Abstract
Objective
We aimed to investigate whether apatinib has an inhibitory effect on the invasion and metastasis of liver cancer in vitro.
Methods
The anti-invasion and antimetastasis effects of apatinib in HepG2, Hep3B,Huh7 and SMMC-7721 liver cancer cell lines were tested by the wound-healing and transwell invasion assays. Real-time PCR and Western blot were used to detect the influence of apatinib on the gene expression of MMPs, TIMPs, and constituents of the NF-κB signaling pathway in Hep3B and HepG2 liver cell lines.
Results
Apatinib has a significant inhibitory effect on the metastasis and invasion of liver cancer cells. The expression levels of MMP-1, MMP-2, MMP-3, MMP-7, MMP-9, MMP-10, MMP-11, and MMP-16 were downregulated, while the expression levels of TIMP-3 and TIMP-4 were upregulated by apatinib treatment at both the mRNA and protein levels. The phosphorylation of IκBα and NF-κB p65 was significantly reduced compared with that in the control group.
Conclusions
Apatinib inhibits the invasion and metastasis of human liver cancer cells by downregulating the expression of MMP-related genes. This may be achieved by inhibiting the activation of the NF-κB signaling pathway.
1. Introduction
Hepatocellular carcinoma (HCC) is the most frequent primary cancer of the liver, which is the sixth most common cancer and the third leading cause of death in the world [1–3]. There is a main reason for the poor prognosis of patients with liver cancer. Liver cancer cells induce the expression of specific proteases, which cause the invasion and metastasis of tumor cells through degradation of the extracellular matrix (ECM) and basement membrane. Matrix metalloproteinases (MMPs) present the most important group of these proteases. As a large family of calcium-dependent, zinc-containing endopeptidases, MMPs play an important role in tumor invasion and metastasis [4–6]. Their activities are posttranslationally regulated by endogenous tissue inhibitors of metalloproteinases (TIMPs) [7, 8]. Therefore, the MMP/TIMP balance is critical for the inhibition of tumor invasion and metastasis.
Apatinib is a new type of small-molecule antiangiogenesis agent and a targeting tyrosine kinase inhibitor that selectively inhibits the vascular endothelial growth factor receptor-2 (VEGFR-2), thus suppressing tumor angiogenesis and being effective against tumors [9]. Currently, clinical studies have confirmed the effectivity and safety of apatinib in the treatment of advanced liver cancer [10, 11]. Furthermore, studies have shown that apatinib led to a survival benefit of patients with advanced gastric cancer [12]. The purpose of this study was to explore the effect of apatinib on metastasis, invasion, and expression of MMPs of liver cancer cells and to provide further theoretical basis for the clinical application of apatinib.
2. Materials and Methods
2.1. Reagents and Antibodies
Apatinib mesylate tablets (AITAN®; 425 mg/tablet) were purchased from Hengrui Medicine Company (Jiangsu, China). Tablets were dissolved in 100% dimethyl sulfoxide (DMSO; MP Biomedicals, Santa Ana, CA, USA) to a concentration of 4 mmol/L and stored at –20°C. The stock solution was diluted to a working concentration with DMEM (Gibco, Grand Island, NY, USA), and the final DMSO concentration in cell culture was less than 0.1%. The BCA kit was purchased from Google Biotechnology Ltd. (Wuhan, China). The RNA extraction reagent TRIzol and the PCR reagent Power SYBR® Green PCR Master Mix were purchased from Life Technologies (Grand Island, NY, USA), and the PCR primers were synthesized by Google Biotechnology Ltd. (Wuhan, China). Anti-MMP-1, anti-MMP-2, anti-MMP-3, anti-MMP-7, anti-MMP-9, anti-TIMP-1, anti-TIMP-2, and anti-TIMP4 antibodies were purchased from Proteintech Group (Chicago, USA), and anti-MMP-10, anti-MMP-11, and anti-MMP-16 antibodies were purchased from Biorbyt Ltd. (Cambridge, UK). Anti-TIMP-3 was purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). An NF-κB pathway sampler kit (9936T) was purchased from Cell Signaling Technology Inc. (Danvers, MA, USA).
2.2. Cells and Cell Culture
The HepG2, Hep3B, Huh7, and SMMC-7721 human liver cancer cell lines were purchased from the China Center for Type Culture Collection (Wuhan, China). Cells were cultured in DMEM cell culture medium containing 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin. The medium was changed every three days, and the cells were passaged with a split ratio of 1 : 4 when they were 80–90% confluent.
2.3. Analysis of the Effect of Apatinib on Invasion and Metastasis of Human Liver Cancer Cells
2.3.1. Wound-Healing Assay
HepG2, Hep3B, Huh7, and SMMC-7721 cells were homogeneously seeded in 6-well plates (2 × 105 per well). After 24 h of growth, a monolayer of cells was attached to the bottom of the wells. The monolayer was gently scratched with a sterile 10 μL pipette tip across the center of the well. The wells were washed three times to remove detached cells and replenished with fresh DMEM medium without serum. Cells were cultured in serum-free DMEM containing 20 or 40 μmol/L apatinib for 48 h. Untreated cells were used as the blank control. Cells cultured only in serum-free DMEM for 48 h were used as the control group. Then, all cells were kept at 37°C in a humidified atmosphere containing 5% CO2. Optical microscopy was performed after 0, 24, and 48 h. The migration rate was calculated using the following formula: migration rate = (width at 0 h–width at 24 or 48 h)/width at 0 h. All experiments were performed in triplicate.
