Summary
New, effective therapies are needed for pancreatic ductal adenocarcinoma. Ipilimumab can mediate an immunologic tumor regression in other histologies. This phase II trial evaluated the efficacy of Ipilimumab for advanced pancreatic cancer. Subjects were adults with locally advanced or metastatic pancreas adenocarcinoma with measurable disease, good performance status, and minimal comorbidities. Ipilimumab was administered intravenously (3.0 mg/kg every 3 wk; 4 doses/course) for a maximum of 2 courses. Response rate by response evaluation criteria in solid tumors criteria and toxicity were measured. Twenty-seven subjects were enrolled (metastatic disease: 20 and locally advanced: 7) with median age of 55 years (27 to 68 y) and good performance status (26 with Eastern Cooperative Oncology Group performance status = 0 to 1). Three subjects experienced ≥ grade 3 immune-mediated adverse events (colitis:1, encephalitis:1, hypohysitis:1). There were no responders by response evaluation criteria in solid tumors criteria but a subject experienced a delayed response after initial progressive disease. In this subject, new metastases after 2 doses of Ipilimumab established progressive disease. But continued administration of the agent per protocol resulted in significant delayed regression of the primary lesion and 20 hepatic metastases. This was reflected in tumor markers normalization, and clinically significant improvement of performance status. Single agent Ipilimumab at 3.0 mg/kg/dose is ineffective for the treatment of advanced pancreas cancer. However, a significant delayed response in one subject of this trial suggests that immunotherapeutic approaches to pancreas cancer deserve further exploration.
Keywords: pancreatic cancer, immunotherapy, natural killer cells, ipilimumab, CTLA-4, gastrointestinal malignancies
Current treatment options for ductal adenocarcinoma of the pancreas continue to have a limited effect on patient survival.1 The greatest effect is with intervention during early-stage disease. For stage I disease, analysis of the National Cancer Database suggests operative resection may prolong median survival by as much as 10.9 months,2 and prospective randomized trials suggest adjuvant therapy after a resection may incrementally extend median survival for early-stage patients an additional 0 to 12 months.3–10 The combination of resection and adjuvant therapy can be curative, albeit infrequently.11
Therapy has even less effect for advanced disease. In locally advanced disease, early placebo-controlled trials demonstrated radiation with concomitant chemotherapy could extend median survival by 4.1 to 6.1 months.12,13 In patients with metastatic disease gemcitabine administration resulted in a prolongation of median survival by 1.24 months,14 and combination therapy adding erlotinib to gemcitabine increased median survival by an additional 2 weeks.15
Immunotherapy is a therapeutic approach in which the patient’s adaptive immune system is stimulated to target the cancer. With the administration of immunologic mediators such as interleukin-2, an objective responses as high as 16.3%16 and 20.0%17 have been reported in patients with advanced metastatic melanoma and renal cell cancer, respectively. In patients with melanoma experiencing a complete response to high-dose interleukin-2, 59% to 82% never recur.18,19 Vaccination currently mediates tumor regression rarely.20 Adoptive T-cell therapy mediates objective tumor regressions in 49% to 72% of patients with melanoma.21 These examples validate the further development of more effective immunotherapy for melanoma and kidney cancer, and justify extending the application of this modality to other forms of cancer.
The tumor microenvironment of pancreas cancer has been analyzed to understand its effect on local immune reactivity. In an immunocompetent mouse model in which murine pancreas cancers develop spontaneously, monocytic/lymphocytic infiltrates are prevalent in premalignant lesions and invasive cancers. In this model, immunosuppressive cell types dominate early infiltrates and persist in invasive lesions.22 In humans, a lymphocytic infiltrate predominates in the microenvironment of pancreatic adenocarcinoma, and T regulatory lymphocytes, which exert an immunosuppressive response to antigenic stimuli, comprise an abnormally high percentage of these cells.23 Manipulation of this tumor microenvironment, by blocking immunoregulatory signals mediated by coinhibitory molecules such as CTLA-4 on effector T cells, may enhance tumor destruction.
