Abstract
Introduction
Clinical investigation is a critical component of clinical medicine. Yet, other than mentorship by an experienced senior physician, young physicians have few formal training opportunities that fit into their clinical training and convey the pre-requisite clinical investigator competencies. To address this training gap, we designed the Clinical Investigator Training Program (CITP); a practical and pragmatic curriculum weaved into the constant pressures of balancing patient care with academic pursuit required of the academic practitioner.
Methods
Between January 2016 and December 2018, we conducted four CITP courses, with each comprised of four 4-h sessions that included didactic lectures, group projects including the development of a mock clinical protocol, and expert's panel discussions. Each course enrolled 15 participants from an average of 28 applicants. We assessed the knowledge acquired following each course via a pre- and post-course test (t-test with positive scores indicating improvement in knowledge base). In addition, we also tracked which participants became first time principal investigator following completion of CITP.
Results
A total of 60 participants enrolled in the 4 CITP courses, and there was a statistically significant improvement in mean post-test scores (p < 0.01). The number of participants achieving first time principal investigator status nearly doubled following CITP from 17 to 33. Conversely, applicants not selected for CITP demonstrated no similar improvement during the same follow up period.
Conclusion
The improvement in test scores and the substantive uptake in first time principal investigator responsibilities following CITP affirms that CITP provides a viable option to convey investigator competencies and encourage clinicians to take on the role of principal investigators.
Keywords: Clinical investigation, Training program, Academic, Outcome
1. Introduction
Clinical investigation is the art of applying clinical knowledge, experience, and experimental methodology to answer a specific set of disease- or treatment-related questions predicated on a hypothesis. Clinical investigation differs from clinical care in that the former is focused on addressing a specific scientific question, studying a specific patient population, and following a strict protocol, whereas the latter is the application of collective medical knowledge and clinical experience and judgement directed to address a given individual clinical scenario [1]. Training in clinical investigation has traditionally been passed down from mentor to mentee in an apprenticeship-type of manner [2,3]. In recent years, there is a growing movement to establish a set of pre-defined clinical trial competencies as a requirement for principal investigators (PI) [4,5]. Some institutions and medical organizations provide brief mentorship opportunities for new investigators, but these are available to only a handful of applicants [[4], [5], [6], [7]]. For those committed to a career in clinical investigation, a master's program or a fellowship in clinical research can provide formal specialized training. However, most institutions only expect clinical investigators to undergo basic Good Clinical Practices (GCP) and compliance training [8]. In general, basic GCP training does not adequately prepare a PI for their role enumerated in the code of federal regulations (CFR) title 45, part 46 (protection of human research subjects), title 21 part 812 (investigational device exemption application), title 21 part 312 (investigational new drug application), and title 21 part 50 (informed consent process) by the Department of Human and Health Services and in the principles of international conference on harmonization (ICH) [9]. On the other hand, formal education in clinical investigation often requires substantial time commitment and while very beneficial, does not prepare the clinician for the hands-on PI responsibilities [[10], [11], [12], [13], [14], [15]].
A 2012 Institute of Medicine workshop report indicated that most clinical investigators were trained by ‘ad-hoc’ or ‘on-the-job’ training due to lack of uniformity in investigator training [16]. Currently nearly 40% of investigators are dropping out of clinical trials responsibilities while the number of clinical investigators in North America is lagging behind those numbers in Europe [17]. This has changed the landscape of clinical research, leading to increasing trials being managed by fewer investigators, which could ultimately compromise compliance and impact the integrity of clinical trials. In addition, the number of FDA audits have increased in recent years with approximately 350 inspections conducted each year between 2000 and 2010 [18]. About two thirds of the audited trials had at least one finding that required voluntary investigator or FDA action [18]. In over 2,300 inspections of clinical trials conducted between 2004 and 2011, the most frequent deficiencies identified by the FDA auditors could be traced to the PI and were ascribed to failure to follow investigational plan (51%), including failure to obtain and/or document informed consent [19,20]. The most common occurrence was failure to follow investigational plans, which ultimately rests on principle investigator's shoulders, and part of the FDA Form 1572 requirement. Thus, in order to increase quantity and enhance quality of investigators, we found it imperative to train upcoming junior faculty in the skills of clinical trials and to motivate them to become responsible investigators. Although a number of clinical investigators in recent years are becoming PI for the first time, a large number of clinical investigators are at risk of joining the “one-and-done” group, quitting after conducting only one FDA approval clinical trial [21,22]. The ever growing responsibilities of PI with stringent regulatory environment has added to more attrition of PI [23]. Current GCP training for investigators often uses a ‘one-size-fits-all’ approach and lacks the practical and pragmatic skills required to conduct clinical trials [9]. Recent study has shown that there is a need to develop an active learning component for researchers with formal and informal approaches to increasing knowledge [9]. Our program adopts this approach in developing a structured, learner-centered curriculum that includes mentoring, knowledge sharing networks, and mock run through of protocol procedures.
