Abstract
Background & Aims:
Patients with celiac disease (CD) often report inadvertent gluten exposures and challenges reading labels. The most common cause of non-responsive CD is gluten exposure. We aimed to assess whether recently diagnosed CD patients can determine whether a food is gluten-free based on labeling, and to assess skills over time. A secondary aim was to identify factors associated with label reading proficiency.
Methods:
Inception cohort with follow-up at 6, 12, and 24 months after diagnosis. Participants were asked to determine whether 25 food items were gluten-free based on labeling information. Diet adherence was assessed using the Celiac Diet Assessment Tool (CDAT) and the Gluten-Free Eating Assessment Tool (GF-EAT). 144 adults with newly diagnosed celiac disease were enrolled. The initial quiz at 6 months was completed by 83%. Quizzes were completed by 72% at 12 months and 70% at 24 months.
Results:
Median overall accuracy scores were: 23/25, 24/25 and 21/25 at 6, 12 and 24 months respectively. Gluten-free products with explicit “gluten-free” claims had the fewest errors. Quiz scores were not correlated with tTG IgA levels, or CDAT or GF-EAT scores. Diet adherence was generally good (>85% with CDAT <13 suggesting adequate GFD adherence); however, at 24 months, only 11% reported no gluten exposure.
Conclusions:
CD patients may be unable to consistently choose gluten-free foods based on product labeling. Explicit identification of gluten-free products may be helpful. Label reading ability appears stable over time. Further studies are needed to evaluate whether erroneous label reading or misleading labels are associated with persistent villous atrophy.
Keywords: celiac disease, gluten-free diet, treatment adherence and compliance, food labeling, follow-up
Introduction
Celiac disease (CD) is a lifelong disorder characterized by inflammation of the small intestine upon ingestion of gluten by genetically predisposed individuals[1]. Chronic inflammation of the small intestine can lead to malabsorption and cause symptoms such as diarrhea, indigestion, bloating, and weight loss. Untreated CD may have long-term effects, such as delayed puberty, malnutrition, osteoporosis, infertility, and gastrointestinal malignancies[2].
Treatment of CD requires strict lifelong maintenance of a gluten-free diet (GFD), which excludes wheat, barley, spelt, and rye[3]. Patients must also ensure that their food has not been in cross-contact with gluten-containing products, since as little as 50 mg per day (equivalent to a few crumbs) may prevent mucosal recovery[4]. To follow a GFD successfully, persons with CD must learn to identify gluten-containing foods by reading labels, which may list unsafe ingredients in ambiguous ways. The skills necessary to maintain a strictly gluten-free diet, including label reading, must be practiced and perfected if patients with CD are to avoid gluten exposure and consequent intestinal damage. Importantly, the most common cause of non-responsive CD is gluten exposure[5,6], so there is a need to both establish a standard method of monitoring adherence and to reduce gluten exposures.
Several questionnaire-based methods have been proposed to assess GFD adherence, and there is no established best practice[7,8]. Diet recalls and food diaries are subject to multiple limitations, including memory biases and intentional or unintentional inaccuracies. Additionally, there is minimal standardization of GFD assessment systems[9] and proposed scoring systems may not be widely applicable. For example, the questionnaire developed by Biagi et al includes the question, “Do you only eat packaged food guaranteed by the Coeliac Association?”[9] While this may be a useful question to assess adherence in Italy, it is less applicable in North America, where gluten-free labeling is voluntary and certification programs are not well-established.
Another method that is used currently is the Celiac Dietary Adherence Test (CDAT), a 7-item survey that includes questions about energy levels, emotional state, and accidental or purposeful gluten exposures[10]. Responses to the CDAT are correlated with a standardized diet assessment created by a single dietitian, and with tTG IgA levels[10], which are often presented as a measure of GFD adherence. Unfortunately, the sensitivity of serum tests for tTG IgA and EMA IgA for detecting persistent villous atrophy on a GFD is less than 50%[11].
In contrast, label reading skills, which are necessary to successfully avoid gluten, are a direct measurement of individuals’ knowledge regarding gluten content of foods. These skills may be modifiable and can help to address accidental gluten exposure. In order to standardize this assessment, a practical test of label reading proficiency was developed. Participants were presented with a basket of 25 grocery items and asked to determine whether each item was gluten-free or contained gluten based on product labeling information. Our objectives were to assess whether recently diagnosed CD patients could use this information to determine whether a food is safe to eat, and to assess skills over time. We also aimed to identify factors associated with label reading proficiency. Given the difficulties in assessing GFD adherence, we hypothesized that scores would not be correlated with any currently established measure of GFD adherence (e.g., Celiac Symptom Index [CSI] scores, Celiac Diet Adherence Test [CDAT] scores, and Gluten-Free Eating Assessment Tool [GF-EAT]).
Methods
The Manitoba Celiac Disease Cohort includes adults (>16 years) with a new diagnosis of celiac disease based upon villous atrophy (Marsh 3) and elevated serum tissue transglutaminase (tTG) and/or endomysial antibodies (EMA). Participants were recruited within 6 weeks of GFD initiation with additional study visits at 6, 12, and 24 months after diagnosis. Those unable to attend follow-up visits or complete surveys in English were excluded. Each visit included an online questionnaire, interview, and blood draw. The questionnaire and interview included questions related to medical history, symptoms, and diet. Symptom severity was assessed using the Celiac Symptom Index (CSI)[12], a 16-item self-report measure which encompasses information about intestinal as well as extra-intestinal manifestations of CD. GFD adherence was assessed using the Celiac Diet Assessment Tool (CDAT)[10] and the Gluten-Free Eating Assessment Tool (GF-EAT)[13], which includes questions related to frequency of intentional and inadvertent gluten consumption. At each visit, serum was collected and assayed for tTG IgA antibodies in the Immunology Laboratory at St. Boniface Hospital, Winnipeg, Canada. In May 2015, the assay was changed from Immulisa (Immco Diagnostics Inc., Buffalo, NY) to Bioplex 2200 (Bio-rad Laboratories (Canada) Inc., Montreal QC). Results are reported as multiples of the upper limit of normal (ULN) for the assay used (Immulisa 20, Bioplex 15).
Per usual practice, consultation with a dietitian was recommended at diagnosis and referrals were provided, but participants were not required to see a dietitian (visits are covered by Manitoba Health). Rather, participants were asked whether they had seen a dietitian and how useful this was.
