Summary
Antitumor immune response and chemotherapy-induced immunomodulation in colon cancer patients represented the rationale to design new strategies, like GOLFIG chemoimmunotherapy (gemcitabine, oxaliplatin, 5-fluorouracil/folinic acid, granulocyte macrophage colony-stimulating factor, and aldesleukine), that resulted a safe and very active regimen. Antitumor activity and immunity feedback to GOLFIG were strictly correlated with the best outcome observed in patients with autoimmunity signs, increase of central memory T cells, and decrease of regulatory T cells (Treg) in the peripheral blood. We thus investigated a potential correlation between the Treg tumor infiltration at diagnosis and the clinical outcome in a current randomized phase 3 trial aimed to compare the GOLFIG regimen with the standard FOLFOX chemotherapy (GOLFIG-2). An immunohistochemistry study was carried out to quantify the infiltration of Treg/FoxP3+ T lymphocytes in tumor samples of 57 patients enrolled in the GOLFIG-2 trial. Treg tumor infiltration scores were correlated with overall survival, treatment-relative survival, and progression-free survival (PFS). Higher Treg tumor infiltration scores were associated with a better prognosis in the whole series (Treg high score vs. low score: overall survival = mean 43.2 mo vs. 28.6 mo, P = 0.0005) and a better outcome after treatment (Treg high score vs. low score: PFS = mean 15.8 mo vs. 8.8 mo, P = 0.0009; treatment-relative survival = mean 23.1 mo vs. 18.2 mo, P = 0.004). PFS was significantly longer in GOLFIG high versus all other subgroups (mean 18.1 mo vs. 9.9 mo, P = 0.01). Our results suggest that a higher FoxP3+ T-lymphocyte tumor infiltration score is a favorable prognostic factor in colon cancer patients undergoing chemo or chemoimmunotherapy.
Keywords: chemotherapy, immunotherapy, colon cancer, FoxP3+ T-cell, immunoregulatory T cells
Colorectal carcinoma is the second leading cause of cancer-related deaths.1 The best therapeutic option for the advanced disease is represented by combination chemotherapy with 5-fluorouracil (5-FU) ± levofolinic acid (LF) together with irinotecan (FOLFIRI) or oxaliplatin (FOLFOX) given alone, or in combination with bevacizumab or cetuximab, 2 monoclonal antibodies directed against the vascular endothelial growth factor, and the epidermal growth factor receptor, respectively.2 We have recently completed a phase 2 trial (GOLFIG-1) in metastatic colon carcinoma patients aimed to test the antitumor activity of a novel polychemoimmunotherapy regimen combining gemcitabine, oxaliplatin, LF, and 5-FU (GOLF)3 with an immunoadjuvant treatment with subcutaneous granulocyte macrophage colony-stimulating factor followed by metronomic low-dose subcutaneous interleukin (IL)-2 (granocyte-macrophage colony-stimulating factor).4 GOLFIG regimen resulted reasonably safe and showed a very promising antitumor activity.5,6 The phase 2 disclosed an objective response rate and a disease control rate of 56.5% and 91.3%, respectively, and a median time to tumor progression of 12.26 months. These results were highly suggestive, taking in account that the majority of patients were in second/third chemotherapy line and offered the rationale to design a phase 3 trial aimed to compare GOLFIG with FOLFOX-4, which is a standard treatment with oxaliplatin+LF+5-FU in bolus followed by infusional 5-FU,7 regimen as frontline treatment of advanced colorectal tumors (GOLFIG-2 protocol code 457/05).
In the previous GOLFIG-1 clinical study, a multivariate analysis validated the occurrence of autoimmunity signs as the most relevant independent predictive variable of prolonged time to tumor progression and overall survival (OS).6 During the treatment there was a progressive increase in the number of peripheral lymphocytes and an improved tumor antigen-specific cytotoxic T lymphocytes (CTL) response (in terms of either tumor antigen-specific CTL precursor’s frequency or cytolytic activity). We observed a significant increase in the number of peripheral and tumor-infiltrating lymphocytes (TIL) with central memory (CD8+CCR7+CD27+CD62L+) immunophenotype. This particular lymphocyte subset drives the immune response into the tumor site and lymphoid organs where the immune attack takes place.8 All these events occurred in parallel with a progressive decrease of peripheral and TIL with a immunoregulatory T (Treg) cell immunophenotype (CD4+CD25+ FoxP3+). This immune suppressive lymphocyte subset is strictly related to preexisting immune attack9–14 and is sensitive to the cytotoxic effects of GOLF regimen.15
All together these findings prompted us to investigate a potential correlation between the Treg tumor infiltration (FoxP3+ T cells) at diagnosis and the clinical outcome of metastatic colon cancer patients enrolled in the ongoing GOLFIG-2 phase 3 trial randomized to receive standard FOLFOX-4 chemotherapy or GOLFIG chemoimmunotherapy.