As T lymphocytes become activated, CTLA-4 is transiently expressed on the T-cell surface. CTLA-4 engagement by B7–1 or B7–2 on antigen presenting cells or target tissues can result in apoptosis of activated lymphocytes thereby down-modulating the immune response. Interruption of this coupling could theoretically block apoptosis allowing an effector cell to continue to recognize and lyse its target. Ipilimumab is a fully humanized antibody that recognizes CTLA-4 and blocks the ligand-receptor interaction of B7–1/B7–2 and CTLA-4, and thereby has the potential to augment antigen-specific immune responses.
Ipilimumab as a single agent is effective for the treatment of metastatic melanoma [partial response (PR) = 14%, complete response (CR) = 2%],24 and renal cell cancer (PR = 10%, CR = 0%),25 and can mediate prostate-specific antigen decreases in prostate cancer.26,27 The agent triggers unique toxicities referred to as immune mediated adverse events. These include the onset of inflammatory disorders such as colitis, hypophysitis, dermatitis, arthritis, and hepatitis,28,29 which are reminiscent of autoimmune phenomena and the occurrence of these toxicities may be correlated with tumor regression.30
In this single arm phase II study, we explored whether Ipilimumab administered at a dosage of 3.0mg/kg, could mediate the regression of advanced pancreatic cancer in human subjects.
METHODS
This trial was conducted on a protocol approved by the National Cancer Institute Institutional Review Board and the US Food and Drug Administration. Eligible patients were ≥ 18 years of age with locally advanced or metastatic ductal adenocarcinoma of the pancreas (characterized by histologic confirmation of adenocarcinoma in the primary or metastatic lesion and a pattern of disease consistent with pancreatic cancer). All patients with locally advanced tumors had unresectable tumors; those with resectable lesions or borderline tumors31 were not included in this trial. Imaging showed at least 1 site of measurable disease. At least 3 weeks elapsed since any previous treatment with recovery from any toxicity of those treatments. Laboratory data at enrollment had to meet the following parameters: white blood cell count ≥ 2500/mL, absolute neutrophil count ≥ 1500/mL, platelets ≥ 100 × 103/mL, hemoglobin ≥ 9 g/dL, hematocrit ≥ 27%, creatinine < 2.0mg/dL. No limitation was placed on transaminases or bilirubin due to the nature of this disease. Patients were excluded for an uncontrolled pancreatic, biliary, or enteric obstruction or fistula, Eastern Cooperative Oncology Group (ECOG) performance status > 2, pregnancy, HIV infection, steroid dependence, autoimmune disease, uncontrolled infection, or a comorbidity that might obscure the interpretation of adverse events. All subjects signed an informed consent and were treated by the Surgery Branch of the Center for Cancer Research, National Cancer Institute at the National Institutes of Health Mark Hatfield Clinical Research Center, Bethesda, MD.
Ipilimumab (MDX-010; Medarex, Inc, Bloomsbury, NJ) was administered intravenously at a dosage of 3 mg/kg over 90 minutes every 3 weeks with 4 doses per course for a maximum of 2 courses of treatment. Diagnostic imaging was completed after every 2 doses of treatment or sooner when clinically indicated. Patients were assessed for toxicity, immune-mediated adverse events, steroid use, and alterations in health status including symptoms from the disease. Toxicity was monitored and severity assigned in accordance to the Cancer Therapy Evaluation Program Common Toxicity Criteria (CTCAE 3.0). Response was measured using standard response evaluation criteria in solid tumors (RECIST) criteria. Objective progression of disease was not a criterion for cessation of treatment due to the possibility of delayed response to this agent as well as limited treatment alternatives for this patient population. Treatment was discontinued for severe symptoms from progressive disease, immune-mediated adverse events, steroid use, subject withdrawal, or death. Ophthalmologic examination, thyroid function, pituitary function, and autoimmunity were monitored during the treatment phase of the study to identify immune-mediated adverse events.
Because the response of the primary tumor may differ from that of metastases, subjects were stratified into 2 cohorts: those with locally advanced pancreatic cancer or metastatic disease. The trial was designed as a phase II clinical trial with objective response by RECIST criteria (PR + CR) as the primary end point. Initially an enrollment of 21 patients was planned for each cohort to rule out an undesirably low response probability of 5% (P0 = 0.05) in favor of a level demonstrating potentially useful activity of 20% (P1 = 0.20), with α = 0.05 (5% probability of accepting a poor agent), and β = 0.10 (10% probability of rejecting a good agent). If 2 or more patients showed clinical response by RECIST criteria in either cohort, that cohort was expanded to 41 patients.