Grocery quiz: At each follow-up visit, participants were asked to determine whether each of 25 groceries procured locally was gluten-free based on product labeling and which ingredient(s) they thought contained gluten. Different products were used at each study visit. Gluten-containing ingredients (as defined by Canadian regulations) were identified by registered Dietitians with expertise in gluten-free diets[14,15]. Scoring was based upon yes/no responses; thus, a participant would be scored as having identified a gluten-containing food item correctly even if the specific ingredient they thought contained gluten was gluten-free. This was done in order to simulate a real-life scenario, wherein individuals with CD must decide whether to consume a particular food based on an evaluation of product labeling and the ingredient list.
Data analysis was performed using RStudio Version 1.1.414[16] with R software version 3.4.1[17]. Descriptive statistics were used to characterize the group at baseline. Univariate analysis (Fisher’s exact test or Pearson’s correlation coefficient as appropriate) was performed to assess whether quiz scores were correlated with various factors, including gender, education level, history of reactions to gluten, whether the home kitchen is kept gluten-free, and frequency of purchasing packaged gluten-free convenience foods. In addition, quiz scores were analyzed for correlation with: self-rated anxiety related to food and ingredients; self-rated attention to ingredients; self-rated confidence in following a gluten-free diet; reported gluten-free diet education with a dietitian; perceived usefulness of information about a gluten-free diet received from various sources rated on a 4-point categorical scale (poor, fair, good, very good); reported support from family and friends; and feelings of being a bother due to the gluten-free diet. Scores were also analyzed for correlation with changes in tTG IgA levels, CSI, CDAT, and GF-EAT scores. The University of Manitoba Bannatyne Campus Research Ethics Board approved the study protocol and all participants provided written informed consent.
Results
Between December 2012 and October 2015, 201 eligible participants were approached, of whom 12 could not be contacted, 33 declined to participate, 10 were ineligible due to being on a GFD, and 1 was ineligible because of a language barrier. Thus, a total of 144 participants enrolled, of whom 138 were at a study site where the quiz was administered. Of the 144 participants enrolled in the study, the majority were female (65%), the median age was 37 years (interquartile range [IQR] 30–54 years), and most were HLADQ2 (84%) positive (Table 1). Participant retention was good with 91% returning for at least one follow-up visit. Quiz completion was 115 (83%) at 6 months, 100 (72%) at 12 months, and 97 (70%) at 24 months.
Table 1 :
Participant characteristics at diagnosis (N=138)
| % (N) | |
|---|---|
| Age (years) [median(IQR)] | 37 (30–54) |
| Female % (N) | 65% (90) |
| HLA genotype (N=137) % (N) | |
| DQ2 | 84% (115) |
| DQ8 | 7% (9) |
| DQ2/DQ8 | 9% (13) |
| Marsh classification % (N) | |
| Marsh 3a | 36% (50) |
| Marsh 3b | 41% (57) |
| Marsh 3c | 23% (31) |
| Celiac serology at diagnosis | |
| tTG IgA level multiples of upper limit of normal [median (IQR)] | 8.5 (3.2->10) |
| GFD Education from a Dietitian (N=123) % (N) | 73% (90) |
GFD adherence and symptoms
At 6 months, the median Celiac Symptom Index (CSI) score was 31 (IQR 26–38) and only 10 participants (9%) had CSI scores > 45, which are associated with poorer adherence and worse quality of life (Table 2)[12]. The median Celiac Diet Adherence Test (CDAT) score was 9 (IQR 7–10), and 95% had scores ≤ 13, which have been associated with adequate adherence[10]. Using the Gluten-Free Eating Assessment Tool (GF-EAT), 75% of participants reported rare accidental gluten ingestion less than once per month. At 12 months, the median CSI score was 29 (IQR 25–37), 7% had CSI scores > 45 and 86% had CDAT scores suggestive of adequate adherence. At 24 months, the median CSI score was 27, with only 4% having scores above 45. Nevertheless, 76% reported accidental gluten exposure.
Table 2:
Celiac disease symptoms, gluten-free diet adherence, serology and grocery quiz scores
| Diagnosis (N=117) | 6 months (N=115) | 12 months (N=100) | 24 months (N=97) | |
|---|---|---|---|---|
| Celiac Symptom Index (CSI) | ||||
| Median[IQR] | 35 (29–44) | 31 (26–38) | 29 (25–37) | 27 (24–32) |
| Above symptom threshold1 [%(N)] | 22 (26) | 9% (10) | 7% (7) | 4%(4) |
| Gluten-free diet adherence | ||||
| Celiac Diet Adherence Test (CDAT)2 | ||||
| Median[IQR] | --- | 9 (7–10) | 10 (9–12) | 10 (9–12) |
| Adequate adherence [%(N)]3 | --- | 95% (109) | 86% (86) | 86%(83) |
| Gluten-Free Eating Assessment Tool (GF-EAT) [% (N)] | ||||
| Gluten unrestricted | --- | 2% (3) | 1%(1) | 4%(4) |
| Occasional gluten (1–4/month) | --- | 2% (2) | 6%(7) | 4%(4) |
| Rare intentional gluten (<1/month) | --- | 15% (22) | 15%(16) | 5%(5) |
| Rare accidental gluten (<1/month) | --- | 75% (108) | 71%(76) | 76%(72) |
| No Gluten | --- | 6% (8) | 7%(8) | 11%(10) |
| Celiac serology | ||||
| tTG IgA level MULN median[IQR] | 7.3 (2.9, >12.5) | 0.92 (0.45–1.8) | 0.65 (0.35–1.5) | 0.47 (0.20–1.4) |
| Quiz Scores median[range] | ||||
| Total (/25) | --- | 23 (8–25) | 24 (18–25) | 22 (14–23) |
| Gluten-free items | --- | 11 (0–11) n = 11 | 11 (4–11) n = 11 | 11 (7–12) n = 12 |
| Gluten-containing items | --- | 13 (0–14) n = 14 | 13 (8–14) n = 14 | 11 (6–12) n = 13 |
CSI scores > 45 are associated with poorer adherence and lower quality of life (possible score range 16 to 80);
Persons not restricting gluten were considered non-adherent and CDAT scores were not calculated;
CDAT scores ≤ 13 are associated with adequate adherence (possible score range 7 to 35). tTG tissue transglutaminase antibody, MULN multiples upper limit of normal.
