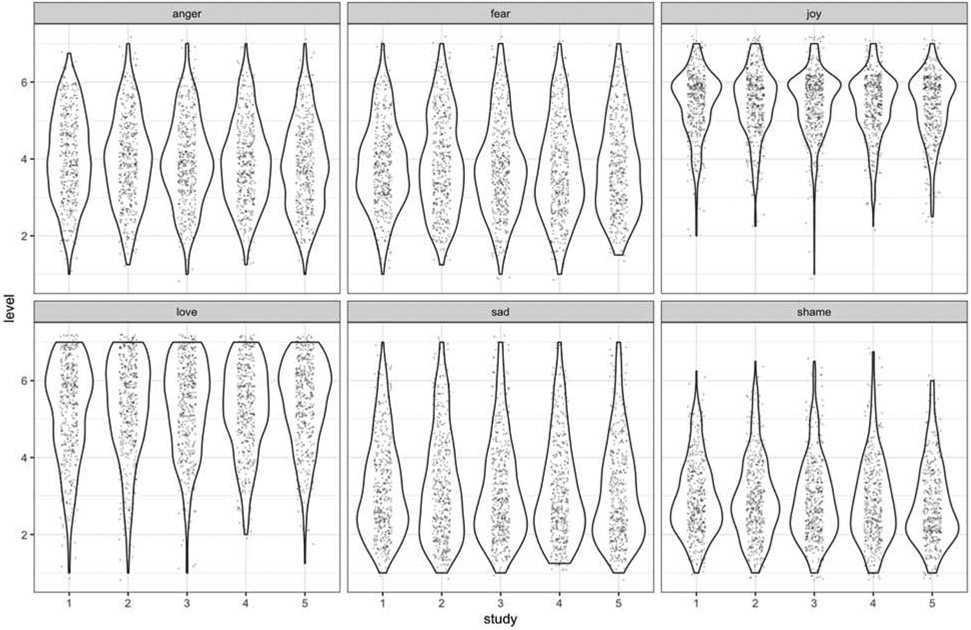
In the analysis correlating emotion expression with MCS, we observed that MCS correlated with the positive emotions love (r = .22, CI.95 [.179, .260]) and joy (r = .19, CI.95 [.151, .233]. No correlation was observed between MCS and negative emotions (which varied from -.107 to .035) (Table 2). We note that the Bayes factor in favor of H1 was above 2.25e+21 for love and above 7.39e+15 for joy, meaning that the data were 2.25e+21 and 7.39e+15 times, respectively, more likely under H1 than under H0. For negative emotions, the Bayes factor in favor of HO was above 34 for fear (BF01 = 34.4) and BF01 = 4.41 for sadness. Scores for other the other negative emotions—anger and shame—were inconclusive (see Fig. 2); under other assumptions, the result may moderately support the hypothesis that a very weak, negative relationship exists between MCS and anger.
Table 2.
Results of Bayesian Correlation Pairs – MCS and emotions
| Bayesian Pearson Correlation | 95% Credible interval |
|||||
|---|---|---|---|---|---|---|
| r | BF10 | BF01 | Lower | Upper | p value | |
| Love | .220 | 2.252e+21 | <.001 | 0.179 | 0.260 | <.001 |
| Joy | .192 | 7.399e +15 | <.001 | 0.151 | 0.233 | <.001 |
| Sadness | −.045 | .227 | 4.411 | −0.087 | −0.002 | .039 |
| Fear | −.008 | .029 | 34.622 | −0.050 | 0.035 | .725 |
| Anger | −.064 | 2.280 | 0.439 | −0.107 | −0.022 | .002 |
| Shame | −.051 | .412 | 2.430 | −0.093 | −0.008 | .020 |
Fig. 2.
Prior and posterior distributions of correlations between MCS and emotions: A – Love, B – Joy, C – Sadness, D – Fear, E - Anger, F – Shame. Pie charts shows the probability ratio of H1 and H0 for the observed data; whiskers over the graphs show 95% credible interval over correlation coefficients. The dotted line is the prior distribution, and the solid line is the posterior distribution. The data reduced the uncertainty about the true value of the correlation (i.e., the posterior is more narrow than the prior), and the posterior peaks near zero.