2.4. Transwell Invasion Assay
The invasion capacity of HepG2, Hep3B, Huh7, and SMMC-7721 cells was evaluated by the transwell invasion assay (Falcon, Oxford, UK) using a polycarbonate membrane filter with a pore size of 8.0 μm. The transwell compartments were prepared by incubation with Matrigel (diluted to 1 : 100) at 37°C for 2 h. Then, the compartments were placed into 24-well plates. To the upper compartments, 1 × 105 HepG2, Hep3B, Huh7, and SMMC-7721 cells were introduced, and 0, 20, or 40 μmol/L apatinib in DMEM medium was added up to a total volume of 200 μL. To the lower compartments, 600 μL DMEM containing 10% FBS was added. All experiments were performed in triplicate. After incubation for 24 h, the transwell compartments were taken out and washed with PBS. Cells were fixed by polyformaldehyde at room temperature for 20 min, followed by crystal violet staining for 5 min and washing with deionized distilled water (ddH2O). Cells on the lower side of the filter were counted under a microscope, and the average counting from five random views was determined.
2.5. Quantitative Real-Time Reverse Transcription-PCR (qRT-PCR)
The effect of apatinib on the mRNA expression levels of MMP and TIMP genes was analyzed using real-time PCR. The cells were seeded in 6-well plates at a concentration of 105 cells/mL and cultured for 24 h. Then, cells were exposed to 0, 20, or 40 μmol/L apatinib for 48 h. The total RNA of the cells was extracted using the RNA extraction reagent TRIzol, and concentration and purity were determined using an ultraviolet spectrophotometer. The same amount of mRNA was used as a reverse transcriptional template to obtain the cDNA. Primers and SYBR Green Mix (2x) were used for quantitative PCR, and each reaction was performed in triplicate.
Fluorescence quantitative PCR was carried out in 20 μL of a mixture containing 1.5 μL cDNA template, 1 μL of each primer (10 μmol/L), 10 μL SYBR Green Mix, and 10 μL ddH2O. PCR for each sample was performed in triplicate. The following thermal cycling conditions were applied: initial denaturation at 94°C for 5 min, followed by 33 PCR amplification cycles at 94, 58, and 72°C for 1 min at each temperature, and termination by an extension step at 72°C for 10 min. GAPDH was used as the loading control. Relative mRNA expression levels were assessed using the 2−ΔΔCT method, as previously described [13]. Gene-specific primers of MMPs were designed according to published articles [14]. The used primers are listed in Table 1.
Table 1.
Characteristics of the primers used for real-time PCR.
| Genes | Primers (forward and reverse) | Products (bp) |
|---|---|---|
| MMP-1 | 5′-CTGCTTACGAATTTGCCGACAGA-3′ | 130 |
| 5′-GTTCTAGGGAAGCCAAAGGAGCTG-3′ | ||
| MMP-2 | 5′-GTTCATTTGGCGGACTGTGACG-3′ | 146 |
| 5′-ATTCATTCCCTGCAAAGAACACAGC-3′ | ||
| MMP-3 | 5′-GGACAAAGGATACAACAGGGACCA-3′ | 140 |
| 5′-GAACCGAGTCAGGTCTGTGAGTG-3′ | ||
| MMP-7 | 5′-GATGGGCCAGGAAACACGC-3′ | 107 |
| 5′-CCTAGACTGCTACCATCCGTCCA-3′ | ||
| MMP-9 | 5′-CACGACGTCTTCCAGTACCGAGA-3′ | 115 |
| 5′-CATAGGTCACGTAGCCCACTTGGT-3′ | ||
| MMP-10 | 5′-TCTGTTCCTTCGGGATCTGAGATG-3′ | 146 |
| 5′-TGAAATTCAGGTTCAGGGTTCCAGT-3′ | ||
| MMP-11 | 5′-ACCTGGACTATCGGGGATGA-3′ | 188 |
| 5′-GGCTGGCCATATAGGTGTTG-3′ | ||
| MMP-16 | 5′-GACAGGCCAAAACCTCCTCGG-3′ | 149 |
| 5′-TTTCTCACTCGCCAAAACCACTG-3′ | ||
| TIMP-1 | 5′-AAGGCTCTGAAAAGGGCTTC-3′ | 161 |
| 5′-GAAAGATGGGAGTGGGAACA-3′ | ||
| TIMP-2 | 5′-TTGACCCAGAGTGGAACG-3′ | 101 |
| 5′-ACCAAAGACGGGAGACGA-3′ | ||
| TIMP-3 | 5′-CCTGCTACTACCTGCCTTGC-3′ | 182 |
| 5′-TGTGGCATTGATGATGCTTT-3′ | ||
| TIMP-4 | 5′-TCTGGACAGACTGGCTGTTG-3′ | 176 |
| 5′-TTGAAGGGATGTGATGGTCA-3′ | ||
| NF-κB p65 | 5′-CAAGTGGCCATTGTGTTCCG-3′ | 149 |
| 5′-TGGCGATCATCTGTGTCTGG-3′ | ||
| GAPDH | 5′-TCGACAGTCAGCCGCATCTTCTTT-3′ | 148 |
| 5′-GCCCAATACGACCAAATCCGTTGA-3′ |
MMP: matrix metalloproteinase; TIMP: tissue inhibitors of metalloproteinase.