Grocery Quiz
At 6 months, the median quiz score was 23/25 (IQR 22–24); 11/11 (IQR 9.5–11) for gluten-free items and 13/14 (IQR 12–14) for gluten-containing items (Table 2). The lowest scoring food items were almond crackers (62% correct, “may contain” statement) and granola bars (63% correct, contained non-gluten-free oats; Figure 1). At 12 months, the median quiz score was 24/25 (IQR 22–24); 11/11 (IQR 10–11) for gluten-free items and 13/14 (IQR 12–14) for gluten-containing items. Granola bars (71% correct, contained non-gluten-free oats) and Worcestershire sauce (74% correct, contained malt vinegar) were the lowest scoring items. At 24 months, the median quiz score was 22/25 (IQR 14–22); 11/12 (IQR 10–11) for gluten-free items and 11/13 (IQR 11–11) for gluten-containing items. The lowest scoring items were potato chips (3% correct) and butterscotch candies (7% correct), both of which were gluten-free. Overall, participants scored highest on items with explicit gluten-free claims (97% at 6 months and 98% at 24 months; Figure 2) and lowest on items with a “contains” statement pertaining to wheat or gluten (e.g., “contains wheat”).
Figure 1. Mean score for each item on 6, 12, and 24 month grocery quizzes.
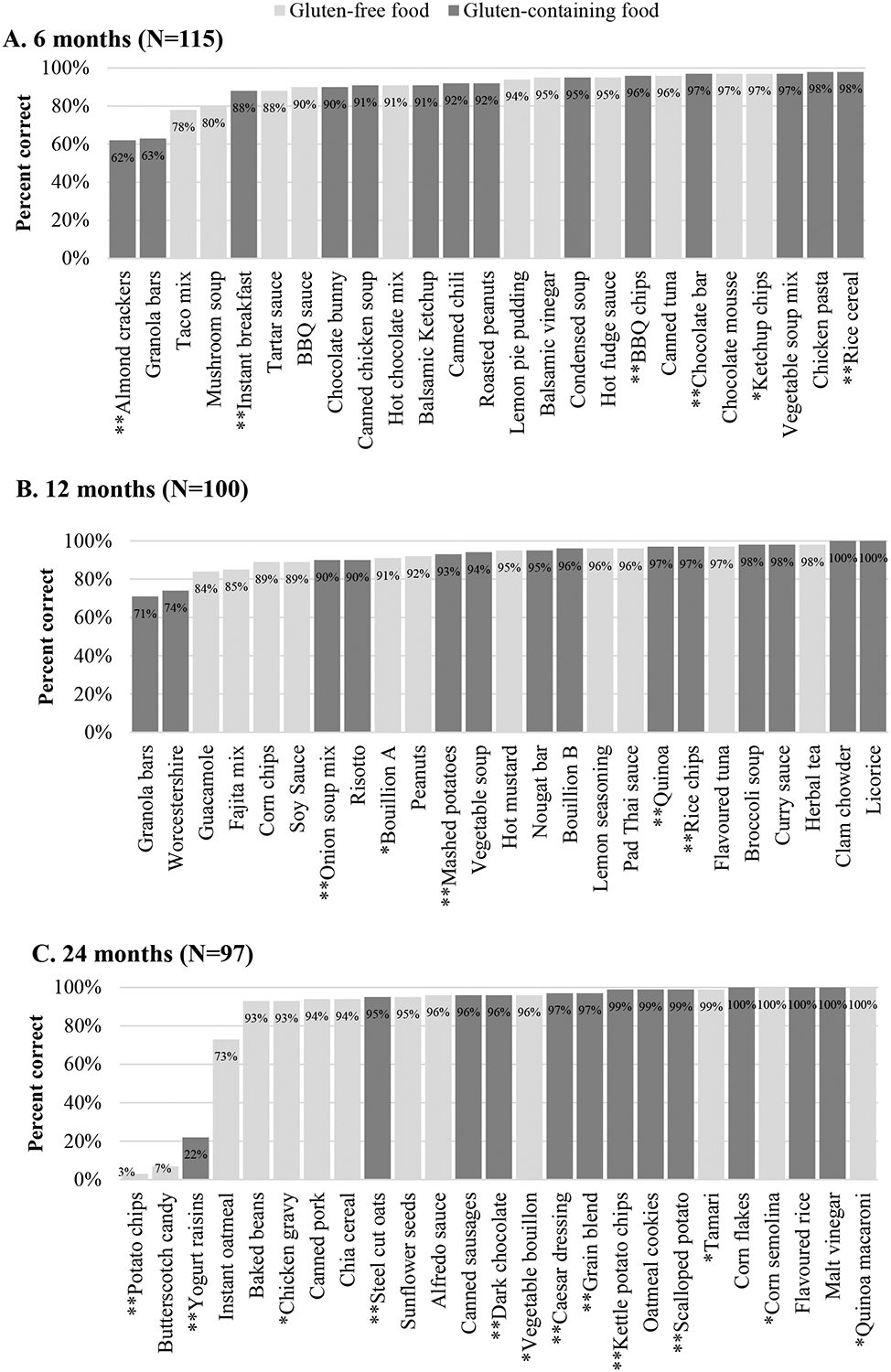
Scores for individual items at each timepoint. Shading indicates whether item was gluten free (light grey) or gluten-containing (dark grey). * Items with gluten-free claim on label; ** Items with gluten/wheat statement on label.
Figure 2. Label reading scores at 6, 12 and 24 months.