MATERIALS AND METHODS
Study Design
The phase 3 trial, designated with the code number 457/05 GOLFIG-2, was authorized by the University Committee (equivalent to Human Subject Committee of Investigational Review Board) and by the Italian Ministry of Health. All enrolled patients were untreated for metastatic disease and signed informed consent.
Patient Characteristics
The inclusion criteria were: a histologic diagnosis of colorectal carcinoma, an Eastern Cooperative Oncology Group performance status ≤ 2, normal renal and hepatic functions, white blood cell count ≥ 2500/mm3, hemoglobin levels ≥ 9 g/dL, platelet cell count ≥ 100,000/mm3, and normal cardiac function. The exclusion criteria were: any major organ failure, central nervous system involvement, other malignancies, active infectious diseases, major autoimmune diseases, and acquired immune suppression. Before the enrolment in this trial, some patients had undergone adjuvant chemotherapy. Data from all participant centers were collected at the Section of Medical Oncology, Department Giorgio Segre of Pharmacology and Pathology Section, Human Pathology and Oncology, Siena University School of Medicine, Italy.
Treatment Schedules and Patients’ Evaluation
All patients in the FOLFOX-4 arm received biweekly chemotherapy with oxaliplatin (85 mg/m2 on day 1), LF (100 mg/m2 on days 1 and 2), and 5-FU (400 mg/m2 as a bolus and 600 mg/m2 as a 24 h infusion on days 1 and 2); all patients in the GOLFIG arm received biweekly chemotherapy with gemcitabine (1000 mg/m2 on days 1 and 15), oxaliplatin (85 mg/m2 on days 2 and 16), LF (100 mg/m2 on days 1, 2, 15, and 16), and 5-FU (400 mg/m2 as a bolus and 800 mg/m2 as a 24 h infusion on days 1, 2, 15, and 16), followed by subcutaneous granulocyte macrophage colony-stimulating factor (100 μg on days 3 to 7) and ultra-low dose subcutaneous IL-2 (0.5 × 106 IU twice a day on days 8 to 14 and 17 to 29).5 Standard assessments (clinical history, physical examination, hematochemical analysis, carcino-embryonic antigen and carbohydrate antigen 19.9 assays, chest x-ray, and ultrasound scans) were performed at baseline and repeated every 4 weeks. High-definition, multi-slice computed tomography scans with contrast medium were recorded every 3 months. The objective of the GOLFIG-2 trial was to compare efficacy of GOLFIG with FOLFOX-4 regimen as frontline treatment of advanced colorectal tumors. The primary end point of the study was the progression-free survival (PFS; calculated from trial enrolment to disease progression or death or loss to follow up). Patients were also evaluated for OS (calculated from diagnosis of cancer to death), treatment-relative survival (rOS; calculated from trial enrolment to death). The analysis was performed by ”intention to treat.“ The median follow-up of patients included in the present analysis was 15 months.
Pathology Study
Tumor tissues derived from biopsy or radical surgery was fixed in 10% buffered-neutral formalin and paraffin-embedded for histology and immunohistochemistry. Sections of each specimens were stained with hematoxylin and eosin and histologically examined by an expert pathologist.
Immunohistochemistry
Immunohistochemical staining was performed on 3-μm thick sections of each block by the streptoavidinbiotin method. Cores were taken at random from within the tumor block face and at least 3 different samples for each patients were evaluated to be more representative of the tumor tissue. After being dewaxed and rehydrated, sections were incubated with 3% H2O2 in Tris buffered-saline to inhibit endogenous peroxidase and processed with different methods for each antibody. To show CD4+ T cells the sections were unmasked with Wcap buffer (pH 6.0 for 40 min at 98°C; Bio-Optica, Milan, Italy) and were incubated with antihuman monoclonal antibody CD4 (clone 4B12; 1:50; Menarini, Florence, Italy). For CD8+ T cells, pretreatment with a microwave oven in citrate buffer (0.01M, pH 6.0) at 750W for 5 minute was performed for 3 cycles, and the sections were incubated with antihuman monoclonal antibody CD8 (clone CD8–144B; 1:50; Dako, Milan, Italy); the epitopes were detected with the Ultravision Detection System and revealed with the diaminobenzidine for 5 minute (Dako, Milan, Italy). For detection of FoxP3+ T cells we used pretreatment with a microwave oven in ethylenediaminetetraacetic acid (0.05M, pH 8.0) at 750W for 5 minutes, 3 cycles, and the sections were incubated with monoclonal antibody antihuman FoxP3 (clone 22510; 1:50; 60min; Abcam, Cambridge, UK); the primary antibody enhancer was associated with AP polymer (Ultravision LP Detection System AP Polymer, LAB Vision) and revealed with fucsin (DakoCytomation). Negative controls were obtained by replacing the specific antibody with nonimmune serum immunoglobulins at the same concentration of the primary antibody. Immunostaining was examined with a Zeiss Axioplan 2 microscope (Carl Zeiss Microscopy, Jena, Germany). Blind reanalysis was carried out to confirm the results.