2.6. Western Blot Analysis
The effect of apatinib on the expression of MMP and TIMP proteins was determined by Western blot analysis, using the same experimental grouping as for the real-time PCR assay. The cells were lysed to extract the total protein, and the protein concentration was determined by the BCA kit according to its instructions. Each protein sample (20 μg) was loaded, and SDS-PAGE was performed on a 12% separation gel. Afterwards, the proteins were transferred from the gel to a PVDF membrane and treated with the respective antibodies: mouse anti-human MMP-3, MMP-9, and MMP-10; mouse anti-human TIMP-3 (1 : 200); rabbit anti-human MMP-1 (1 : 200); and rabbit anti-human MMP-2, MMP-7, MMP-10, MMP-16, TIMP-1, TIMP-2, and TIMP-4 (1 : 500) at 4°C overnight. GAPDH was used as the loading control. The next day, the membrane was incubated with the corresponding secondary antibodies labeled with horseradish peroxidase (purchased from Wuhan Google Biotechnology Ltd., Wuhan, China) and visualized by a chemiluminescent immunoassay.
3. Results
3.1. Apatinib Inhibits the Migration of HepG2, Hep3B, Huh7, and SMMC-7721 Cells
Wound gaps in the HepG2, Hep3B, Huh7, and SMMC-7721 cell monolayers were observed under a microscope at 0, 24, and 48 h after apatinib treatment. The liver cancer cell lines were incubated with apatinib (20 and 40 μmol/L, respectively) for 48 h, and the results are shown in Figure 1. In contrast to cells in the control group, cells with 24 and 48 h of apatinib treatment obviously migrated into the wound gaps, leading to a significantly reduced migration distance compared with that in the control group (P < 0.05). In addition, the migration rate of cells treated with a high concentration (40 μmol/L) of apatinib was significantly lower than that of cells treated with a low apatinib concentration (20 μmol/L), suggesting that apatinib has a significant and dose-dependent inhibitory effect on the migration of liver cancer cells.
Figure 1.
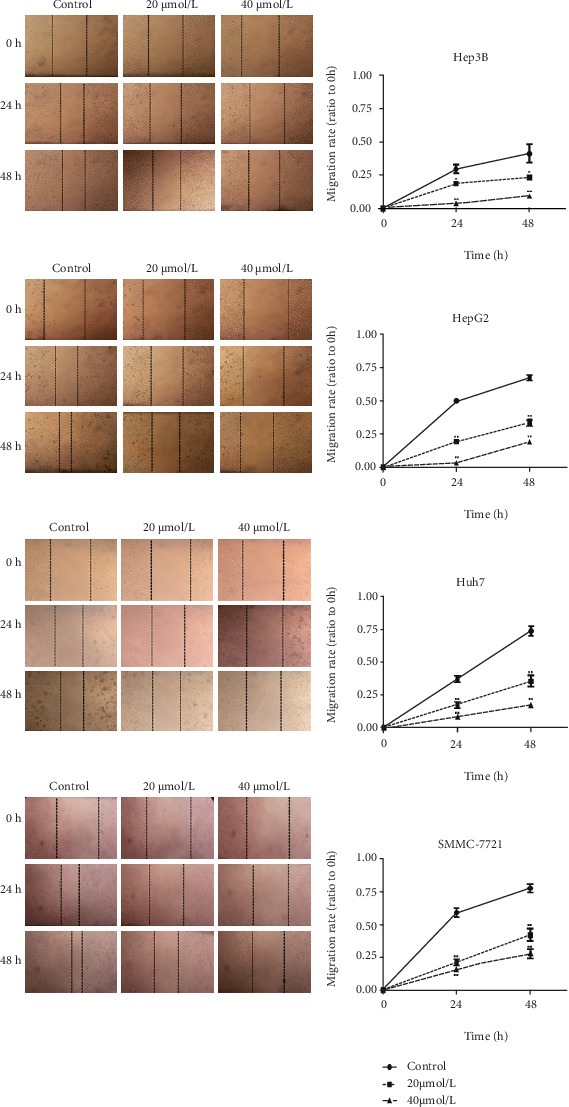
Apatinib inhibits the migration of HepG2, Hep3B, Huh7, and SMMC-7721 cells. Results of the wound healing assay of Hep3B, HepG2, Huh7, and SMMC-7721 cells following treatment with different doses of apatinib at 24 and 48 h (original magnification, ×200). Analysis of the relative migration rate curves showed that apatinib treatment inhibited the migration of Hep3B, HepG2, Huh7, and SMMC-7721 cells in a dose-dependent manner (∗ indicates p < 0.05 and ∗∗p < 0.01 vs. 0 μmol/L (control)).
3.2. Apatinib Inhibits the Invasion of HepG2, Hep3B, Huh7, and SMMC-7721 Cells
The transwell invasion experiment showed that after apatinib treatment, fewer HepG2, Hep3B, Huh7, and SMMC-7721 cells migrated across the filter membrane (Figure 2; P < 0.05). In addition, the number of invading cells treated with a high concentration of apatinib was significantly less than that of invading cells treated with a low concentration of apatinib. The results of the four liver cancer cell lines are consistent and indicate that apatinib inhibits the invasion of human liver cancer cells.