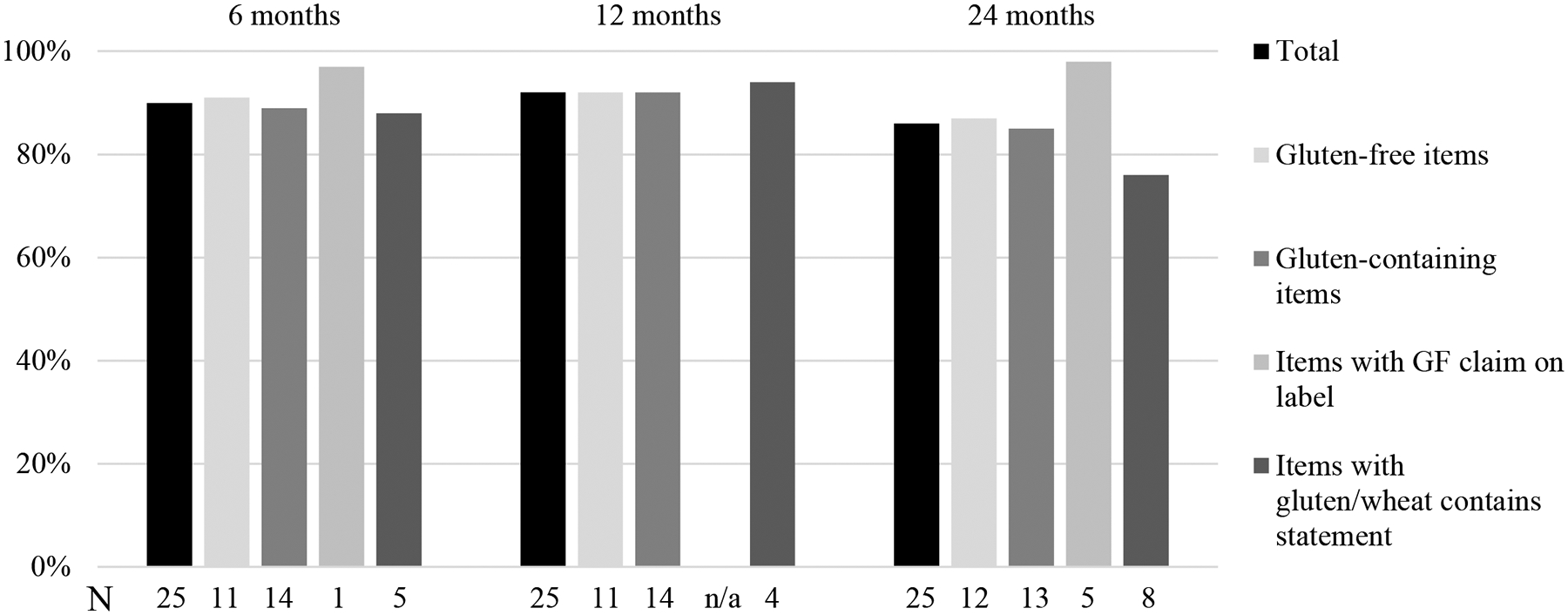
For each timepoint, the height of the horizontal bars corresponds to the percent of respondents who correctly identified the gluten-free status of the associated product type. The numbers along the horizontal axis indicate how many products were in each category at each timepoint. There were 115 participants at 6 months, 100 participants at 12 months and 97 participants at 24 months.
Soy products, maltodextrin, and yeast extract were commonly misidentified as gluten-containing at all time points (Table 4). Gluten-free products containing wheat derivatives (i.e., wheat dextrose, glucose syrup derived from wheat, wheat fiber, and wheat maltodextrin) included at 12 and 24 months were among the lowest scoring items. Other ingredients mistaken to contain gluten included natural and artificial flavor, modified cornstarch, spices and seasonings, mustard flour, buckwheat, and MSG. Notably, many of these ingredients were present in multiple gluten-free products at a given time point and individual participants were not consistent in indicating these ingredients as gluten-containing.
Table 4:
Gluten-free ingredients most commonly thought to contain gluten
| Visit | Gluten-free ingredient | Foods containing this ingredient |
|---|---|---|
| 6 months | Maltodextrin | Chocolate bar, instant breakfast, ketchup chips, kettle peanuts, taco mix |
| Flavor (natural and artificial) | Balsamic vinegar, BBQ chips, BBQ sauce, canned chicken chili, canned soup, canned tuna, chocolate bar, chocolate bunny, chicken pasta, crackers, mushroom soup, hot chocolate, kettle chips, kettle peanuts, lemon pie pudding, rice cereal, soup mix, taco mix, tartar sauce | |
| Soy products* | Canned chicken soup, canned chili, chicken pasta, chocolate bar, chocolate bunny, condensed soup, granola bars, hot chocolate, instant breakfast, kettle peanuts, rice cereal, taco mix, tartar sauce | |
| (autolyzed) yeast extract | Chicken pasta, condensed soup, mushroom soup, soup mix, taco mix | |
| Modified cornstarch | BBQ sauce, canned chicken soup, canned chili, canned tuna, mushroom soup, hot fudge, kettle peanuts, lemon pie pudding, soup mix, tartar sauce | |
| Spices/seasonings | BBQ chips, BBQ sauce, canned chicken soup, canned chili, chicken pasta, condensed soup, mushroom soup, ketchup, ketchup chips, kettle peanuts, soup mix, taco mix, tartar sauce | |
| Mustard flour | Canned chili, tartar sauce | |
| MSG | BBQ chips, condensed soup, mushroom soup, soup mix | |
| 12 months | (autolyzed) yeast extract | Bouillon A, bouillon B, fajita mix, mashed potatoes, peanuts, rice chips, risotto, vegetable soup, |
| Soy products* | Bouillon B, clam chowder, curry sauce, fajita mix, guacamole dip, granola bars, lemon seasoning, licorice, Mars bar, mashed potatoes, onion soup, Pad Thai sauce, peanuts, rice chips, risotto, soy sauce, vegetable soup, Worcestershire sauce | |
| Maltodextrin/corn maltodextrin | Broccoli soup, corn chips, fajita mix, guacamole dip, lemon seasoning, mashed potatoes, peanuts | |
| Caramel color | Broccoli soup, curry sauce, soy sauce, Worcestershire sauce | |
| Whey | Corn chips, guacamole dip | |
| Glucose syrup (wheat) | Licorice | |
| Spices | Bouillon A, bouillon B, clam chowder, corn chips, curry sauce, guacamole dip, lemon seasoning, rice chips, vegetable soup, Worcestershire sauce | |
| Wheat fiber | Licorice | |
| 24 months | Glucose syrup (wheat) | Butterscotch candies |
| Wheat dextrose | Potato chips | |
| Wheat maltodextrin | Kettle potato chips | |
| Oats | Grain blend, instant oatmeal, oatmeal cookies, steel cut oats | |
| Soy products* | Butterscotch candies, Caesar dressing, canned pork, chia cereal, corn flakes, dark chocolate, flavored rice, oatmeal cookies, scalloped potato, steel cut oats, tamari sauce, yogurt raisins | |
| Buckwheat | Chia cereal, grain blend | |
| Yeast extract | Chicken gravy, flavored rice, scalloped potatoes, sunflower seeds, vegetable bouillon | |
| Modified cornstarch | Alfredo sauce, baked beans, Caesar dressing, canned pork |
Soy products include: soy, soy lecithin, soybean, soybean oil, hydrolyzed soy protein, and hydrogenated soybean oil.