Quantitative Evaluation of T Lymphocyte Subtypes
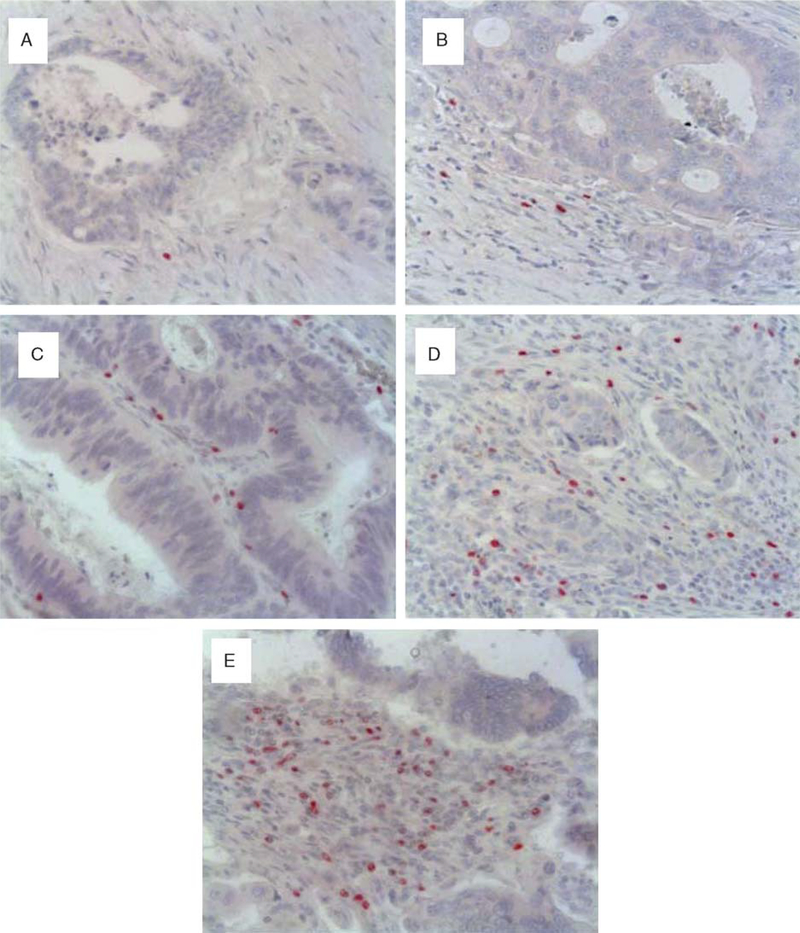
Sections stained for CD4+, CD8+, or FoxP3+ lymphocytes were scored in coded slides by one observer (C.G.). The numbers of positive cells for each marker were recorded in 5 randomly chosen high-power fields in the stroma adjacent to neoplastic glands. FoxP3+ tumor tissue infiltration was scored as follows: 1 to 10 positive cells=0, 11 to 15=1, 16 to 30=2, 31 to 50=3, 51 to 70=4, and >70=5 (Fig. 1).
FIGURE 1.
Immunohistochemistry scores of T lymphocytes expressing FoxP3; panels A represents score 1; B, score 2; C, score 3;D, score 4; and E, score 5 (original magnification × 200).
Statistical Analysis
Survival plots were constructed using the Kaplan-Meyer method, and the survival data were analyzed by using the GraphPad Instat 3.2 statistic software. Regression analysis was performed by the GraphPad Instat 3.2 statistic software. Cox analysis was performed by the NCSS statistical software. Using this program, we performed a multivariate Cox (proportional hazards) regression analysis, which models the relationship between a set of one or more covariates and the hazard rate. We studied the impact of various factors on survival. We specified categorical independent variables. Time variable contains the length of time where an individual was observed. This may represent a failure time or a censor time.
RESULTS
Demography and Study Design
Fifty-seven consecutive patients with metastatic colon carcinoma, 35 men and 22 women, with a median age of 64 years, were included in this study. These patients had been enrolled in the GOLFIG-2 phase 3 trial and had been randomized to receive first-line treatment with FOLFOX-4 chemotherapy (27 patients) or GOLFIG (30 patients) chemoimmunotherapy.