RESULTS
Twenty-seven subjects were enrolled in the trial, 20 with metastatic disease and 7 with locally advanced disease. The target of 21 patients per cohort was not fulfilled due to the low response rate by established criteria. Subject accrual to the metastatic cohort was halted when 0/20 patients responded by RECIST criteria (It was clear 2/21 could not respond so a 21st subject was not enrolled). At that time, 7 patients with locally advanced disease had been enrolled and no additional patients were added to the locally advanced disease cohort at the discretion of the investigators due to low response rate in patients with metastatic disease.
Demographic characteristics of the study subjects are shown in Table 1. Notably, the patients tended to be younger than characteristic for pancreatic cancer with good performance status. All patients were encouraged to pursue standard treatment when feasible before participation in the study. Twenty patients were treated with a standard gemcitabine-based chemotherapy regimen before enrollment on this trial. The remaining 7 patients were not eligible for standard treatment, or refused standard treatment after in-depth review of the options.
TABLE 1.
Patient Characteristics
| Sex (M/F) | 15/12 |
| Median age (range) | 55 (27 to 68) |
| Extent of disease | |
| Locally advanced | 7 |
| Metastatic | 20 |
| ECOG performance status | |
| 0 | 12 |
| 1 | 14 |
| 2 | 1 |
| Prior treatment | |
| Surgery | 24 |
| Chemotherapy | 20 |
| Radiation | 12 |
| Two or more | 19 |
ECOG indicates Eastern Cooperative Oncology Group.
The majority of patients experienced severe side effects from disease progression that limited the number of doses of Ipilimumab administered in the trial (Table 2). In the 20 subjects with metastatic disease only 8 completed a single course (4 doses) of treatment.
TABLE 2.
Treatment Characteristics: Few Doses Tolerated Before Debilitating Disease Progression
| Total Doses | No. Patients |
|---|---|
| 1 | 3 |
| 2 | 8* |
| 3 | 4 |
| 4 (1 course) | 7† |
| 5 | |
| 6 | 2 |
| 7 | 1 |
| 8 (2 courses) | 2 |
| Grade 3 to 4 Immune-mediated adverse events | |
| Colitis | 1 |
| Hypophysitis | 1 |
| Encephalitis | 1 |
1 stopped for colitis, 1 stopped for encephalitis.
1 stopped for hypophysitis.
Three episodes of grade 3 to 4 immune-mediated adverse events were noted in the 27 subjects, one of which culminated in treatment-related death. A patient with locally advanced pancreatic cancer developed colitis after 2 doses of Ipilimumab. The sole measurable disease was a locally advanced tumor, which showed a maximal regression of 29% (near PR). The colitis was treated unsuccessfully with steroids mandating treatment with antitumor necrosis factor antibody. Thereafter, a mixed Aspergillus and staphylococcal pneumonia developed that was fatal. A second patient with a locally advanced tumor received 2 doses of Ipilimumab and developed confusion and lethargy without abnormalities on central nervous system imaging, or in the cerebrospinal fluid. Symptoms resolved after steroid treatment, and the measurable disease remained stable until the patient withdrew from the trial 7 weeks after the treatment. A third patient with widespread hepatic metastases developed hypophysitis after 3 doses of Ipilimumab. This was adequately managed with hormonal replacement, but the metastases demonstrated rapid progression. A subsequent experimental treatment (percutaneous hepatic perfusion with melphalan) resulted in disease stabilization, and the patient remained on hormonal replacement until death 22 months after Ipilimumab treatment.