3. Methods: Study 2 - Relationship between MCS and anhedonia
3.1. Participants
Thirty-one healthy volunteer participants (15 males, 16 females; ages 18-65) were recruited via community advertising and internet advertising including the ClinicalTrials.gov website (NCT00397111). The study was approved by the Combined Neuroscience Institutional Review Board of the National Institutes of Health (NIH), and all participants gave written informed consent prior to participation.
All participants were healthy as determined by a thorough evaluation including a medical and psychiatric history, negative SCID-NP (First et al. 2002), laboratory testing, drug screening, and physical examination. Additional inclusion criteria were related to MRI safety (no contraindications) and English language fluency. Thirty-one individuals signed informed consent forms and were enrolled in the study. One participant was subsequently excluded due to anxiety related to MRI scanning and one because of technical difficulties with the equipment. Of the 29 participants who completed the scans, one was excluded from the imaging analysis due to excessive head movement, but their performance data were included in the behavioral analysis. The final sample comprised 14 males and 15 females (average age: 32.44 years (SD=10.32); average education: 16.96 years (SD=2.24).
3.2. Study procedures and materials
Each study session included self-rating measures, experimental task training, and scanning, and lasted about three hours. The participants were administered the MSCQ-21, which was translated to English and then proofread by native speakers of American English. In addition, participants completed self-report scales to assess depressive, anxiety, and anhedonic symptoms, including the Beck Depression Inventory (BDI-II) (Beck & Beamesderfer, 1974), the State-Trait Anxiety Inventory (STAI-Y1, Y2) (Spielberger et al. 1983), the Snaith-Hamilton Pleasure Scale (SHAPS) (Snaith et al., 1995), and the Temporal Experience of Pleasure Scale (TEPS) (Gard, Gard, Kring, & John, 2006). Prior to scanning, participants had an opportunity to practice the tasks and to ask questions. The fMRI portion of the study was conducted on a 3T scanner (see below).
Task description
A detailed description of the task has been reported elsewhere (Szczepanik et al., 2019). Briefly, during scanning, participants performed a decision-making task requiring them to rate stimuli representing activities that people may engage in on a 5-point Likert scale. The activities were represented by either pictures (walking on the beach, people bowling) of activities or words describing activities (hiking, bowling). A wide variety of activities was presented, and no assumptions were made regarding what an individual might find potentially pleasurable, allowing for a highly individualized rating selection; that is, bowling might be a favorite pastime for one person but not at all appealing to someone else, and the ratings would reflect that preference. Three runs of the task were administered. Each run included 40 word presentations, 40 picture presentations, eight control (pixelated and scrambled) images, and eight scrambled words. All stimuli were presented randomly within runs, and the order of the runs was also randomized. Between stimulus presentations, participants were instructed to focus on the fixation cross that appeared on the screen for an average of 2000 ms (jittered between 1500 and 3500ms).
Imaging protocol
Images were collected on a 3 Tesla General Electric scanner (GE Signa, Milwaukee, WI) with an eight-channel, phased-array head coil. An echo-planar imaging (EPI) sequence was used to measure blood-oxygen-level dependent (BOLD) signal with axial acquisition (echo time (TE)=30ms; repetition time (TR)= 2000ms; 35 3.5mm axial slices=35; 90 degree flip angle, inplane resolution = 3.75mm*3.75mm; 238 timepoints per run). Five volumes were discarded from the beginning of each of the three runs. A magnetization prepared rapid gradient echo (MPRAGE) structural sequence was used for co-registration.
3.3. Statistical analysis
Behavioral analysis
The primary goal of this study was to measure a relationship between a cognitive variable (MCS) and mood and anhedonia measures via correlation coefficient analyses (Pearson’s r). Significance was set at p < 0.05, bidirectional.