Figure 2.
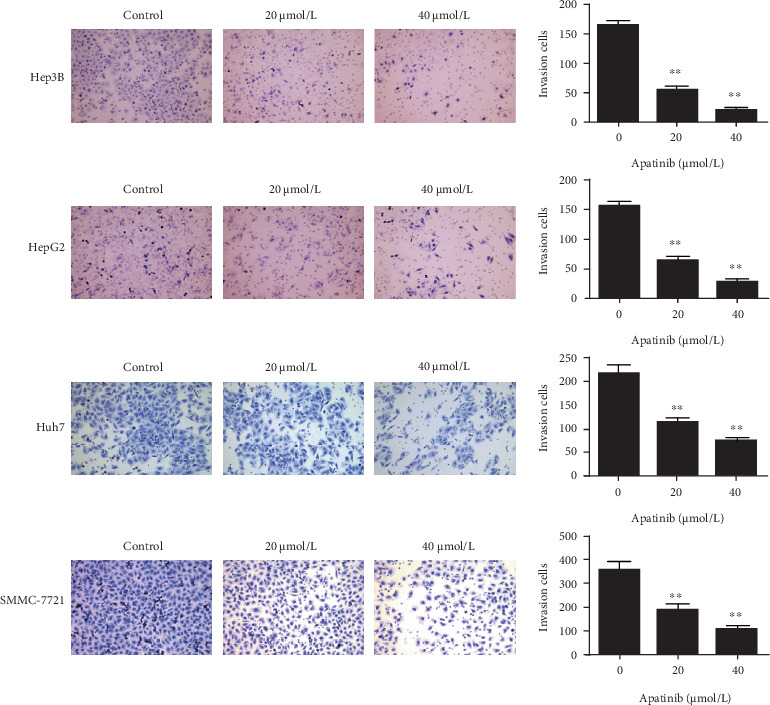
Apatinib inhibits the invasion of HepG2, Hep3B, Huh7, and SMMC-7721 cells. Results of transwell invasion assays of Hep3B, HepG2, Huh7, and SMMC-7721 cells following treatment with apatinib for 24 h (original magnification, ×200). Quantification of the invasion in Hep3B, HepG2, Huh7, and SMMC-7721 cells (∗∗ indicates p < 0.01 vs. 0 μmol/L (control)).
3.3. Apatinib Downregulates MMP Expression Levels in HepG2 and Hep3B Cells
Real-time PCR showed that apatinib treatment obviously reduced the expression levels of MMP-1, MMP-2, MMP-3, MMP-7, MMP-9, MMP-10, MMP-11, and MMP-16 in HepG2 and Hep3B cells compared with the corresponding levels in the control group, and these reductions were statistically significant (P < 0.05; Figure 3(a)). Western blot analysis showed that apatinib also downregulated the expression of the abovementioned MMPs at the protein level (Figure 3(b)).
Figure 3.
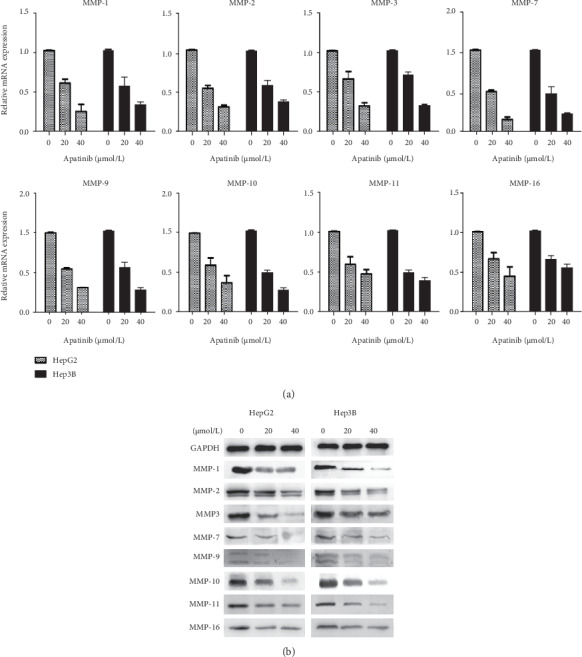
Real-time PCR and Western blot analysis of expression levels of MMP family genes in Hep3B and HepG2 liver cells. (a) Real-time PCR analysis of mRNA levels of MMP-1, MMP-2, MMP-3, MMP-7, MMP-9, MMP-10, MMP-11, and MMP-16. Compared with the control group, the expression levels of the mRNAs of MMP-1, MMP-2, MMP-3, MMP-7, MMP-9, MMP-10, MMP-11, and MMP-16 in the apatinib-treated group were significantly decreased (p < 0.05). (b) Western blot was used to screen the level of MMP expression in the HCC cell lines, showing that apatinib reduced the expression of MMP-1, MMP-2, MMP-3, MMP-7, MMP-9, MMP-10, MMP-11, and MMP-16. GAPDH was used as the loading control. MMP: matrix metalloproteinase.