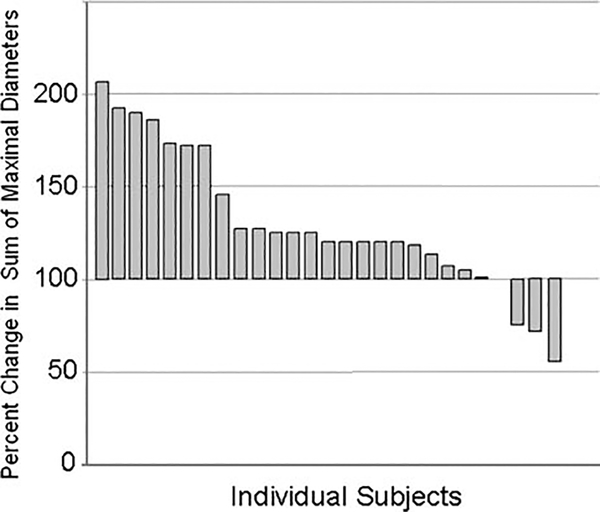
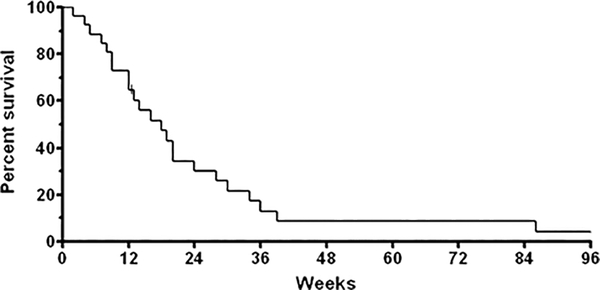
By RECIST criteria, there were no responders to single agent Ipilimumab (Fig. 1). Two patients with locally advanced disease showed a minor response. But, in most patients, progression was rapid with a short survival; as is characteristic for this disease (Fig. 2).
FIGURE 1.
Maximal response in sum of maximal diameters of index lesions for subjects in this study. The single patient with a decrease exceeding 30% was not a responder by RECIST criteria due to new lesions at evaluation. These lesions subsequently regressed. Two patients with locally advanced disease showed a minor response.
FIGURE 2.
Overall survival of patients on trial. One patient is lost to follow-up after progression of disease at 12 weeks. Many patients entered with metastatic disease receiving Ipilimumab as second line therapy. Short survival is characteristic for these patients.
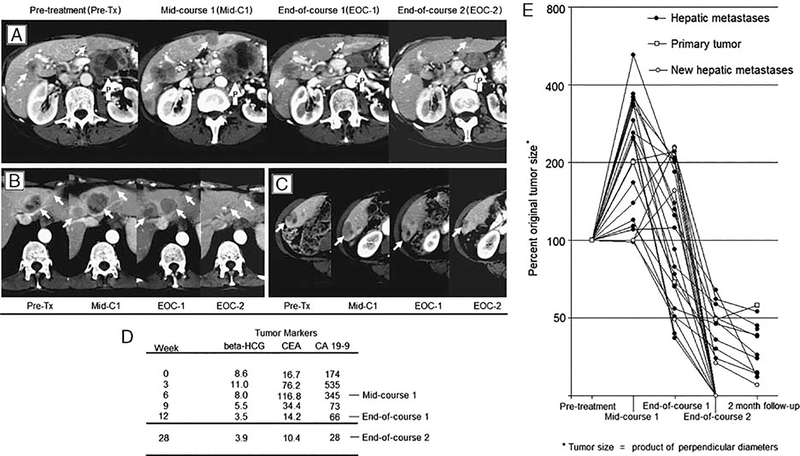
However, 1 patient experienced a delayed response that was both measurable and clinically significant. This subject was a 67-year-old woman who was diagnosed with metastatic pancreas cancer to the liver 8 months before enrollment on the trial. Her disease had transiently responded to gemcitabine but then progressed. Before receiving Ipilimumab, the patient had an ECOG performance status of 1 (fatigue, mild abdominal pain) and imaging showed a 5.4-cm diameter primary pancreatic mass with 16 hepatic metastases. During the first course, her ECOG performance status declined to 3 with worsening fatigue and inability to ambulate more than 50 feet. Her serum tumor markers increased and an interim scan confirmed progression of disease in the pancreas and liver along with the appearance of 4 new hepatic metastases.
In contrast, at the end of course 1, the patient reported improved energy with ECOG performance status returning to 0: ambulating several miles daily. Serum tumor markers declined correlating to regression of the primary and all metastases compared to the interim scan (Fig. 3). Her tumor continued to regress through a second course though one of the new liver metastases never resolved completely.
FIGURE 3.