Imaging analysis
The images were pre-processed and analyzed using AFNI (Cox, 1996). Briefly, BOLD images were aligned to a high resolution structural scan data with an affine transform using the AFNI LPCcost function, slice timing corrected, spike corrected, smoothed to 6 mm full width at half max, and normalized to the mean. Regressors were created for each task component based on timing and type of button press to indicate decision about an activity for all pictures and word stimuli rated as liked. Each stimulus event was modeled as a 3.5-second block from stimulus onset, equal to the time the stimulus remained on the screen, regardless of how quickly the participant responded. A fixation cross was presented during the inter-stimulus interval, with duration randomized between 1500 and 3500 ms, and was included as baseline in the imaging analysis. Timepoints with participant motion greater than 0.3 mm were discarded, as were timepoints with outlying values, and participants were excluded from the analysis if more than 15% of volumes were censored. For the exploratory purpose of evaluating brain activity underlying the processing of hedonic value of activities in relation to MCS, we sought to determine regions where BOLD activity during decisions where a picture or a word, in separate analyses for each modality, represented an individually preferred (liked) activity was associated with MCS using the AFNI 3dttest. For exploratory purposes, we set the voxelwise threshold at p<0.01 and applied a family wise error (FWE) correction for clusters using the ACF method within AFNI’s 3dClustSim. We also conducted an analysis using p<0.001 voxelwise threshold.
3.4. Results
Demographics
As expected given our eligibility criteria, the participants had very low levels of depression (BDI-II; M=0.65, SD=1.04), anxiety (STAI-Y1=24.96, SD=4.28; STAI-Y2=25.07, SD=4.24), and anhedonia. The mean scores for the anhedonia scales were 4.44 (SD=0.69) for the TEPS Anticipatory Anhedonia scale, 4.68 (SD=0.85) for the TEPS Consummatory Anhedonia scale, and 18.54 (SD=4.53) for the SHAPS.
Behavioral Results
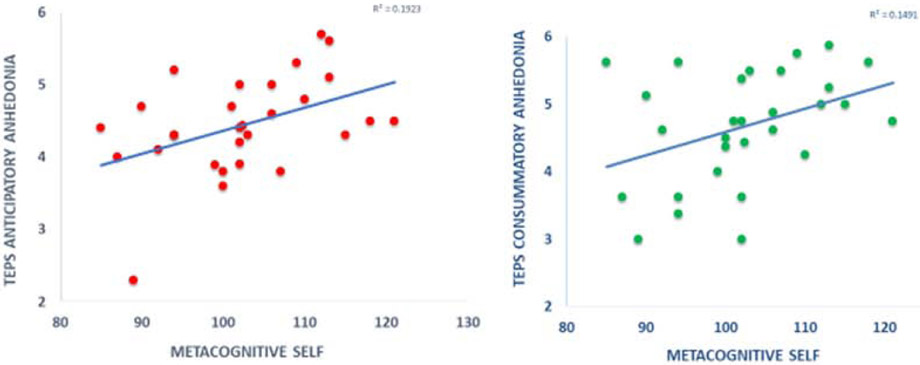
MCS level correlated positively with measures of hedonic capacity: TEPS Anticipatory Anhedonia (N=28, r=0.438, p=0.02), TEPS Consummatory Anhedonia (N=28, r=0.387, p=0.042), and at a trend level with SHAPS score, a measure of hedonic potential (N=28, r=−0.349, p=0.069). The nature of this relationship was consistent given that higher TEPS scores imply greater hedonic potential and higher SHAPS scores indicate greater anhedonia.
Imaging Results
MCQ score was associated with ‘liking’ decisions about activities presented both as pictures and as words. For pictures, three clusters were identified: left cerebellum, 47 voxels, −19.2,−60,−11.0 p <0.02, left precentral gyrus, 44 voxels, −29.8, −29. +62.5, p <0.02, and right postcentral gyrus, 37 voxels, +50.8, −32.5, +55.5, p<0.05. For words, the clusters were found in left middle frontal gyrus, 68 voxels, −29.8, +20.0, +34.5, p<0.01, right supramarginal gyrus, 51 voxels, +61.2, −22.0, +41.5, p<0.01, and in left middle temporal gyrus extending to the temporoparietal junction, 49 voxels, −43.8, −50.0, +17.0, p<0.01. P values are reported for 0.01 voxelwise threshold, FWE corrected. The cerebellar cluster and the left middle temporal gyrus cluster survived voxelwise threshold of p<0.001 with the FWE corrected values of p<0.01.