The gluten-containing foods that were most commonly misidentified contained barley, yeast extract, or oats which were not certified gluten-free and/or were processed in a shared facility or had a “contains” statement pertaining to wheat and/or gluten (Table 3). The lowest scoring food item in this category at 24 months was yogurt raisins (22% correct), whose label consisted of a listing of ingredients in English, ingredients in French, a “contains” statement for milk and soy in English and French, and a separate section labeled “Allergen Information,” declaring that “this product is processed on equipment that is also used for nuts, tree nuts and wheat.” Each of these was in a different font. The gluten-containing foods with the highest scores contained wheat flour and/or had a precautionary allergen statement pertaining to wheat or barley.
Table 3:
Gluten-containing foods most commonly misidentified as gluten-free
| Visit | Food | Percent correct | Gluten-containing ingredients |
|---|---|---|---|
| 6 months | Almond crackers | 62 | (Shared facility) |
| Granola bars | 63 | Oats (not certified gluten-free) | |
| 12 months | Granola bars | 71 | Oats (not certified gluten-free) |
| Worcestershire sauce | 74 | Malt vinegar | |
| 24 months | Yogurt raisins | 22 | (Shared equipment) |
Grocery quiz scores did not differ significantly according to gender or whether patients had consulted a dietitian regarding a GFD. Although there was no clear association between quiz scores and GF-EAT scores, the distribution of scores did tend to be skewed with an over-representation of lower scores among those who reported accidental gluten exposure. CSI scores were negatively correlated with quiz scores (Table 5). This was significant only at 6 months, suggesting that those who were more symptomatic were also more adept at label reading. There was a weak negative correlation between CDAT score and grocery quiz score, which was significant at 6 months (r= −0.26) and 12 months (r= −0.25). As expected, tTG IgA levels were not correlated with quiz scores; however, those who had a persistently elevated tTG IgA at 12 months scored significantly lower on the quiz than those who had seroconverted (22.3 vs 23.5, 95% CI for difference 0.4–2.1). All 6 participants who scored <80% on the 12-month quiz had a persistently elevated tTG IgA.
Table 5:
Correlation between grocery quiz scores and other measures of celiac disease activity
| 6 months | 12 months | 24 months | |
|---|---|---|---|
| Celiac Symptom Index (CSI)1 | −0.35* | −0.15 | −0.006 |
| Celiac Diet Adherence Test (CDAT)2,3 | −0.26* | −0.25* | −0.17 |
| TTG IgA level (MULN) | 0.002 | 0.04 | −0.005 |
P< 0.01.
CSI scores > 45 are associated with poorer adherence and lower quality of life (possible score range 16 to 80);
Persons not restricting gluten were considered non-adherent and CDAT scores were not calculated;
CDAT scores ≤ 13 are associated with adequate adherence (possible score range 7 to 35). tTG tissue transglutaminase antibody, MULN multiples upper limit of normal.
Discussion
This prospective longitudinal study reveals that patients with CD are unable to consistently ascertain correctly whether foods contain gluten based upon the available product information. Incomplete gluten-free food identification was present at all time points of the study, suggesting that this knowledge is not necessarily acquired as individuals gain more experience with a GFD, and inadvertent gluten ingestion can occur even up to two years post-diagnosis. Although performance was generally high overall, the risk of accidental consumption cannot be completely eliminated without perfect label reading accuracy.
Label reading has been identified as an important topic by patients with celiac disease[18] and understanding of food labels has been associated with self-reported gluten-free diet adherence[8,19,20] and CDAT scores[21]. Label reading is more complex than simply identifying individual ingredients; however, there is no standardized method of assessing label reading skills that is part of systematic GFD education or the routine follow-up of patients with CD[2,22]. Correlation between quiz scores and other measures of celiac disease activity (e.g., CDAT, CSI) was weak. This study highlights the ability of a grocery quiz to provide unique insight into factors that influence day-to-day decision making, help estimate risk of gluten ingestion, and identify areas that need improvement. Participants scored highest on products with explicit labeling identifying the food as gluten-free; however, placement and style of such claims is clearly important, as participants often failed to recognize gluten-free claims if they were not in a particularly conspicuous location. In a Polish study, women with celiac disease selected food items with written information about a “gluten-free” claim with greater frequency than a front-of-package gluten-free logotype alone[23], presumably due to a greater degree of trust in written information as opposed to an emblem alone.
Failing to identify gluten in a product is an error of obvious significance because it could lead to inadvertent gluten ingestion. The grocery quiz identified several areas of confusion with respect to gluten identification in a product label that may exist for patients with celiac disease. Product labeling was not always clear and unambiguous despite regulations governing declaration of allergens, including gluten and wheat. For instance, on the 6-month quiz, only 62% correctly identified that the almond crackers may contain gluten. The errors may have been due to unclear labeling. The product packaging advertised support for the Celiac Disease Foundation; however, the crackers were produced in a facility that makes products containing wheat. Notably, the same product has a gluten-free claim with similar packaging when sold in the United States (where the Celiac Disease Foundation is based). Similarly, the label for yogurt raisins on the 24-month quiz included ingredient lists and non-overlapping allergen statements in two different languages, each in a different font, and many participants did not recognize the “Allergen Information” statement. These mixed messages were likely confusing to participants and may have contributed to the low score of 22%.
Oats were another ingredient associated with errors. Although oats are a gluten-free grain, they are commonly grown, harvested, and transported alongside wheat, and are often contaminated with gluten. Canadian laws require that gluten-free oats be labeled as such, thus the unqualified term “oats” implies that they may have been contaminated[24]. Participants appeared unaware of this nuance as they consistently performed poorly on oat-containing products without clear labeling. At 6 months, the granola bars (63% correct) did not contain wheat, rye or barley ingredients; however, the oats were not certified gluten-free. The statement “May contain peanuts, sesame, tree nuts & soy” at the end of the ingredient list may also have caused confusion. As gluten and wheat were excluded from this statement, participants may have believed that the food was gluten-free. In contrast, participants scored highly on oats-containing items that either also had an obviously gluten-containing ingredient (such as wheat flour) or explicitly stated that the product “may contain wheat”.