An immunohistochemical study was performed on tumor samples obtained from these patients before any systemic treatment. The study was carried out to evaluate the amount of tumor-infiltrating CTLs and Treg lymphocytes. A previous double-colour immunohistochemistry characterization performed on 10 samples showed FoxP3 expression only in CD4+ T cells (data not shown). Further experiments also showed in these FoxP3+ T cells high intracellular expression of transforming growth factor-b (data not shown). These data allowed us to identify FoxP3+ T cells as active Tregs with immunosuppressive status.
Different levels of lymphocyte infiltration were classified by setting an arbitrary score based on the number of lymphocytes expressing the specific marker (CD8 for CTLs and FoxP3 for Tregs) counted in each high power slide field. Patients were then assigned to low (0 to 2; 1 to 30 FoxP3 positive cells/field) or high (3 to 5; more than 30 FoxP3 positive cells/field) infiltration score group, respectively (Fig. 1). A statistical analysis was performed to evaluate a potential correlation among a specific lymphocyte subset infiltration score and OS (survival since cancer diagnosis), rOS (survival since trial enrolment), or PFS of these patients.
Prognostic Value of FoxP3+ (Treg) Tumor Infiltration Score
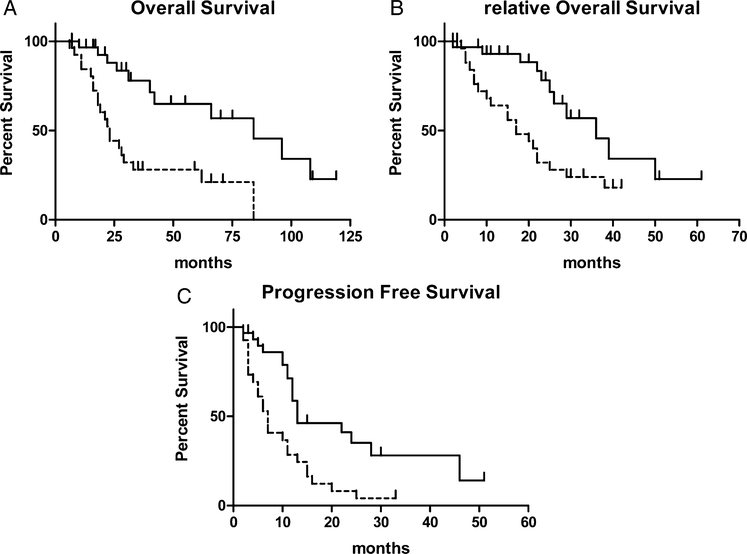
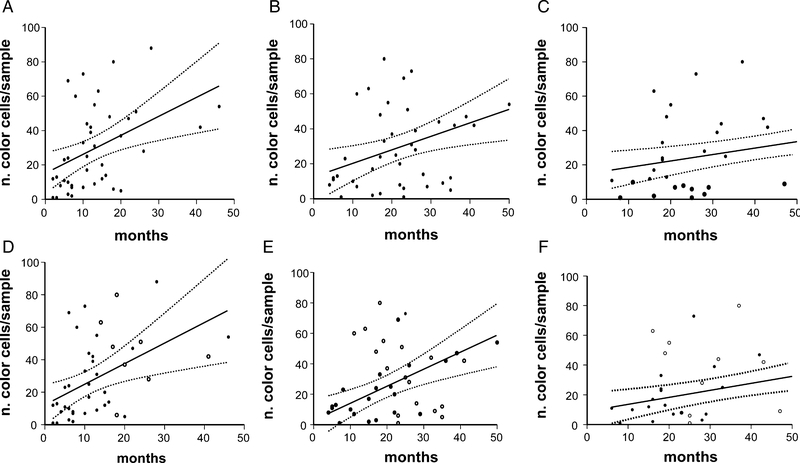
Our analysis in the whole patients’ series revealed that a high Treg tumor infiltration score at the diagnosis correlated with a prolonged OS (high, > 30 cells/field FoxP3+ vs. low score, < 30 cells/field FoxP3+: mean 43.2 mo vs. 28.6 mo; P = 0.0005), and a better outcome to the frontline treatment in term of rOS (high score vs. low score: mean 23.1 mo vs. 18.2 mo, P = 0.004) and PFS (high score vs. low score: mean PFS = 15.8 mo vs. 8.8 mo, P = 0.0009) (Fig. 2). The correlation between FoxP3 expression and PFS, OS, and rOS was also evaluated by linear regression analysis which was performed both by considering events (relapse or death) alone or events together with follow up length in patients without events. In all cases there was a statistical correlation between the average number of FoxP3 cells/field and PFS, OS, and rOS (r2 0.2132 P ≤ 0.006, r2 0.3233 P ≤ 0.004, and r2 0.3999 P ≤ 0.001 for analysis including events only and r2 0.1918 P ≤ 0.004, r2 0.2011 P ≤ 0.003, and r2 0.1554 P ≤ 0.01 for analysis including also follow up length in patients without events) (Fig. 3). A Cox regression model demonstrated that a high Treg tumor infiltration score (score 3 to 5; more than 30 FoxP3 positive cells/field) and a good electrocochleography performance status are 2 independent variables of prolonged survival and that the high Treg tumor infiltration score alone is an independent variable of treatment-related better outcome (evaluated as PFS) (Table 1).