4. Discussion
Cognitive and emotional processes are naturally intertwined as humans interact with their own thoughts and feelings, with others, and while decisions about the environment are made. We observed that the particular metacognitive ability—that is, insight into one’s own common biases—was related to the way emotions are experienced and decisions of hedonic valence are made. In our student sample, those scoring higher in MCS, hence more aware of their biases, consistently endorsed more positive emotions. Among the emotions we assessed, love, joy, sadness, and shame were all remarkably stable over the five time points (2.5 years) despite possible developmental and environmental factors of emerging adulthood. We observed more variability in feelings of fear, but no significant differences between any timepoints, and a small but significant change in anger between the first and fifth sessions, suggesting that these two emotions may be more state-dependent in our population of undergraduate students. We also established that MCS is consistent over time and is associated with feelings of love and joy but has no association with sadness, fear, or anger. This finding is consistent with our hypothesis and supports the adaptive role of insight in healthy individuals. Why this is the case should be investigated further; it is possible that accepting one’s own vulnerability to error and ability to accept the negative feedback bound to be encountered during college years may reflect greater psychological wellbeing (Stamp et al., 2015), thus allowing individuals to experience a more positive affect.
Our second study allowed us to further examine the relationship between high awareness of biases and hedonic tone. We found significant positive correlations between MCS and hedonic capacity, the ability to anticipate and enjoy pleasurable events, and negative correlations between MCS and anhedonia. This latter relationship is of particular interest and potential significance for clinical research on anhedonia, a common symptom in mood, addiction, and psychotic disorders. While metacognition has been investigated in major depressive disorder, as have factors related to cognitive-behavioral approaches to treatment (Wells et al., 2012), MCS has not yet been studied in clinical populations. Because pro-health and positive behavior and emotions are associated with high MCS individuals in the general population, greater self-knowledge of one’s biases and being receptive to feedback could imply openness to experiences and ease in perceiving potential for pleasure consistent with one’s own preferences. Our exploratory study of neural correlates of hedonic decision making and MCS suggests that signal in several cortical areas supportive of higher order cognitive processes was positively associated with higher MCS. Notably, we observed a cluster with the voxels in the temporoparietal junction, an area consistently implicated in social cognition, including empathy, perspective taking, and pro-social behaviors (Frith & Frith, 2006; Kestemont, Vandekerckhove, Ma, Van Hoeck, & Van Overwalle, 2013; Morishima, Schunk, Bruhin, Ruff, & Fehr, 2012; Saxe & Kanwisher, 2003). Other regions where BOLD signal during hedonic valence decision making was associated with MCS scores have been related to movement and action imitation processing, embodied cognition and emotion, imagery, and reward valuation (Delgado, Gillis, & Phelps, 2008; Guillot et al., 2008; Lamm, Fischer, & Decety, 2007, Saxbe, Yang, Borofsky, & Immordino-Yang, 2013). Thus, we found evidence that during hedonic decision-making, metacognitive self is linked with the recruitment of neural resources to perform the task. Although somewhat speculative, we surmise that the evaluation of potential pleasure, which requires identifying the stimulus, relating the given activity to one’s own experiences and rating its value, binds elements of self, pleasure, and decision-making, and this process may be enhanced by the capability for metacognitive insight. Given the behavioral result of higher hedonic capacity associated with MCS, the BOLD signal correlates may indicate that insight into one’s own biases supports the processing of pleasure.
We are aware of several limitation of our studies. First, the samples in studies one and two had noticeable differences. The large sample of Polish undergraduate students allowed for a longitudinal analysis of the relationship between MCS and emotions, while the small, homogeneous sample of healthy American adults across a much wider age range allowed us to explore potential biobehavioral correlates of MCS during hedonic decision-making assessed with fMRI. Second, the longitudinal study correlations observed between MCS and positive emotions were at the r=.2 level, which has to be considered a weak correlation, possibly due to the heterogeneity of that sample. Nevertheless, we are confident in reporting this relationship because the effect was stable across multiple measures for positive emotions only. Third, the MCSQ administered in English was translated but not otherwise adapted for an American population, which should be considered for future studies. Given the universal nature of biases, however, and no reported problems with understanding or responding to questions, we consider our results to reflect the same construct as the original instrument. Finally, the neuroimaging study was not prospectively designed to elucidate the neural correlates of MCS, which limits any interpretation of the neurobiology of the construct itself.
In conclusion, cognitive processes including metacognition about biases interact with individual experiences of emotion and enjoyment. Greater insight into one’s own common psychological biases is associated with more positive feelings and ability to enjoy environmental reinforcers. Future research on metacognition of biases should further characterize this construct as a personal quality with distinct behavioral and neural characteristics. Given the findings of associations between positive emotional states and pro-health behaviors, MCS could be of interest as a protective factor for mental health. If modifiable, this cognitive ability could be potentially fostered for mental health benefit, and as such could be evaluated for inclusion in cognitive therapeutic approaches.