A patient with pancreas adenocarcinoma was treated with Ipilimumab (3 mg/kg) every 3 weeks with immune-mediated tumor regression evident after the mid-course 1 evaluation. A–C, The primary tumor (white arrows with the letter “P”) and liver metastases (white arrows) regress after mid-course 1 evaluation in representative tumors. Four new metastases developed between start of treatment and the midcourse evaluation. A new metastasis is shown by the right hepatic vein at midcourse evaluation in (B). D, Tumor markers during the 2 courses of treatment show a decline in all measures after mid course-1 evaluation. E, Change in tumor size relative to size at the presentation of the lesion. Four new hepatic metastases are depicted at mid course-1 evaluation with baseline tumor size measured at 100% at this time point
By RECIST definition, she always demonstrated progressive disease due to the presence of at least 1 new lesion compared with baseline. Approximately 6 days after each dose the patient developed a grade 2 rash, which peaked in severity at day 8 to 9 and resolved to brownish macules before the next dose. This was consistent with immune-mediated rashes seen in other patients treated with Ipilimumab. No other immune-mediated toxicities were exhibited by this patient.
Nine weeks after the last dose of the drug, 9 months after the initiation of Ipilimumab, both CA19–9 and CEA levels increased. At that time, cross-sectional imaging showed the primary tumor progressed and new disease was evident in the small bowel mesentery. The patient was removed from the study and went on to receive other therapy. The hepatic metastases progressed 20 weeks after the last dose of the drug and the patient died of disease 30 weeks after Ipilimumab treatment.
DISCUSSION
Few investigators have explored the use of immunotherapy for pancreas cancer. Jaffee and colleagues delivered vaccination in the adjuvant setting using a genetically modified granulocyte macrophage colony-stimulating factor secreting allogeneic irradiated whole tumor cell preparation. Both a delayed type hypersensitivity response to autologous tumor challenge and the generation of mesothelin-specific T cells were seen in these patients. As this was used as an adjuvant treatment, objective tumor response could not be assessed. Some patients on this trial experienced survival of several years, but the contribution of vaccination to this survival is unknown.32
Similarly, other investigators have vaccinated patients against mutated RAS,33 an autologous heat shock protein,34 MUC-1,35 gastrin,36 or telomerase37 in the adjuvant or metastatic disease setting. In all instances, patients with measurable disease showed no tumor regression. However, a correlation was reported in all of these studies between the duration of survival and the development of immunity, as analyzed by different methods. The disappointing clinical responses seen to date with pancreatic cancer vaccines are in line with those reported for vaccines used against other tumor histologies.20
In contrast to these vaccination studies, Wobser and colleagues reported that a single patient vaccinated against survivin in the face of hepatic metastases from a low-grade mucinous pancreatic adenocarcinoma showed complete resolution of hepatic metastases after 9 months of vaccination.38 The patient vaccinated against survivin and the delayed response highlighted in this report, are the only reported regressions of pancreatic cancer from an immunotherapeutic approach.
Aside from vaccination, the Virginia Mason regimen for adjuvant therapy of resectable pancreas cancer uses an immunologic agent, interferon-alpha (IFN), as a component of a multidrug regimen that mediates extended survival in single arm trials.39 IFN can increase tumor antigenicity, but it has many mechanisms of action40; and an immunotherapeutic effect is unlikely in this marrow suppressive regimen. In this regimen, IFN is delivered in the adjuvant setting, when no visible tumor is present and no tumor regression can be confirmed.