Most of the available clinical investigator training programs for physicians are didactic, focus on theoretical concepts and lack practical application. During their clinical residency and fellowship training, physicians learn and gain valuable experience through interaction with their peers and experienced mentors. We attempted to retain a similar format of imparting education through in-person interactive discussions between peers and with experienced investigators from within the institutions.
To address this gap in investigator training and the growing demands for well-trained clinical investigators at our institution, we developed a pragmatic, hands-on Clinical Investigator Training Program (CITP). The CITP course had following objectives [1]: to provide a basic overview of clinical research, implementation of protocol and the expected role and responsibilities of the PI [2], to educate the clinical investigators about various components of the clinical trial activation processes and subsequent clinical trial management, and [3] to introduce the investigators to the vast institutional infrastructure and supportive mechanisms, thus contributing to more rapid Time-To-Trial Activation (TTA), a leading benchmark in conduct of clinical trial.
Having conducted four CITP courses (CITP 1.0 – CITP 4.0) over the past 2 years, we present our early experience and demonstrate outcomes from this pilot project.
2. Methods
Each course consisted of four 4-h sessions and held every other week, for a total of 16 h of didactic and hands-on training over a 2-month period (Table 1).
Table 1.
CITP Curriculum and covered core competencies.
| Session/Module | Topic | Group Activities | Course materials | Core Competencies Covered |
|---|---|---|---|---|
|
Session #1 Nuts & Bolts of a Clinical Trial |
Types of clinical studies Key Components of a clinical trial
|
|
Presentation Handouts
Research querieshttps://www.youtube.com/watch?v=dl6C_GrZrbE |
1, 4, 5, & 6 |
| Session #2 Human Subjects Research |
|
|
Presentation Handouts Other tools
Good Consenting process https://www.youtube.com/watch?v=Vb7e_0Mw4ps |
2, 4 & 5 |
| Session #3 Clinical Project Management |
|
|
Presentation Handouts Other tools
|
3 & 5 |
| Session #4 What does it take to be successful? |
More than just Science! Expert Panel with Key Members of Investigative Team How Did You Get Here? Expert Panel with experienced investigators
Post-course Test |
group project presentations end of session test | 7 & 8 |
The course was offered biannually to senior fellows (subspecialty residents) and new junior faculty. Our curriculum took into account the 8 competencies outlined by the Joint Task Force for Clinical Trial Competency [1]: Scientific concepts and research design [2], Ethical and participant safety [3], Medicines development and regulation [4], Clinical trial operations [5], Study and site management [6], Data management and informatics [7], Leadership and professionalism, and [8] Communication and teamwork [24]. In addition to the above competencies, we also focused on: a) understanding the roles and responsibilities of a PI in providing clinical trial oversight; b) clinical, regulatory and fiscal compliance; c) pre- and post-award processes; d) non-clinical aspects of clinical investigation (contract, budget, payment schedule etc.); and e) audit preparedness [25]. Six months following each course, we contacted the participants to obtain feedback. Overall, the outcome of all four CITP courses was assessed, one year after the fourth course and this outcome formed the basis of this submission.