Fig. 1.
Violin plots representing emotions x study timepoint. Each dot represents a personal score. The width of each violin shape represents density of a specific emotion at each time point.
Fig. 3.
Correlation of MCS with anticipatory and consummatory anhedonia (TEPS). For both types of anhedonia, greater ability to enjoy was correlated with higher awareness of biases.
Fig. 4.
Brain regions active while evaluating stimuli for pleasure from engagement associated with metacognitive strength. A. While viewing pictures, clusters were observed in the left cerebellum, left precentral, and right postcentral gyrus. B. During word processing, clusters were identified in the left middle frontal gyrus, right supramarginal gyrus, and the left middle temporal gyrus extending to the temporoparietal junction.
Table 1.
Number of participants in all five waves of the study
| Wave | Number enrolled |
From first wave |
|---|---|---|
| 1 | 415 | - |
| 2 | 428 | 366 |
| 3 | 439 | 348 |
| 4 | 417 | 339 |
| 5 | 430 | 349 |
Highlights:
Awareness of own common cognitive biases is defined as ‘metacognitive self’ and is a distinct metacognitive ability.
Stronger metacognitive self is associated with experience of positive emotions, such as love and joy.
Ability to experience pleasure is also positively correlated with greater awareness of biases.
During decision making about pleasant experiences, higher metacognition is associated with greater recruitment of cortical activity in temporoparietal junction and other areas involved in both self-referential and empathetic processing.
Self awareness of biases may be a metacognitive factor important for psychological well-being.
Acknowledgments
Funding: Funding for this work was supported in part by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH; ZIA MH002927) to Drs. Szczepanik, Nugent, and Zarate; by a NARSAD Independent Investigator Award to Dr. Zarate; and by a Brain and Behavior Mood Disorders Research Award to Dr. Zarate. This work was also supported by a National Science Centre (Narodowe Centrum Nauki), Poland grant 2013/11/B/HS6/01463 awarded to Dr. Brycz.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: Dr. Zarate is listed as a co-inventor on a patent for the use of ketamine in major depression and suicidal ideation; as a co-inventor on a patent for the use of (2R,6R)-hydroxynorketamine, (S)-dehydronorketamine, and other stereoisomeric dehydro and hydroxylated metabolites of (R,S)-ketamine metabolites in the treatment of depression and neuropathic pain; and as a co-inventor on a patent application for the use of (2R,6R)-hydroxynorketamine and (2S,6S)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation, and post-traumatic stress disorders. He has assigned his patent rights to the U.S. government but will share a percentage of any royalties that may be received by the government. Drs Szczepanik, Brycz, Kleka, Fanslau and Nugent have no conflict of interest to disclose, financial or otherwise.
Ethical Approval: All procedures performed in studies involving human participants were conducted in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent (USA): The study was approved by the Institutional Review Board of the National Institute of Mental Health, National Institutes of Health (NIMH-NIH), and all participants gave written informed consent for participation. The study was conducted under the trial registered at ClinicalTrials.gov (NCT00397111).
Informed Consent (Poland): The study was approved by the Institutional Review Board of the University of Gdansk, Poland. All participants provided written informed consent which was approved by the National Classified Board and its division, the Personal Data Protection Council at the University of Gdansk, Poland.