FIGURE 2.
Actuarial Kaplan-Meyer survival curves of 57 colon cancer patients undergone FOLFOX or GOLFIG treatment whose tumor was immunohistochemically scored for Treg infiltration. Panels compare overall survival (A), relative overall survival (from trial enrolment to death; B), and progression-free survival (C) with infiltrating lymphocytes expressing FoxP3.
FIGURE 3.
Regression curves (solid line) (with 95% confidence interval) (dotted line) according to the number of tumor-infiltrating lymphocytes positive for the expression of FoxP3. Panels compare overall survival (A), relative overall survival (from trial enrolment to death; B), and progression-free survival (C) with the actual number of tumor-infiltrating lymphocytes positive for the expression of FoxP3. Panels compare overall survival (D), relative overall survival (E), and progression-free survival (F) with the actual number of tumor-infiltrating lymphocytes positive for the expression of FoxP3 in patients with (●) or without events (○).
TABLE 1.
Cox Regression Model (With 95% CI) According to Different Variables
| Prognostic Variable | P | Risk Ratio (95% CI) |
|---|---|---|
| (A) Represents the risk of disease progression | ||
| FoxP3 expression | 0.007* | 0.34 (0.15–0.75) |
| Grading | 0.34 | 1.43 (0.68–3.00) |
| Disease free survival | 0.73 | 0.99 (0.97–1.01) |
| Basal lymphocytes number | 0.52 | 1.00 (0.99–1.0005) |
| Performance status | 0.07 | 1.80 (0.94–3.45) |
| Sex | 0.26 | 1.58 (0.70–3.54) |
| (B) Represents the risk of death | ||
| FoxP3 expression | 0.02* | 0.28 (0.09–0.83) |
| Grading | 0.89 | 0.93 (0.36–2.40) |
| Disease free survival | 0.71 | 0.99 (0.96–1.02) |
| Basal lymphocytes number | 0.66 | 1.00 (0.99–1.0008) |
| Performance status | 0.009* | 2.66 (1.27–5.55) |
| Sex | 0.07 | 2.63 (0.91–7.62) |
Statistically significant values.
CI indicates confidence interval.
In this study the different patient treatment groups were also analyzed. In either treatment arms, patients with higher Treg scores showed a better outcome. In fact, it reached a statistical significance in: (1) high score FOLFOX versus low score FOLFOX in OS (P = 0.0025), rOS (P = 0.0088), PFS (P = 0.0421) and (2) high score GOLFIG versus low score GOLFIG only for PFS (P = 0.0340) (Table 2). Finally, high score GOLFIG had a prolonged PFS which was significantly compared with all other subgroups (mean 18.1 mo vs. 9.9 mo, P = 0.01). Our analysis did not show any correlation among Treg score with: (1) lymphocyte infiltration density, (2) CD8+/CD4+ T-cell tumor infiltration score, (3) tumor grading, and (4) tumor stage at the diagnosis. Finally, we were also unable to demonstrate any statistical correlation among CD8+ or CD4+ tumor infiltration score, CD8+/FoxP3+ TIL ratio, and CD4+/FoxP3+ TIL ratio, respectively, with OS, rOS, and PFS (data not shown).
TABLE 2.
PFS, OS, and rOS for the 2 Treatment Arms GOLFIG and FOLFOX Stratified for FoxP3 Expression High or Low
| Treatment | FoxP3+ Expression Score | OS | rOS | PFS |
|---|---|---|---|---|
| FOLFOX | Low | Mean 25.4 (95% CI 17–33.8), median 21 | Mean 17.5 (95% CI 11.4–23.5), median 15 | Mean 8.3 (95% CI 4.5–12.2), median 5.0 |
| High | Mean 31.4 (95% CI 18.2–44.5), median 28 | Mean 19.2 (95% CI 13.8–24.6), median 22.5 | Mean 12.4 (95% CI 8.6–16.2), median 12.5 | |
| P = 0.0025* | P = 0.0088* | P = 0.0421* | ||
| GOLFIG | Low | Mean 39.8 (95% CI 9.9–69.6), median 27.5 | Mean 20.6 (95% CI 10.2–31.1), median 20.5 | Mean 10.6 (95% CI 5.9–15.3), median 11.0 |
| High | Mean 51 (95% CI 32.5–69.5), median 41 | Mean 25.7 (95% CI 17.3–34), median 24.5 | Mean 18.1 (95% CI 11.1–25.1), median 12.0 | |
| P = 0.1528 | P = 0.0828 | P = 0.0340* |
Statistically significant values.