3.4. Downregulation of MMPs by Apatinib Is Associated with Upregulation of TIMP3/4 Expression
We further investigated the mechanisms underlying the inhibitory effect of apatinib on HepG2 and Hep3B cell metastasis. The expression levels of members of the TIMP gene family, including TIMP-1, TIMP-2, TIMP-3, and TIMP-4, were analyzed using real-time PCR and Western blot. As shown in Figures 4(a) and 4(b), apatinib treatment had no effect on the expression of TIMP-1 and TIMP-2 in HepG2 or Hep3B cells, although the mRNA and protein levels of TIMP-3 and TIMP-4 were markedly increased. These results indicate that apatinib treatment upregulated the expression levels of members of the TIMP gene family.
Figure 4.
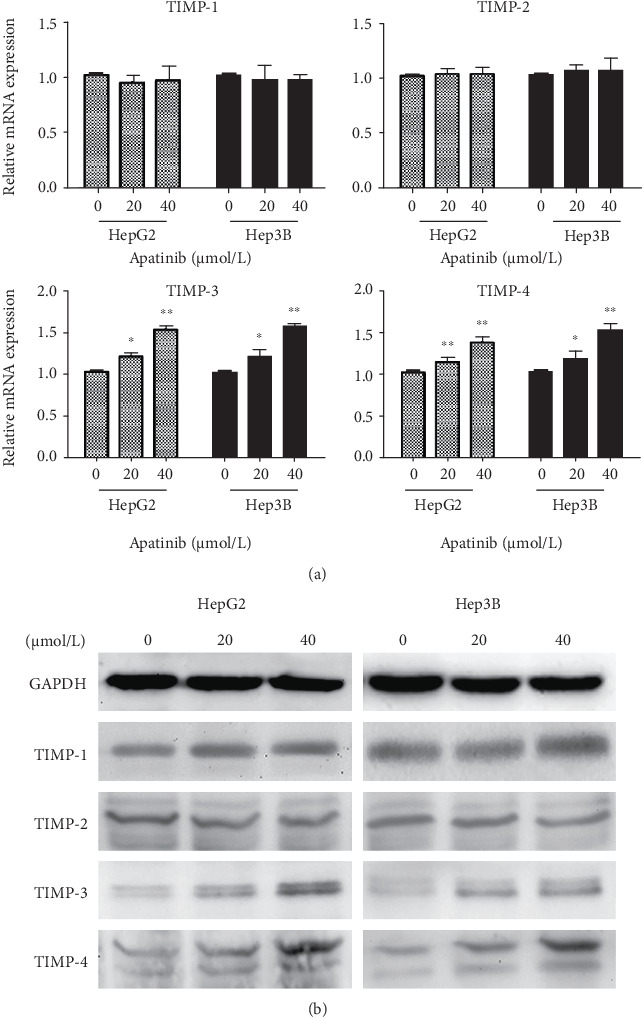
Real-time PCR and Western blot analysis of expression levels of TIMP family genes in Hep3B and HepG2 liver cells. (a) Real-time PCR analysis of the expression levels of TIMP-1, TIMP-2, TIMP-3, and TIMP-4. The results show that apatinib increased the expression of TIMP-3 and TIMP-4. (b) Protein expression levels of TIMPs following treatment with apatinib in Hep3B and HepG2 cells analyzed by Western blot, confirming an increased TIMP-3 and TIMP-4 expression upon apatinib treatment. GAPDH was used as the loading control. TIMP: tissue inhibitors of metalloproteinase.
3.5. Apatinib Downregulates the Activation of the NF-κB Signaling Pathway
The NF-κB signaling pathway plays an important role in cancer migration [15]; therefore, we explored whether apatinib could affect this pathway. As shown in Figure 5, Western blot analysis revealed that the expression levels of p-IκBα and p-p65 were decreased in a dose-dependent manner in liver cancer cells treated with apatinib when compared with the levels in the control group. In addition, the ratios of p-IκBα/IκBα and p-p65/p65 in HepG2 and Hep3B cells were significantly lower than those in the control group. These results indicated that apatinib inhibits NF-κB signaling pathway activation.
Figure 5.
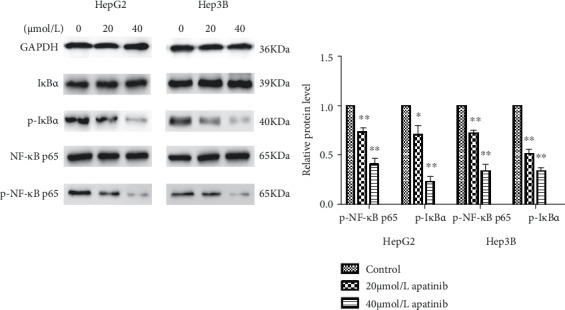
Western blot analysis of expression levels of the NF-κB signaling pathway in Hep3B and HepG2 liver cells. The protein expressions of p65, p-p65, IκBα, and p-IκBα in Hep3B and HepG2 liver cells measured by Western blotting. Results revealed that the expression levels of p-IκBα and p-p65 were decreased in HepG2 and Hep3B liver cancer cells treated with apatinib when compared with the control group in a dose-dependent manner. And the ratio of p-IκBα/IκBα and p-p65/p65 in cells was significantly lower compared with that of the control group. ImageJ software was used to analyze the gray values. ∗ indicates p < 0.05, ∗∗p < 0.01.