Hesitation to use immunotherapy may in part be due to the suspicion that patients with pancreatic cancer are unable to mount an immune response against any antigen. In this study, we did not measure whether the subjects included were anergic, but previous studies suggest these patients were able to respond to antigen. Tseng and colleagues analyzed the response of subjects with pancreatic adenocarcinoma to a standard anergy panel and found patients are able to mount both humoral and cellular responses to standard vaccines.41 Similarly, Horig and colleagues found subjects with pancreatic cancer were able to consistently develop elevated antibody titers against virus when vaccinated with an altered fowlpox virus.42
The immunosuppressive tumor microenvironment observed in pancreas cancer raises the possibility that altering immunoregulation may be an effective means of immunotherapy in these patients. We used the anti-CTLA-4 antibody as an agent to block the immunosuppressive CTLA-4 signal. In the current study of 27 patients, we documented a significant delayed regression of metastatic pancreas cancer in one of the subjects. This case illustrates 2 important points: (1) under specific conditions, pancreas cancer may be susceptible to an immunotherapeutic approach. Exploration of alternative immunotherapies to treat pancreas cancer, or combinatorial approaches, is therefore warranted. (2) Tumor regression in response to Ipilimumab-based therapy may be delayed and may indeed follow transient progression of disease. In the case highlighted in this report, marked progression of the tumor was noted prior to the striking regression of disease. This is in contrast to most antineoplastic therapies in which mediate early tumor regression, and may reflect the indirect the mechanism of action of Ipilimumab which acts through immune modulation rather than direct antitumor activity. Standard RECIST criteria may be inadequate for assessing responses due to Ipilumumab and guiding treatment decisions for patients receiving the agent.
This is the first report of the use of Ipilimumab against advanced gastrointestinal cancer. We recorded the regression of a pancreatic adenocarcinoma in response to Ipilimumab. However, this report should not be interpreted as an endorsement of single agent Ipilimumab at a dose of 3.0 mg/kg as an effective therapy for pancreas adenocarcinoma. We did not, in this study, show acceptable response rates to advocate its use for therapy in this disease.
The agent may prove to be more effective against pancreas cancer under conditions that differ from those used in this study. For example, Ipilimumab may be efficacious (1) at higher doses, (2) in a minimal disease setting or (3) as a component of combination therapy. A dose of 10 mg/kg is now routinely used to treat patients with advanced melanoma. The trial reported was designed and initiated when the lower dose was generally used but treatment with the 10 mg/kg dose may allow for regression of tumor before symptoms from pancreas cancer preclude further dosing. Secondly, in this study, patients with advanced disease rarely tolerated a full 12-week course of Ipilimumab and few patients developed immune mediated adverse events (suggesting suboptimal CTLA-4 blockade). Patients with lesser disease burdens would potentially tolerate longer treatment duration. For example, Ipilimumab therapy could be initiated after neoadjuvant therapy and resection of early pancreas cancers or after stabilization of locally advanced disease with radiation and chemotherapy. These are time points where symptoms are usually low, and no standard therapy is delivered. Thirdly, preclinical studies suggest the efficacy of anti-CTLA-4 is increased when combined with vaccination.43 Combining Ipilimumab with an effective pancreas cancer vaccine deserves study. In addition, studies suggest combination of chemotherapy44 or radiation45 with vaccination may enhance the development of immunity against tumor targets. Likewise, combination of Ipilimumab with chemotherapy or radiation may increase Ipilimumab activity. These concepts require further development in preclinical models before advancing to combinatorial therapies in clinical trials.
At present, patients with pancreas cancer should only receive Ipilimumab in the context of well designed clinical trials, and clinical trials should build on the lessons learned from this initial study. Our study shows Ipilimumab in this dose scheme is not efficacious against pancreatic cancer. The fact that some tumor regression was seen supports testing of this agent at higher doses and at earlier disease stages, possibly with combination agents. In addition, RECIST response criteria are inadequate to measure some responses to Ipilimumab and need to be modified to allow for the delayed mechanism of action of this drug.
ACKNOWLEDGMENTS
The authors thank the Immunotherapy Fellows, Research Nurses, Data Managers, and Protocol Support team of the Surgery Branch at the National Cancer Institute. Without their tireless effort, attention to detail, and thoughtful devotion the authors would not have been able to complete this trial.
Richard E. Royal has received honoraria from Medarex, Inc. Suzanne L. Topalian has received honoraria and research funding from Medarex, Inc. Israel Lowy is employed by Medarex, Inc. Steven A. Rosenberg has received research funding from Medarex, Inc.
Supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health
Footnotes
All other authors have declared there are no financial conflicts of interest in regard to this work.