The quiz also identified errors related to mistakenly identifying gluten-free ingredients as gluten-containing (Figure 3). Such errors may lead to avoidance of products that are safe, as well as unnecessary and excessive dietary restriction which may increase the risk of nutrient deficiencies[25]. Gluten-free ingredients most commonly mistaken to contain gluten included wheat starch, glucose syrup (wheat), wheat dextrose, corn maltodextrin/maltodextrin, soy products (including soy, soy lecithin, and hydrolyzed soy protein), oats, and yeast extract. Ingredients derived from wheat but still considered gluten-free (e.g., wheat starch, glucose syrup from wheat, and wheat dextrose), were a particular source of confusion, with some of the lowest scores. This may be attributable both to participants overlooking or not seeing the “contains” statement on a product without gluten-containing ingredients and because the statement pertained to wheat derivatives that are considered to be gluten-free. Under Canadian regulations, gluten refers to protein; therefore, highly processed non-protein components of gluten-containing grains, such as dextrose and maltodextrin, are considered to be gluten-free[15]. If these products are derived from wheat and may contain other wheat proteins, then manufacturers may voluntarily declare that the product “may contain wheat” or “contains wheat ingredients” in consideration of those with wheat allergies. Nevertheless, these foods are in fact safe for persons with CD. This situation applies to some of the two lowest scoring items. At 24 months, the potato chips (3% correct) listed “wheat dextrose” as the last ingredient, followed by a “contains wheat ingredients” statement. The butterscotch candies (7% correct) listed “glucose syrup (wheat)” as the first of many ingredients, along with allergen information that related only to nuts, not to wheat or gluten. Canadian regulations require that all priority allergens be included if there is a “contains” statement; therefore, the absence of gluten in the “contains” statement indicates that the product is safe for individuals with celiac disease.
Figure 3. Gluten-free ingredients most commonly mistaken to contain gluten.
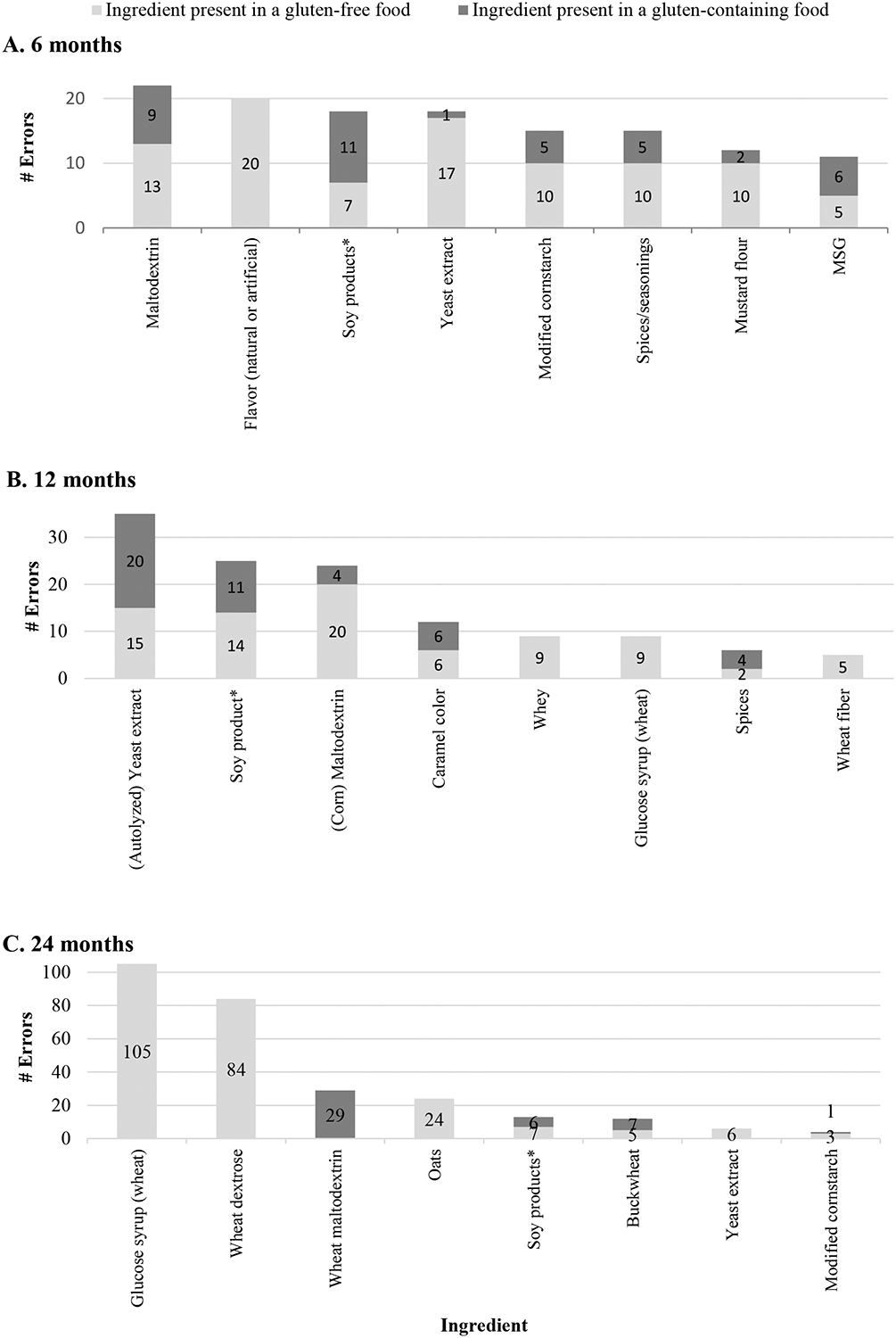
Horizontal bars indicate the number of individuals making an erorr related to an ingredient. Dark grey shading indicates the ingredient was in a gluten-containing food contained gluten and light grey shading indicates ingredient was in a gluten-free food. Only the eight items with the highest number of errors are shown. Numbers overlying horizontal bars indicate the number of individuals who made the corresponding error. * Soy products include: soy, soy lecithin, soybean, soybean oil, hydrolyzed soy protein, and hydrogenated soybean oil.
Multiple errors involved (corn) maltodextrin, potentially due to confusion of maltodextrin with malt, as they sound similar. Additionally, soy products (including soybeans, soy lecithin, and hydrolyzed soy protein) were the source of many errors, possibly because soy and wheat are both allergens, and participants may have grouped them together. Notably, participants who indicated that these ingredients contained gluten did not consistently do so for all items in a particular quiz. This suggests that factors other than the label, such as index of suspicion for gluten, may influence interpretation of ingredient lists. Further studies are needed to determine why some ingredients are conditionally considered gluten-containing.