4. Discussion
More than half of liver cancer patients are diagnosed after progressing to the advanced stages of liver cancer where surgery is impossible. Many liver cancer patients who do undergo operation also experience local recurrences and distal metastases [16, 17]. MMP proteins degrade extracellular matrix (ECM) proteins and therefore play a key role in the invasion and metastasis of tumor cells, including liver cancer cells [18].
In this study, we confirmed the effect of apatinib on the metastasis and invasion of HepG2, Hep3B, Huh7, and SMMC-7721 cells through a wound-healing assay and transwell invasion assay, respectively. Our results showed that apatinib has a significant and dose-dependent inhibitory effect on the metastasis and invasion of these four liver cancer cells. A recently published study by Li et al. also confirmed the anti-invasion and metastasis effects of apatinib on multiple liver cancer cell lines (Hep3B, BEL-7402, HepG2, Huh7, and HCCC-9810) [19]. To further explore the underlying mechanism, we performed real-time PCR and Western blot analysis and verified that apatinib downregulates the expression of MMPs. Therefore, we believe that apatinib probably inhibits the metastasis and invasion of liver cancer cells by downregulating the expression of MMPs. Previous studies have found that apatinib plays a role in the downregulation of MMP-2 and MMP-9 in gastric cancer [20, 21]. However, our study was the first to verify apatinib-induced downregulation of members of the MMP gene family in liver cancer cells. Tumor angiogenesis includes tube formation and proliferation of endothelial cells. Tube formation is mainly mediated by MMPs, whereas proliferation primarily involves VEGF family members; for example, VEGF-induced angiogenesis is mainly mediated by VEGF-2 [22–24]. As a small molecule inhibitor of VEGFR-2, apatinib inhibits tumor angiogenesis in the two ways mentioned above, explaining its good antitumor efficacy. Currently, it is believed that VEGF promotes the metastasis of tumor cells synergistically with MMPs; this phenomenon has been demonstrated in multiple tumors [25–27]. These data suggest that apatinib can also inhibit the invasion and metastasis of liver cancer by inhibiting the VEGF/VEGFR-2 signaling pathway.
Tissue inhibitors of metalloproteinases (TIMPs) are natural inhibitors of MMPs and play an important role in regulating the activation of MMPs [22]. The balance between these two gene families is critical to the invasion and metastasis of tumor cells [28]. Despite evidence of its antitumor effects, whether apatinib can affect the expression of the TIMP gene family has not yet been reported. Our study showed that apatinib regulates the expression of TIMP-3 and TIMP-4. Therefore, we demonstrated for the first time that apatinib upregulates TIMP family members in liver cancer. It has been reported that the expression of TIMPs significantly inhibits the expression of all active MMP family members [29]. These results suggest that the decrease in MMP gene expression levels in response to apatinib administration may be due to the upregulation of TIMP-3 and TIMP-4. Moreover, studies have found that TIMPs are involved in inhibiting cell migration, invasion, and angiogenesis, which is independent of their inhibitory activity on MMPs [30]. Our study indicates that apatinib reduced the invasive and metastatic capabilities of liver cancer cells, at least in part, by downregulating MMP expression and simultaneously upregulating TIMP expression.
Our study involved eight members of the MMP family—MMP-1, MMP-2, MMP-3, MMP-7, MMP-9, MMP-10, MMP-11, and MMP-16—and the expression of all these MMPs was significantly downregulated by apatinib. Previous research has shown enhanced levels of various MMPs in several types of tumors, including gastric cancer and liver cancer. Łukaszewicz-Zając et al. also demonstrated that high MMP expression in the liver is related to a poorer prognosis of liver cancer patients [31]. MMPs have been indicated to mainly affect the invasion and metastasis of cancer, but studies have also found that some MMPs participate in multiple stages of carcinogenesis [32, 33], such as the destruction of the host immune response, promotion of early tumor progression, and inhibition of cancer cell apoptosis and death. Among all MMPs, MMP-1, MMP-2, MMP-3, MMP-7, and MMP-9 are involved in almost all stages of tumor growth, angiogenesis, and metastasis [34]. Therefore, in addition to inhibiting VEGFR-2, apatinib can further enhance its antitumor effects by downregulating the expression of MMPs.
Studies have reported that upregulation of the nuclear factor-kappaB (NF-κB) signaling pathway can cause the invasion and metastasis of human cancer [35, 36]. Further studies confirmed that inhibiting NF-κB signaling pathway activity significantly reduced invasion of HCC cells and downregulated the expression of the VEGF and MMP gene families, the latter including MMP-1, MMP-2, MMP-3, and MMP-9 [37, 38]. These observations indicate that apatinib inhibits the migration and invasion of liver cancer cells by decreasing MMP-related gene expression via suppression of the NF-κB signaling pathway.
In conclusion, apatinib inhibits the invasion and metastasis of liver cancer via regulation of the MMP/TIMP balance. Therefore, apatinib is a potential antimetastasis drug for liver cancer. Next, we will validate this conclusion in animal models and further explore the antimetastasis mechanism of apatinib to provide a basis for a better application of apatinib in the clinic.
Acknowledgments
This work was supported by the Research Project of Hubei Provincial Science and Technology Department (2018CFC846), Henry CSCO-Cancer Research Fund (Y-HR2017-033), and Henry research Fund for hepatobiliary and pancreatic malignancies of Chen Xiao-ping foundation for the development of Science and Technology of Hubei Province (CXPJJH11800001-2018317).