References
- Almeida OP, MacLeod C, Ford A, Grafton B, Hirani V, Glance D and Holmes E (2014). "Cognitive bias modification to prevent depression (COPE): study protocol for a randomised controlled trial." Trials 15: 282–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Tal Y, Brycz H, Dolinska B, & Dolinski D (2017). When saying that you are biased means that you are acurate? The moderating effect of cognitive structuring on relationship between metacognitive self and confirmation bias use. Current Psychology, 1–7. [Google Scholar]
- Beck AT, & Beamesderfer A (1974). Assessment of depression: the depression inventory. ModProbl Pharmacopsychiatry, 7(0), 151–169. [DOI] [PubMed] [Google Scholar]
- Beer N, & Moneta GB (2010). Construct and concurrent validity of the Positive Metacognitions and Positive Meta-Emotions Questionnaire. Personality and Individual Differences, 49(8), 977–982. [Google Scholar]
- Brycz H, & Karasiewicz K (2011). Metacognition and Self-Regulation: The Metacognitive Self Scale. Acta Neuropsychologica, 9(3), 263–281. [Google Scholar]
- Brycz H, Karasiewicz K, & Klimaszewska J (2014). Wspolzaleznosc Metapoznawczego Ja i Wybranych Aspektow Funkcjonowania Poznawczego [Interdependence of metacognitive self and selected aspects of cognitive functioning]. Polish Psychological Bulletin, 19, 421–428. [Google Scholar]
- Brycz H, Konarski R, Kleka P, & Wright R (2019). The Metacognitive Self: The Role of Motivation and an Updated Measurement Tool. Economics & Sociology, 12(1), 208–232. [Google Scholar]
- Brycz H, Wyszomirska-Góra M, Konarski R, & Wojciszke B (2018). The metacognitive selffosters the drive for self-knowledge: The role of the metacognitive self in the motivation to search for diagnostic information about the self.
- Buckner RL, Andrews-Hanna JR and Schacter DL (2008). The brain's default network: Anatomy, function, and relevance to disease The year in cognitive neuroscience 2008. Malden, Blackwell Publishing: 1–38. [DOI] [PubMed] [Google Scholar]
- Cartwright-Hatton S, & Wells A (1997). Beliefs about worry and intrusions: the Meta-Cognitions Questionnaire and its correlates. J Anxiety Disord, 11(3), 279–296. [DOI] [PubMed] [Google Scholar]
- Chapman LJ (1967). Illusory correlation in observational report. Journal of Verbal Learning and Verbal Behavior, 6(1), 151–155. [Google Scholar]
- Cocks M, Shaw CS, Shepherd SO, Fisher JP, Ranasinghe A, Barker TA, &. Craske MG, Meuret AE, Ritz T, Treanor M, Dour H and Rosenfield D (2019). "Positive affect treatment for depression and anxiety: A randomized clinical trial for a core feature of anhedonia." Journal of Consulting and Clinical Psychology 87(5): 457–471. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Gillis MM, & Phelps EA (2008). Regulating the expectation of reward via cognitive strategies. Nature neuroscience, 11(8), 880–881. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3077128/. doi: 10.1038/nn.2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMarree KG, Loersch C, Brinol P, Petty RE, Payne BK, & Rucker DD (2012). From Primed Construct to Motivated Behavior: Validation Processes in Goal Pursuit. Personality and Social Psychology Bulletin, 38(12), 1659–1670. [DOI] [PubMed] [Google Scholar]
- Dewey J (1933). How We Think: A Restatement of the Relation of Reflective Thinking to the Educative Process. Boston, MA: D.C. Heath & Co Publishers. [Google Scholar]
- Efklides A, & Vlachopoulos SP (2012). Measurement of metacognitive knowledge of self, task, and strategies in mathematics. European Journal of Psychological Assessment, 28(3), 227–239. [Google Scholar]
- First MB, Spitzer RL, M. G, & Williams JB (2002). Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P). New York, New York State Psychiatric Institute: Biometrics Research. [Google Scholar]
- Flavell JH (1979). Meta-Cognition and Cognitive Monitoring - New Area of Cognitive-Developmental Inquiry. American Psychologist, 34(10), 906–911. [Google Scholar]
- Frith CD, & Frith U (2006). The neural basis of mentalizing. Neuron, 50(4), 531–534. [DOI] [PubMed] [Google Scholar]
- Gard DE, Gard MG, Kring AM, & John OP (2006). Anticipatory and consummatory components of the experience of pleasure: A scale development study. Journal of Research in Personality, 40(6), 1086–1102. [Google Scholar]
- Ghorbani N, Watson PJ, & Hargis MB (2008). Integrative Self-Knowledge Scale: correlations and incremental validity of a cross-cultural measure developed in Iran and the United States. J Psychol, 142(4), 395–412. [DOI] [PubMed] [Google Scholar]