Finally, the grocery quiz identified knowledge gaps related to particular ingredients. Allergen labeling requirements are not regulated in all countries, which may contribute to confusion regarding the safety of particular foods. Yeast extract is unique in that it may or may not contain gluten depending on the substrate used and was responsible for a significant number of errors. Canadian labeling laws require the declaration of ingredients derived from gluten, wheat, and other priority allergens (including barley), so yeast extract should be considered gluten-free unless gluten-containing ingredients are also declared[26]. Thus, “yeast extract” without a “contains” statement that includes gluten or wheat is considered gluten-free, whereas “yeast extract (barley)” is a gluten-containing ingredient. This is clearly a source of confusion indicating a need for better labeling and education regarding this common ingredient.
There are several limitations to this study. Firstly, only 25 grocery items were included at each time point, which cannot adequately cover the scope of labels and ingredients encountered by patients with CD. Additionally, to reduce any learning effects related to retesting, there were 25 different items at each time point. Thus, it was difficult to determine if participants’ skills (and scores) for the same foods improved over time. This trade-off is partially mitigated by including common ingredients across all time points. Additionally, scoring for each item was based on yes/no responses. While this most closely models participants’ day-to-day decision making, an unintended consequence was that once a gluten-containing ingredient was identified, participants proceeded to the next item without evaluating the remaining ingredients. This may have led to certain ingredients towards the end of the list being under-recognized merely because participants did not evaluate them. However, if these ingredients had shown up earlier on the ingredient lists, they may have been correctly identified more often. This may partially explain why some products with a particular gluten-free ingredient (e.g., yeast extract), which also contained wheat or barley, were correctly identified as gluten-containing, even though the ingredient thought to contain gluten was in fact gluten-free. Lastly, the Manitoba Celiac Disease Cohort includes only adults older than 16 years with a new diagnosis of CD. The grocery quiz has not been tested in children with CD or their caregivers, or those who have been following a GFD for more than 24 months. Further study of these patient groups is needed along with evaluation of how label reading proficiency correlates with objective markers of gluten exposure and mucosal recovery.
A gluten-free label quiz has the potential to be a valuable adjunct to diet assessment in the follow-up of individuals with celiac disease. The grocery quiz offers a simple way to determine how patients with CD are deciding which foods are safe or unsafe to eat, which may have long-lasting impacts on their health and quality of life. This standardized assessment challenges patients in a realistic scenario, and provides important information to healthcare providers regarding their ability to determine if foods are gluten-free. The quiz may identify unintentional gluten consumption related to misconceptions regarding gluten-free foods. Observation of this skill in a clinic also affords the opportunity to intervene and provide targeted education if necessary.
The present study suggests that a grocery quiz is a simple tool to assess patients’ ability to identify gluten in food products, which provides information not captured by other measures. This is important to determine, given that understanding of food labels has been associated with GFD adherence and gluten consumption is the most common cause of persistent symptoms in individuals with celiac disease who are trying to follow a GFD. Quiz administration also provides an opportunity for intervention to address knowledge gaps and should be an area of focus when following patients with celiac disease. This study also highlights the need for clearer labeling with unambiguous declaration of allergens, and, when possible, explicit labeling of gluten-free items. Accurate knowledge of gluten-free ingredients may have important implications for the degree and speed of mucosal recovery. Further studies are needed to evaluate how label reading proficiency correlates with objective markers of gluten exposure and persistent villous atrophy.
Acknowledgements:
The authors thank the participants in the Manitoba Celiac Disease Cohort who generously provided their time and shared their experiences.
Funding Sources: This study was funded by the Canadian Institutes of Health Research, the Manitoba Health Research Council (now Research Manitoba), and the Canadian Celiac Association JA Campbell Fund. JAS received salary support from the Canadian Institutes of Health Research, Canadian Association of Gastroenterology, Harvard Medical School Eleanor and Miles Shore Fellowship and the National Institutes of Health (T32 DK 07760).
Footnotes
Conflict of Interest Statement: Dr. Bernstein reports grants from Canadian Institutes of Health Research, grants from Canadian Celiac Association JA Campbell Fund, during the conduct of the study; grants and personal fees from Takeda Canada, personal fees from Mylan Pharmaceuticals, grants, personal fees and other from AbbVie Canada, personal fees from Ferring Canada, grants and personal fees from Janssen Canada, grants and other from Shire Canada, grants from Pfizer Canada, grants, personal fees and other from Takeda Canada, outside the submitted work; and Speaker’s Bureau - AbbVie Canada, Ferring Canada, and Shire Canada. Dr. Duerksen reports grants from Canadian Institutes of Health Research, grants from Manitoba Health Research Council, grants from Canadian Celiac Association JA Campbell Fund, during the conduct of the study; personal fees from Takeda Canada, grants from Biomedal SL, other from Canadian Celiac Association, outside the submitted work. Dr. Graff reports grants from Canadian Institutes of Health Research, grants from Manitoba Health Research Council, grants from Canadian Celiac Association JA Campbell Fund, during the conduct of the study. Ms. Green reports grants from Canadian Institutes of Health Research, grants from Manitoba Health Research Council, grants from Canadian Celiac Association JA Campbell Fund, during the conduct of the study. Ms. Gutowski has nothing to disclose. Ms. Rigaux reports grants from Canadian Institutes of Health Research, grants from Manitoba Health Research Council, grants from Canadian Celiac Association JA Campbell Fund, during the conduct of the study. Dr. Silvester reports grants from Canadian Institutes of Health Research, grants from Manitoba Health Research Council, grants from Harvard Medical School Eleanor and Miles Shore Fellowship, grants from Canadian Celiac Association JA Campbell Fund, grants from Canadian Association of Gastroenterology, grants from National Institutes of Diabetes, Digestive and Kidney Disease, during the conduct of the study; personal fees from Takeda Pharmaceuticals, grants from Glutenostics LLC, grants from Biomedal SL, outside the submitted work. Dr. Walker reports grants from Canadian Institutes of Health Research, grants from Manitoba Health Research Council, grants from Canadian Celiac Association JA Campbell Fund, during the conduct of the study. Ms. Weiten reports grants from Canadian Institutes of Health Research, grants from Manitoba Health Research Council, grants from Canadian Celiac Association JA Campbell Fund, during the conduct of the study; other from Canadian Celiac Association, outside the submitted work.