REFERENCES
- 1.Royal RE, Wolff RA, Crane CH. Pancreatic cancer In: DeVita VT, Lawrence TS, Rosenberg SA, eds. Cancer: Principles and Practice of Oncology. 8th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2008:1098–1136. [Google Scholar]
- 2.Bilimoria KY, Bentrem DJ, Ko CY, et al. National failure to operate on early stage pancreatic cancer. Ann Surg. 2007;246: 173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakkevold KE, Arnesjo B, Dahl O, et al. Adjuvant combination chemotherapy (AMF) following radical resection of carcinoma of the pancreas and papilla of Vater–results of a controlled, prospective, randomised multicentre study. Eur J Cancer. 1993;29A:698–703. [DOI] [PubMed] [Google Scholar]
- 4.Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120:899–903. [DOI] [PubMed] [Google Scholar]
- 5.Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg. 1999;230:776–782. discussion 782–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–1210. [DOI] [PubMed] [Google Scholar]
- 7.Neuhaus H, Riess S. CONKO-001: final results of the randomized, prospective, multicenter phase III trial of adjuvant chemotherapy with gemcitabine versus observation in patients with resected pancreatic cancer (PC). J Clin Oncol 2008;26:LBA4504. [Google Scholar]
- 8.Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267–277. [DOI] [PubMed] [Google Scholar]
- 9.Regine WF, Winter KA, Abrams RA, et al. Fluorouracil vs. gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA. 2008;299:1019–1026. [DOI] [PubMed] [Google Scholar]
- 10.Takada T, Amano H, Yasuda H, et al. Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer. 2002;95:1685–1695. [DOI] [PubMed] [Google Scholar]
- 11.Ferrone CR, Brennan MF, Gonen M, et al. Pancreatic adenocarcinoma: the actual 5-year survivors. J Gastrointest Surg. 2008;12:701–706. [DOI] [PubMed] [Google Scholar]
- 12.Moertel CG, Childs DS Jr, Reitemeier RJ, et al. Combined 5-fluorouracil and supervoltage radiation therapy of locally unresectable gastrointestinal cancer. Lancet. 1969;2:865–867. [DOI] [PubMed] [Google Scholar]
- 13.Moertel CG, Frytak S, Hahn RG, et al. Therapy of locally unresectable pancreatic carcinoma: a randomized comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4000 rads + 5-fluorouracil), and high dose radiation + 5-fluorouracil: The Gastrointestinal Tumor Study Group. Cancer. 1981;48:1705–1710. [DOI] [PubMed] [Google Scholar]
- 14.Burris HA III, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. [DOI] [PubMed] [Google Scholar]
- 15.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. [DOI] [PubMed] [Google Scholar]
- 16.Royal RE, Steinberg SM, Krouse RS, et al. Correlates of response to IL-2 therapy in patients treated for metastatic renal cancer and melanoma. Cancer J Sci Am. 1996;2:91–98. [PubMed] [Google Scholar]
- 17.Klapper JA, Downey SG, Smith FO, et al. High-dose interleukin-2 for the treatment of metastatic renal cell carcinoma: a retrospective analysis of response and survival in patients treated in the surgery branch at the National Cancer Institute between 1986 and 2006. Cancer. 2008;113: 293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–2116. [DOI] [PubMed] [Google Scholar]
- 19.Rosenberg SA, Yang JC, White DE, et al. Durability of complete responses in patients with metastatic cancer treated with high-dose interleukin-2: identification of the antigens mediating response. Ann Surg. 1998;228:307–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10: 909–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dudley ME, Yang JC, Sherry R, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clark CE, Hingorani SR, Mick R, et al. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 2007;67:9518–9527. [DOI] [PubMed] [Google Scholar]
- 23.Liyanage UK, Goedegebuure PS, Moore TT, et al. Increased prevalence of regulatory T cells (Treg) is induced by pancreas adenocarcinoma. J Immunother. 2006;29:416–424. [DOI] [PubMed] [Google Scholar]
- 24.Downey SG, Klapper JA, Smith FO, et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007;13:6681–6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang JC, Hughes M, Kammula U, et al. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother. 2007;30:825–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Small EJ, Tchekmedyian NS, Rini BI, et al. A pilot trial of CTLA-4 blockade with human anti-CTLA-4 in patients with hormone-refractory prostate cancer. Clin Cancer Res. 2007;13: 1810–1815. [DOI] [PubMed] [Google Scholar]