- Ghorbani N, Watson PJ, Salimian M, & Chen Z (2013). Shame and Guilt: Relationships of Test of Self-Conscious Affect Measures With Psychological Adjustment and Gender Differences in Iran. Interpersona: An International Journal on Personal Relationships, 7(1), 13. [Google Scholar]
- Guillot A, Collet C, Nguyen VA, Malouin F, Richards C, & Doyon J (2008). Functional neuroanatomical networks associated with expertise in motor imagery. Neuroimage, 41(4), 1471–1483. [DOI] [PubMed] [Google Scholar]
- Kestemont J, Vandekerckhove M, Ma N, Van Hoeck N, & Van Overwalle F (2013). Situation and person attributions under spontaneous and intentional instructions: an fMRI study. Soc Cogn Affect Neurosci, 8(5), 481–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Fischer MH, & Decety J (2007). Predicting the actions of others taps into one's own somatosensory representations--a functional MRI study. Neuropsychologia, 45(11), 2480–2491. [DOI] [PubMed] [Google Scholar]
- Morishima Y, Schunk D, Bruhin A, Ruff CC, & Fehr E (2012). Linking brain structure and activation in temporoparietal junction to explain the neurobiology of human altruism. Neuron, 75(1), 73–79. [DOI] [PubMed] [Google Scholar]
- Nisbett RE, & Borgida E (1975). Attribution and the psychology of prediction. Journal of Personality and Social Psychology, 32(5), 932–943. doi: 10.1037/0022-3514.32.5.932 [DOI] [Google Scholar]
- Nisbett RE, & Ross L (1980). Human Inference: Strategies and Shortcomings of Social Judgment: Prentice-Hall. [Google Scholar]
- Pizzagalli DA, Jahn AL and O’Shea JP (2005). "Toward an objective characterization of an anhedonic phenotype: A signal-detection approach." Biological Psychiatry 57(4): 319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxbe DE, Yang XF, Borofsky LA, & Immordino-Yang MH (2013). The embodiment of emotion: language use during the feeling of social emotions predicts cortical somatosensory activity. Soc Cogn Affect Neurosci, 8(7), 806–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe R, & Kanwisher N (2003). People thinking about thinking people. The role of the temporo-parietal junction in "theory of mind". Neuroimage, 19(4), 1835–1842. [DOI] [PubMed] [Google Scholar]
- Schraw G, & Dennison RS (1994). Assessing Metacognitive Awareness. Contemporary Educational Psychology, 19(4), 460–475. [Google Scholar]
- Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, & Trigwell P (1995). A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. British Journal of Psychiatry, 167(1), 99–103. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, & Jacobs GA (1983). Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press.. [Google Scholar]
- Stamp E, Crust L, Swann C, Perry J, Clough P, & Marchant D (2015). Relationships between mental toughness and psychological wellbeing in undergraduate students. Personality and Individual Differences, 75, 170–174. [Google Scholar]
- Szczepanik JE, Reed JL, Nugent AC, Ballard ED, Evans JW, Lejuez CW, & Zarate CA Jr. (2019). Mapping anticipatory anhedonia: an fMRI study. Brain Imaging Behav. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tailarach JT, P. (1988). Co-Planar Stereotaxic Atlas of the Human Brain: 3-D Proportional System: An Approach to Cerebral Imaging. New York. [Google Scholar]
- Taylor SE, & Brown JD (1988). Illusion and well-being: A social psychological perspective on mental health [American Psychological Association; doi: 10.1037/0033-2909.103.2.193]. [DOI] [PubMed] [Google Scholar]
- Tversky A, & Kahneman D (1974). Judgment under Uncertainty: Heuristics and Biases. Science, 185(4157), 1124–1131. [DOI] [PubMed] [Google Scholar]
- Wagenmakers E-J, Verhagen J, & Ly A (2016). How to quantify the evidence for the absence of a correlation. Behavior Research Methods, 48(2), 413–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinert FE, & Kluwe R (1987). Metacognition, motivation, and understanding. Hillsdale, N.J.: L. Erlbaum Associates. [Google Scholar]
- Weinstein ND (1980). Unrealistic optimism about future life events. Journal of Personality and Social Psychology, 39(5), 806–820. [Google Scholar]
- Wells A, & Cartwright-Hatton S (2004). A short form of the metacognitions questionnaire: properties of the MCQ-30. Behav Res Ther, 42(4), 385–396. [DOI] [PubMed] [Google Scholar]
- Wells A, & Matthews G (1996). Modelling cognition in emotional disorder: the S-REF model. Behav Res Ther, 34(11-12), 881–888. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/8990539. [DOI] [PubMed] [Google Scholar]