References
- [1].Lebwohl B, Sanders DS, Green PHR. Coeliac disease. Lancet (London, England) 2017;6736:1–12. [DOI] [PubMed] [Google Scholar]
- [2].Rubio-Tapia A, Hill ID, Kelly CP, Calderwood AH, Murray JA. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol 2013;108:656–76; quiz 677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Theethira TG, Dennis M, Leffler DA. Nutritional consequences of celiac disease and the gluten-free diet. Expert Rev Gastroenterol Hepatol 2014;8:123–9. 10.1586/17474124.2014.876360. [DOI] [PubMed] [Google Scholar]
- [4].Biagi F, Campanella J, Martucci S, Pezzimenti D, Ciclitira PJ, Ellis HJ, et al. A milligram of gluten a day keeps the mucosal recovery away: a case report. Nutr Rev 2004;62:360–3. 10.1301/nr.2004.sept.360. [DOI] [PubMed] [Google Scholar]
- [5].Leffler DA, Dennis M, Hyett B, Kelly E, Schuppan D, Kelly CP. Etiologies and predictors of diagnosis in nonresponsive celiac disease. Clin Gastroenterol Hepatol 2007;5:445–50. 10.1016/j.cgh.2006.12.006. [DOI] [PubMed] [Google Scholar]
- [6].Ciacci C, Cirillo M, Cavallaro R, Mazzacca G. Long-Term Follow-Up of Celiac Adults on Gluten-Free Diet: Prevalence and Correlates of Intestinal Damage. Digestion 2002;66:178–85. 10.1159/000066757. [DOI] [PubMed] [Google Scholar]
- [7].Haines ML, Anderson RP, Gibson PR. Systematic review: The evidence base for long-term management of coeliac disease. Aliment Pharmacol Ther 2008;28:1042–66. 10.1111/j.1365-2036.2008.03820.x. [DOI] [PubMed] [Google Scholar]
- [8].White LE, Bannerman E, Gillett PM. Coeliac disease and the gluten-free diet: a review of the burdens; factors associated with adherence and impact on health-related quality of life, with specific focus on adolescence. J Hum Nutr Diet 2016;29:593–606. 10.1111/jhn.12375. [DOI] [PubMed] [Google Scholar]
- [9].Biagi F, Andrealli A, Bianchi PI, Marchese A, Klersy C, Corazza GR. A gluten-free diet score to evaluate dietary compliance in patients with coeliac disease. Br J Nutr 2009;102:882–7. 10.1017/S0007114509301579. [DOI] [PubMed] [Google Scholar]
- [10].Leffler DA, Dennis M, Edwards George JB, Jamma S, Magge S, Cook EF, et al. A simple validated gluten-free diet adherence survey for adults with celiac disease. Clin Gastroenterol Hepatol 2009;7:530–6, 536.e1–2. 10.1016/j.cgh.2008.12.032. [DOI] [PubMed] [Google Scholar]
- [11].Silvester JA, Kurada S, Szwajcer A, Kelly CP, Leffler DA, Duerksen DR. Tests for Serum Transglutaminase and Endomysial Antibodies Do Not Detect Most Patients With Celiac Disease and Persistent Villous Atrophy on Gluten-free Diets: a Meta-analysis. Gastroenterology 2017;153:689–701.e1. 10.1053/j.gastro.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Leffler DA, Dennis M, Edwards George J, Jamma S, Cook EF, Schuppan D, et al. A validated disease-specific symptom index for adults with celiac disease. Clin Gastroenterol Hepatol 2009;7:1328–34, 1334.e1–3. 10.1016/j.cgh.2009.07.031. [DOI] [PubMed] [Google Scholar]
- [13].Silvester JA, Graff LA, Rigaux L, Walker JR, Duerksen DR. Symptomatic suspected gluten exposure is common among patients with coeliac disease on a gluten-free diet. Aliment Pharmacol Ther 2016;44:612–9. 10.1111/apt.13725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Marketing Authorization for Gluten-free Oats and Foods Containing Gluten-free Oats. 2015.
- [15].Health Canada. Health Canada’s Position on Gluten-Free Claims. 2012.
- [16].RStudio Team. RStudio: Integrated Development for R 2016.
- [17].R Core Team. R: A Language and Environment for Statistical Computing 2016. http://www.r-project.org.
- [18].Madden AM, Riordan AM, Knowles L. Outcomes in coeliac disease: a qualitative exploration of patients ‘ views on what they want to achieve when seeing a dietitian. J Hum Nutr Diet 2016;29:607–16. 10.1111/jhn.12378. [DOI] [PubMed] [Google Scholar]
- [19].Butterworth JR, Banfield LM, Iqbal TH, Cooper BT. Factors relating to compliance with a gluten-free diet in patients with coeliac disease: comparison of white Caucasian and South Asian patients. Clin Nutr 2004;23:1127–34. 10.1016/j.clnu.2004.02.009. [DOI] [PubMed] [Google Scholar]
- [20].Muhammad H, Reeves S, Jeanes YM. Identifying and improving adherence to the gluten-free diet in people with coeliac disease. Proc Nutr Soc 2019:1–8. 10.1017/S002966511800277X. [DOI] [PubMed] [Google Scholar]
- [21].Muhammad H, Reeves S, Ishaq S, Mayberry J, Jeanes YM. Adherence to a gluten free diet is associated with receiving gluten free foods on prescription and understanding food labelling. Nutrients 2017;9:705 10.3390/nu9070705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Internal Clinical Guidelines Team, NICE. Coeliac Disease - Recognition, assessment and management. NICE Guildeline 2015. 10.1093/bmb/ldn044. [DOI] [Google Scholar]
- [23].Zysk W, Glabska D, Guzek D. Role of Front-of-Package Gluten-Free Product Labeling in a Pair-Matched Study in Women with and without Celiac Disease on a Gluten-free Diet. Nutrients 2019;11:398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Health Canada. Celiac Disease and Gluten-Free Claims on Uncontaminated Oats. Ottawa: 2015. [Google Scholar]
- [25].Shepherd SJ, Gibson PR. Nutritional inadequacies of the gluten-free diet in both recently-diagnosed and long-term patients with coeliac disease. J Hum Nutr Diet 2012. 10.1111/jhn.12018. [DOI] [PubMed] [Google Scholar]
- [26].Health Canada. Food and Drug Regulations Règlement sur les aliments et drogues. Canada: 1989. [Google Scholar]