Contributor Information
Zhifan Xiong, Email: xiongzhifan@126.com.
Shengli Yang, Email: yangshengli2014@yahoo.com.
Data Availability
The data used to support the findings of this study are available from the corresponding authors upon request.
Disclosure
The abstracts of this manuscript have been published in the 19th Congress of Gastroenterology China.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
ZFX, SLY, and XXH conceived and designed the project. XXH, ZZH, and PL carried out most of the experiments. QTL and MMW performed the statistical analysis. MJQ gave support in doing experiments and reviewed the manuscript. XXH wrote the manuscript, and all authors read and approved the final manuscript.
References
- 1.Katyal S., Oliver J. H., III, Peterson M. S., Ferris J. V., Carr B. S., Baron R. L. Extrahepatic metastases of hepatocellular carcinoma. Radiology. 2000;216(3):698–703. doi: 10.1148/radiology.216.3.r00se24698. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag H. B., Rudolph K. L. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132(7):2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 3.Forner A., Llovet J. M., Bruix J. Hepatocellular carcinoma. The Lancet. 2012;379(9822):1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 4.Hadler-Olsen E., Winberg J. O., Uhlin-Hansen L. Matrix metalloproteinases in cancer: their value as diagnostic and prognostic markers and therapeutic targets. Tumour Biology. 2013;34(4):2041–2051. doi: 10.1007/s13277-013-0842-8. [DOI] [PubMed] [Google Scholar]
- 5.Kessenbrock K., Wang C. Y., Werb Z. Matrix metalloproteinases in stem cell regulation and cancer. Matrix Biology. 2015;44-46:184–190. doi: 10.1016/j.matbio.2015.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jabłońska-Trypuć A., Matejczyk M., Rosochacki S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. Journal of Enzyme Inhibition and Medicinal Chemistry. 2016;31(sup1):177–183. doi: 10.3109/14756366.2016.1161620. [DOI] [PubMed] [Google Scholar]
- 7.Nagase H., Visse R., Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovascular Research. 2006;69(3):562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Arpino V., Brock M., Gill S. E. The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biology. 2015;44-46:247–254. doi: 10.1016/j.matbio.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Scott A. J., Messersmith W. A., Jimeno A. Apatinib: a promising oral antiangiogenic agent in the treatment of multiple solid tumors. Drugs of Today. 2015;51(4):223–229. doi: 10.1358/dot.2015.51.4.2320599. [DOI] [PubMed] [Google Scholar]
- 10.Qin S. K. Apatinib in Chinese patients with advanced hepatocellular carcinoma: a phase II randomized, open-label trial. Journal of Clinical Oncology. 2014;32(15_Supplement):4019–4019. doi: 10.1200/jco.2014.32.15_suppl.4019. [DOI] [Google Scholar]
- 11.Cidon E. U. Systemic treatment of hepatocellular carcinoma: past, present and future. World Journal of Hepatology. 2017;9(18):797–807. doi: 10.4254/wjh.v9.i18.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang L., Wei Y., Shen S., et al. Therapeutic effect of apatinib on overall survival is mediated by prolonged progression-free survival in advanced gastric cancer patients. Oncotarget. 2017;8(17):29346–29354. doi: 10.18632/oncotarget.12897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Z., Chang X., Dai D., Deng P., Sun Q. RASSF10 is an epigenetically silenced tumor suppressor in gastric cancer. Oncology Reports. 2014;31(4):1661–1668. doi: 10.3892/or.2014.3039. [DOI] [PubMed] [Google Scholar]
- 14.Asano T., Tada M., Cheng S., et al. Prognostic values of matrix metalloproteinase family expression in human colorectal carcinoma. The Journal of Surgical Research. 2008;146(1):32–42. doi: 10.1016/j.jss.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 15.Dolcet X., Llobet D., Pallares J., Matias-Guiu X. NF-kB in development and progression of human cancer. Virchows Archiv. 2005;446(5):475–482. doi: 10.1007/s00428-005-1264-9. [DOI] [PubMed] [Google Scholar]
- 16.Bruix J., Llovet J. M. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35(3):519–524. doi: 10.1053/jhep.2002.32089. [DOI] [PubMed] [Google Scholar]
- 17.Cao W., Li J., Hu C., et al. Symptom clusters and symptom interference of HCC patients undergoing TACE: a cross-sectional study in China. Support Care Cancer. 2013;21(2):475–483. doi: 10.1007/s00520-012-1541-5. [DOI] [PubMed] [Google Scholar]
- 18.Brown G. T., Murray G. I. Current mechanistic insights into the roles of matrix metalloproteinases in tumour invasion and metastasis. The Journal of Pathology. 2015;237(3):273–281. doi: 10.1002/path.4586. [DOI] [PubMed] [Google Scholar]