CI indicates confidence interval; OS, overall survival; rOS, treatment-relative survival; PSF, progression-free survival.
DISCUSSION
We report the results of an immunobiologic investigation which represents a side study of the GOLFIG-2 phase 3 trial, aimed to evaluate in advanced colon carcinoma patients the antitumor efficacy of GOLFIG chemoimmunotherapy regimen compared with the standard FOLFOX-4 chemotherapy.
Our results confirm in a prospective series of colorectal cancer patients the favorable prognostic value of a high FoxP3+ Treg tumor infiltration score and suggest for the first time a favorable upfront treatment-related outcome especially in those patients who have received GOLFIG chemoimmunotherapy.
By starting from a different study design, Salama et al16 have, in fact, already reported that a high Treg cell tumor infiltration score is a favorable prognostic marker for colon cancer patients. Their study, performed on a large sample of 967 colorectal carcinoma patients (593 with American Joint Committee on Cancer stage II and 374 with American Joint Committee on Cancer stage III), however, did not investigate any possible correlation with treatment response. These results were mostly unexpected, considering the known immunosuppressive properties of this lymphocyte subset, which would intuitively suggest a potential correlation with a less favorable outcome. In contrast, the results of a number of different studies have instead suggested a negative prognostic value for either Treg infiltration or FoxP3 expression in patients with melanoma, or breast, ovary, hepatocellular carcinoma, and also colorectal cancer.17–22 Sinicrope et al22 in particular, have recently reported that a low intraepithelial effector CD3+/regulatory (FoxP3+) T-cell ratio can predict an adverse outcome in colon carcinoma patients.
The discrepancy with the results of these studies might take place from the different techniques of tissue analysis and lymphocyte immune characterization but above all, from a completely different patient population. In this contest, in our study, we evaluated the prognostic value of FoxP3+ TIL only in patients who had relapsed and were aimed to receive systemic treatment with FOLFOX regimen or GOLFIG chemoimmunotherapy. We thus excluded by the analysis the larger number of patients with a good prognosis who never relapsed, which was the main target population in the Sinicrope’s study.22 In addition, we do not believe that CD3 may be considered as the best marker to identify an actual effector lymphocyte population as it is also expressed on either Tregs and other immunomodulating T-cell (Th1, Th2, etc).
Another point of discussion is related to the marker chosen to identify Tregs; in fact, many of these studies merely use CD4+ CD25+ high immunophenotype. More recently other authors have demonstrated that the transcription FoxP3 fork-head may be also expressed in cancer cells where it controls the expression of more than 250 genes and that its over expression might represent a negative prognostic factor for breast cancer patients.21
Our results, as well as those reported by Salama et al,16 could be explained by taking in account that Tregs have the specific task of preventing autoimmunity and that they are supposed to attenuate a potentially dangerous over-reactive immune response after viral infections, multiple vaccinations, or prolonged immune reactions.9–14 Their rise in the tumor tissue represents a feedback response to a preexisting cancer cell-directed immune attack and it is therefore a consequence of a specific immune response. Treg expansion is strictly dependent upon IL-2 levels produced by specific antigen-activated effector lymphocytes (either CD4+ or CD8+).9–14 For long time it has been difficult to evaluate this population in the tumor tissues owing to our inability to clearly distinguish them from activated T cells as they were initially identified as lymphocytes with CD3+CD4+ CD25high+ immunophenotype.
More recently, experimental evidence has clearly demonstrated that FoxP3 expression is a valid biologic marker to identify human Treg in tumor tissues23,24 and that this T-cell population accumulates in tumor sites where an immune reaction takes place.24 In addition, before adopting FoxP3 as a marker to identify active Treg infiltration, we had previously verified, by performing double-colour immune histochemistry in a limited number of samples, that its expression only occurred on CD4+ T lymphocytes and that these cells expressed high levels of transforming growth factor-β. On these bases, we believe that FoxP3+ lymphocyte infiltration in tumor tissue might be an indirect and powerful indicator of local antitumor immune response. The detection of a greater Treg tumor infiltration score at the diagnosis, before any treatment takes place, might be consequent to a more immunogenic disease and/or to a preexisting and prolonged antigen specific or unspecific immune reaction. In this case tumor cells have been able of igniting an endogenous anticancer immune response.
However, we cannot exclude that a greater Treg expression may be due to the expression of Treg-specific chemotactic cytokines and/or chemokines associated with a more favorable cancer phenotype.