- 27.Theoret MR, Arlen PM, Pazdur M, et al. Phase I trial of an enhanced prostate-specific antigen-based vaccine and anti-CTLA-4 antibody in patients with metastatic androgen-independent prostate cancer. Clin Genitourin Cancer. 2007;5: 347–350. [DOI] [PubMed] [Google Scholar]
- 28.Beck KE, Blansfield JA, Tran KQ, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol 2006;24:2283–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blansfield JA, Beck KE, Tran K, et al. Cytotoxic T-lymphocyte-associated antigen-4 blockage can induce autoimmune hypophysitis in patients with metastatic melanoma and renal cancer. J Immunother. 2005;28:593–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Attia P, Phan GQ, Maker AV, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol 2005;23:6043–6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Callery MP, Chang KJ, Fishman EK, et al. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol. 2009;16: 1727–1733. [DOI] [PubMed] [Google Scholar]
- 32.Jaffee EM, Hruban RH, Biedrzycki B, et al. Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J Clin Oncol. 2001;19:145–156. [DOI] [PubMed] [Google Scholar]
- 33.Toubaji A, Achtar M, Provenzano M, et al. Pilot study of mutant ras peptide-based vaccine as an adjuvant treatment in pancreatic and colorectal cancers. Cancer Immunol Immunother. 2008;57:1413–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maki RG, Livingston PO, Lewis JJ, et al. A phase I pilot study of autologous heat shock protein vaccine HSPPC-96 in patients with resected pancreatic adenocarcinoma. Dig Dis Sci. 2007;52:1964–1972. [DOI] [PubMed] [Google Scholar]
- 35.Kaufman HL, Kim-Schulze S, Manson K, et al. Poxvirus-based vaccine therapy for patients with advanced pancreatic cancer. J Transl Med. 2007;5:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brett BT, Smith SC, Bouvier CV, et al. Phase II study of antigastrin-17 antibodies, raised to G17DT, in advanced pancreatic cancer. J Clin Oncol. 2002;20:4225–4231. [DOI] [PubMed] [Google Scholar]
- 37.Bernhardt SL, Gjertsen MK, Trachsel S, et al. Telomerase peptide vaccination of patients with non-resectable pancreatic cancer: a dose escalating phase I/II study. Br J Cancer. 2006; 95:1474–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wobser M, Keikavoussi P, Kunzmann V, et al. Complete remission of liver metastasis of pancreatic cancer under vaccination with a HLA-A2 restricted peptide derived from the universal tumor antigen survivin. Cancer Immunol Immunother. 2006;55:1294–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nukui Y, Picozzi VJ, Traverso LW. Interferon-based adjuvant chemoradiation therapy improves survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am J Surg. 2000;179:367–371. [DOI] [PubMed] [Google Scholar]
- 40.Hoffmann K, Mehrle S, Schmidt J, et al. Interferon-alpha restitutes the chemosensitivity in pancreatic cancer. Anticancer Res. 2008;28:1499–1507. [PubMed] [Google Scholar]
- 41.Tseng JF, Willett CG, Fernandez-del Castillo C, et al. Patients undergoing treatment for pancreatic adenocarcinoma can mount an effective immune response to vaccinations. Pancreatology. 2005;5:67–74. [DOI] [PubMed] [Google Scholar]
- 42.Horig H, Lee DS, Conkright W, et al. Phase I clinical trial of a recombinant canarypoxvirus (ALVAC) vaccine expressing human carcinoembryonic antigen and the B7.1 co-stimulatory molecule. Cancer Immunol Immunother. 2000;49: 504–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hodge JW, Chakraborty M, Kudo-Saito C, et al. Multiple costimulatory modalities enhance CTL avidity. J Immunol. 2005; 174:5994–6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nistico P, Capone I, Palermo B, et al. Chemotherapy enhances vaccine-induced antitumor immunity in melanoma patients. Int J Cancer. 2009;124:130–139. [DOI] [PubMed] [Google Scholar]
- 45.Teitz-Tennenbaum S, Li Q, Okuyama R, et al. Mechanisms involved in radiation enhancement of intratumoral dendritic cell therapy. J Immunother. 2008;31:345–358. [DOI] [PMC free article] [PubMed] [Google Scholar]