- 19.Li X., Xu A., Li H., Zhang B., Cao B., Huang J. Novel role of apatinib as a multi-target RTK inhibitor in the direct suppression of hepatocellular carcinoma cells. Biochimica et Biophysica Acta-Molecular Basis of Disease. 2018;1864(5):1693–1701. doi: 10.1016/j.bbadis.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 20.Wu J., Yu J., Wang J., et al. Astragalus polysaccharide enhanced antitumor effects of Apatinib in gastric cancer AGS cells by inhibiting AKT signalling pathway. Biomedicine & Pharmacotherapy. 2018;100:176–183. doi: 10.1016/j.biopha.2018.01.140. [DOI] [PubMed] [Google Scholar]
- 21.Zhao P., Zhang J., Pang X., Zhao L., Li Q., Cao B. Effect of apatinib combined with 5-fluorouracil (5-FU) on proliferation, apoptosis and invasiveness of gastric cancer cells. Annals of Oncology. 2017;28(Supplement 5):v13–v14. doi: 10.1093/annonc/mdx361.047. [DOI] [Google Scholar]
- 22.Li X. M., Tang Z. Y., Zhou G., Lui Y. K., Ye S. L. Significance of vascular endothelial growth factor mRNA expression in invasion and metastasis of hepatocellular carcinoma. Journal of Experimental & Clinical Cancer Research. 1998;17(1):13–17. [PubMed] [Google Scholar]
- 23.Leonardi G. C., Candido S., Cervello M., et al. The tumor microenvironment in hepatocellular carcinoma (review) International Journal of Oncology. 2012;40(6):1733–1747. doi: 10.3892/ijo.2012.1408. [DOI] [PubMed] [Google Scholar]
- 24.Shahneh F. Z., Baradaran B., Zamani F., Aghebati-Maleki L. Tumor angiogenesis and anti-angiogenic therapies. Human Antibodies. 2013;22(1-2):15–19. doi: 10.3233/HAB-130267. [DOI] [PubMed] [Google Scholar]
- 25.Chen K. W., Yang H. L., Lu J., et al. Expression of vascular endothelial growth factor and matrix metalloproteinase-9 in sacral chordoma. Journal of Neuro-Oncology. 2011;101(3):357–363. doi: 10.1007/s11060-010-0263-0. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y. F., Guo S., Zhao R., Chen Y. G., Wang X. Q., Xu K. S. Correlation of vascular endothelial growth factor expression with tumor recurrence and poor prognosis in patients with pN0 gastric cancer. World Journal of Surgery. 2012;36(1):109–117. doi: 10.1007/s00268-011-1192-6. [DOI] [PubMed] [Google Scholar]
- 27.Wen Y. L., Li L. Correlation between matrix metalloproteinase–9 and vascular endothelial growth factor expression in lung adenocarcinoma. Genetics and Molecular Research. 2015;14(4):19342–19348. doi: 10.4238/2015.December.29.44. [DOI] [PubMed] [Google Scholar]
- 28.Bourboulia D., Stetler-Stevenson W. G. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs): positive and negative regulators in tumor cell adhesion. Seminars in Cancer Biology. 2010;20(3):161–168. doi: 10.1016/j.semcancer.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ketsawatsomkron P., Keen H. L., Davis D. R., et al. Protective role for tissue inhibitor of metalloproteinase-4, a novel peroxisome proliferator–activated receptor-γ target gene, in smooth muscle in deoxycorticosterone acetate–salt hypertension. Hypertension. 2016;67(1):214–222. doi: 10.1161/HYPERTENSIONAHA.115.06391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brew K., Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochimica et Biophysica Acta. 2010;1803(1):55–71. doi: 10.1016/j.bbamcr.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Łukaszewicz-Zając M., Mroczko B., Szmitkowski M. Gastric cancer—the role of matrix metalloproteinases in tumor progression. Clinica Chimica Acta. 2011;412(19-20):1725–1730. doi: 10.1016/j.cca.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 32.Shay G., Lynch C. C., Fingleton B. Moving targets: emerging roles for MMPs in cancer progression and metastasis. Matrix Biology. 2015;44-46:200–206. doi: 10.1016/j.matbio.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yadav L., Puri N., Rastogi V., Satpute P., Ahmad R., Kaur G. Matrix metalloproteinases and cancer-roles in threat and therapy. Asian Pacific Journal of Cancer Prevention. 2014;15(3):1085–1091. doi: 10.7314/APJCP.2014.15.3.1085. [DOI] [PubMed] [Google Scholar]
- 34.Deryugina E. I., Quigley J. P. Tumor angiogenesis: MMP-mediated induction of intravasation- and metastasis- sustaining neovasculature. Matrix Biology. 2015;44-46:94–112. doi: 10.1016/j.matbio.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karin M., Greten F. R. NF-κB: linking inflammation and immunity to cancer development and progression. Nature Reviews. Immunology. 2005;5(10):749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 36.Xia Y., Shen S., Verma I. M. NF-κB, an active player in human cancers. Cancer Immunology Research. 2014;2(9):823–830. doi: 10.1158/2326-6066.CIR-14-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu J. M., Sheng H., Saxena R., et al. NF-κB inhibition in human hepatocellular carcinoma and its potential as adjunct to sorafenib based therapy. Cancer Letters. 2009;278(2):145–155. doi: 10.1016/j.canlet.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 38.Chiang I.-T., Liu Y.-C., Wang W.-H., et al. Sorafenib inhibits TPA-induced MMP-9 and VEGF expression via suppression of ERK/NF-κB pathway in hepatocellular carcinoma cells. In Vivo. 2012;26(4):671–681. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding authors upon request.