This is the first study which reports a better frontline treatment-related outcome in patients with a greater Treg tumor infiltration score with the greatest benefit observed in subgroup of patients in the GOLFIG arm in terms of prolonged PFS (mean 18.1mo). We believe that these findings are in line with our previous hypothesis of a chemotherapy-mediated specific killing of Tregs,6,15 whose expansion on the long term could become detrimental. Chemotherapy, therefore, could be able in these patients with a more immunogenic disease to restore and/or empower the original preexisting antitumor immune response and this effect may produce an improved outcome.25 Results from preclinical models suggest that Tregs depletion is induced by cytotoxic drugs, like cyclophosphamide, 5-FU, gemcitabine, and/or oxaliplatin and that this depletion correlates with a better treatment response.26,27 In a previous study, we also observed that GOLFIG treatment induces in colon cancer patients a progressive depletion in peripheral Tregs and in tumor infiltrating Tregs in tumor samples derived from few patients whose metastases could be resected after treatment. This decline was accompanied by a significant increase in activated CTLs, central memory T cells, and sometime by the occurrence of self-limiting autoimmunity signs.5,6 The tight correlation between the latter event and the occurrence of a very good outcome in patients receiving the GOLFIG regimen was suggestive of a chemotherapy-induced distress of Tregs with consequent over stimulation of T-cell mediated immune response. A strong correlation between treatment efficacy and long term survival with autoimmunity in cancer patients was also reported by other authors in different studies based on different immunologic agents.28–31
All together, these findings suggest that tumor-specific antigen expression and the presence of autoantigens in the tumor tissue are critical partners in driving an efficient anticancer immune response. This response might be able to confer protection from tumor progression and might prolong patient survival, even if occurrence of autoimmunity might represent the most important side effect.28–32
In conclusion, the detection of a high FoxP3+-Treg tumor infiltration score identifies a subset of patients with an advanced colorectal cancer with a more favorable outcome which may specifically benefit from chemoimmunotherapy approaches. The understanding of the clinical relevance of the microenvironmental immunologic milieu might provide an important clue for the design of novel strategies in cancer immunotherapy.
Footnotes
All authors have declared there are no financial conflicts of interest in regard to this work.
All the authors indicated no potential conflicts of interest.
REFERENCES
- 1.Ferlay J, Bray F, Pisani P, et al. Cancer Incidence, Mortality and Prevalence Worldwide In: GLOBOCAN 2000 version 1.0 IARC CancerBase N Lyon: 5 IARC Press; 2001. [Google Scholar]
- 2.Meyerhardt JA, Mayer RJ. Systemic therapy for colorectalcancer. N Engl J Med. 2005;352:476–487. [DOI] [PubMed] [Google Scholar]
- 3.Correale P, Messinese S, Caraglia M, et al. A novel biweeklymulti-drug regimen of gemcitabine, oxaliplatin, 5-fluorouracil (5–FU), and folinic acid (FA) in pre-treated patients with advanced colorectal carcinoma. Br J Cancer. 2004;90: 1710–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Correale P, Campoccia G, Tsang KY, et al. Recruitment of dendritic cells and enhanced antigen specific immune-reactivity in cancer patients treated with hrGMCSF (molgramostim) and hr IL-2: results from a Phase Ib Clinical Trial. Eur J Cancer. 2001;37:892–902. [DOI] [PubMed] [Google Scholar]
- 5.Correale P, Cusi MG, Tsang KY, et al. Chemo-immunotherapy of metastatic colorectal carcinoma with gemcitabine plus FOLFOX 4 followed by subcutaneous granulocyte-macrophage colony stimulating factor and interleukin-2 induces strong immunological and antitumor activity in metastatic colon cancer patients. J Clin Oncol. 2005;23:8950–8958. [DOI] [PubMed] [Google Scholar]
- 6.Correale P, Tagliaferri P, Fioravanti A, et al. Immunity feedback and clinical outcome in colon cancer patients undergoing chemo-immunotherapy with gemcitabine+FOLFOX followed by subcutaneous granulocyte macrophage colony-stimulating factor and aldesleukine (GOLFIG-1 trial). Clin Cancer Res. 2008;14:4192–4199. [DOI] [PubMed] [Google Scholar]
- 7.De Gramont A, Figer A, Seymour M, et al. Leucovorinand fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18: 2938–2947. [DOI] [PubMed] [Google Scholar]
- 8.Klebanoff CA, Gattinoni L, Torabi-Parizi P, et al. Centralmemory self/tumor reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci U S A. 2005;102:9571–9576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubois B, Chapat L, Kaiserlian D. CD4+ CD25+ T cells as key regulators of immune responses. Eur J Dermatol. 2003;13: 111–116. [PubMed] [Google Scholar]
- 10.Khazaie K, Von Boehmer H. The impact of CD4+ CD25+ Treg on tumor specific CD8+ T cell cytotoxicity and cancer. Semin Cancer Biol. 2006;16:124–136. [DOI] [PubMed] [Google Scholar]
- 11.Nomura T, Sakaguchi S. Naturally arising CD25+ CD4+ regulatory T cells in tumor immunity. Curr Top Microbiol Immunol. 2005;293:287–302. [DOI] [PubMed] [Google Scholar]
- 12.Beyer M, Schultze JL. Regulatory T cells in cancer. Blood. 2006;108:804–811. [DOI] [PubMed] [Google Scholar]
- 13.Rudensky AY, Campbell DJ. In vivo sites and cellular mechanism of T reg cellmediated suppression. J Exp Med. 2006;203:489–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vojdani A, Erde J. Regulatory T cells, a potent immunoregulatory target for CAM researchers: the ultimate antagonist (I). Evid Based Complement Altern Med. 2006;3:25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Correale P, Del Vecchio MT, La Placa M, et al. Chemotherapeutic drugs may be used to enhance the killing efficacy of human tumor antigen peptide specific CTLs. J Immunother. 2008;31:132–147. [DOI] [PubMed] [Google Scholar]
- 16.Salama P, Phillips M, Grieu F, et al. Tumor-infiltratingFoxP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27:186–192. [DOI] [PubMed] [Google Scholar]
- 17.Clarke SL, Betts GJ, Plant A, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10: 942–949. [DOI] [PubMed] [Google Scholar]
- 18.Bates GJ, Fox SB, Han C, et al. Quantification of regulatory T cells enables the identification of high risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006; 24:5373–5380. [DOI] [PubMed] [Google Scholar]
- 19.Miracco C, Mormouras VM, Biagioli M, et al. Utility of tumor infiltrating CD25+ FoxP3+ regulatory T cell evaluation in predicting local recurrence in vertical growth phase cutaneous melanoma. Oncol Rep. 2007;18:1115–1122. [PubMed] [Google Scholar]
- 20.Gao Q, Qiu SJ, Fan J, et al. Intratumoral balance of regulator yand cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25: 2586–2593. [DOI] [PubMed] [Google Scholar]
- 21.Merlo A, Casalini P, Carcangiu ML, et al. FoxP3 expression and overall survival in breast cancer. J Clin Oncol. 2009;27: 1746–1752. [DOI] [PubMed] [Google Scholar]
- 22.Sinicrope FA, Rego RL, Ansell SM et al. Intraepithelial effector (CD3+)/regulatory (FoxP3+) T-cell ratio predicts a clinical outcome of human colon carcinoma. Gastroenterology. 2009;137:1270–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roncador G, Brown PJ, Maestre L, et al. Analysis ofFoxP3 protein expression in human CD4+ CD25+ regulatory T cells at the single cell level. Eur J Immunol. 2005;35:1681–1691. [DOI] [PubMed] [Google Scholar]
- 24.Ahmadzadeh M, Felipe-Silva A, Heemskerk B, et al. FoxP3 expression accurately defines the population of intratumoral regulatory T cells that selectively accumulate in metastatic melanoma lesions. Blood. 2008;112:4953–4960. Epub 2008 Sep 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zitvogel L, Apetoh L, Ghiringhelli F, et al. The anticancer immunoresponse: indispensable for therapeutic success? J Clin Invest. 2008;118:1991–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghiringhelli F, Larmonier N, Schmitt E, et al. CD4+ CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34: 336–344. [DOI] [PubMed] [Google Scholar]
- 27.Lutsiak ME, Semnani RT, De Pascalis R, et al. Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105:2862–2868. [DOI] [PubMed] [Google Scholar]
- 28.Ji Q, Gondek D, Hurwitz AA. Provision of granulocyte-macrophage colony-stimulating factor converts an autoimmune response to a self-antigen into an antitumor response. J Immunol. 2005;175:1456–1463. [DOI] [PubMed] [Google Scholar]
- 29.Gogas H, Ioannovich J, Dafni U, et al. Prognostic significance of autoimmunity during treatment of melanoma with interferon. N Eng J Med. 2006;354:709–718. [DOI] [PubMed] [Google Scholar]
- 30.Duan-Porter WD, Casciola-Rosen L, Rosen A. Auto-antigens. The critical partner in initiating and propagating systemic autoimmunity. Ann N Y Acad Sci. 2006;1062:127–136. [DOI] [PubMed] [Google Scholar]
- 31.Attia P, Phan GQ, Maker AV, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol 2005;23:6043–6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez-Lecompte JC, Kruth S, Gyorffy S, et al. Cell-based cancer gene therapy: breaking tolerance or inducing autoimmunity? Anim Health Res Rev. 2004;5:227–234. [DOI] [PubMed] [Google Scholar]