2.1. Participants
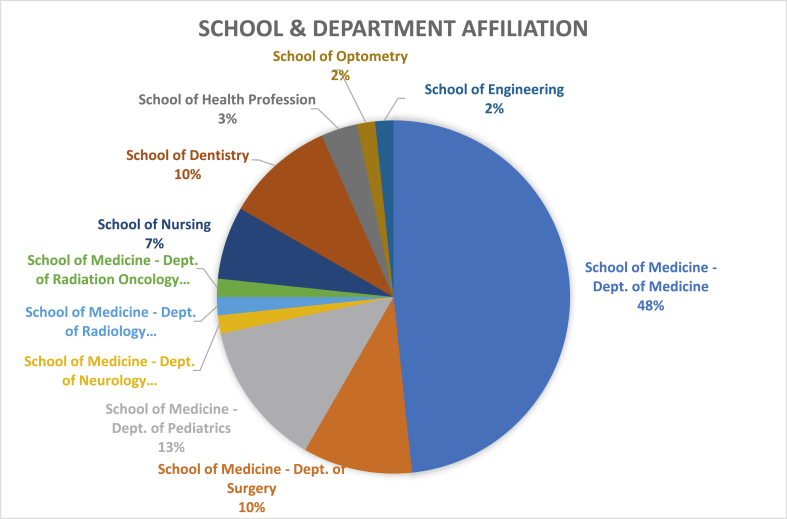
Fig. 1 provides the distribution of the participant class, which is composed of the clinicians and basic researchers from within UAB, where each have a role in patient care, teaching, research and academic pursuit. Participants in our program came from different backgrounds – 42 out of 60 from the school of medicine, 8 from the school of dentistry and 10 from allied health sciences. The participants, who were affiliated to the school of medicine, belonged to various specialties such as oncology, cardiology, neurology, nephrology, gastroenterology, pulmonary medicine, rheumatology, gynecology, radiology, pediatrics, and surgery. All practicing clinicians were part of an academic group practice and received protected time to participate in CITP. We selected participants following nominations by department/division directors in response to a university-wide promotion through the offices of the Deans and academic units. We gave priority to new junior clinical faculty interested in a clinical investigational career and basic science faculty interested in translational research. To promote cross-institutional collaboration, we actively encouraged participants from outside the School of Medicine, including non-MD investigators. CITP faculty reviewed nominations and selected participants with a goal to foster diversity and select those candidates most inclined to follow an academic career in clinical investigation. We accepted no more than two candidates per division from the School of Medicine, and accepted at least one nominee from each school outside the School of Medicine. Prior to the course, participants were required to complete the Institutional Review Board (IRB) mandated Collaborative Institutional Training Initiative (CITI) and to meet the requirements of the Health Insurance Portability and Accountability Act (HIPAA).
Fig. 1.
Distribution of selected participants based on School and department affiliation.
To promote peer competitiveness, we introduced Kaizen, an app-based program designed to encourage recall of training material, foster group interaction, and inject a competitive spirit within the participants [26]. A pre-course test was conducted to introduce participants, form groups, select clinical protocol topics, provide handouts, and complete the pre-course assessment.
During the coursework, participants were divided in 3 groups of 5 each. Each group was expected to select a hypothetical clinical trial question and jointly design, present and defend a mock protocol at the last session. This exercise was intended to provide a tangible avenue for the application of the didactic learnings.
2.2. Faculty
CITP was conducted by six core faculty members and a senior biostatistician, with significant expertise in scientific, regulatory, and administrative aspects of clinical research. Guest speakers included representatives of the Institutional Review Board (IRB), Office of Sponsored Programs (OSP), and experienced clinical research coordinators (CRCs) with hands-on experience in team science. The final session of each CITP course included two panels: 1) Seasoned investigators and mentors who provided their personal perspective on career paths and lessons learned, and 2) A panel of experienced regulatory, budgetary and administrative research personnel who provided a “team science” approach to clinical investigation.
2.3. Faculty outcome metrics/assessment
We assessed programmatic success through pre- and post-course tests to gauge information acquisition and knowledge enhancement following each course. CITP faculty scored the mock clinical protocols presented by each group and provided critical feedback. We also tracked the clinical investigational activities of the participants following CITP as part of our outcomes.
Each participant completed a 20-questions pre-course test to assess baseline core competencies and knowledge of clinical investigation, and this was repeated at the end of the course (post-course test). Questions covered a wide array of conceptual knowledge about clinical trials and research designed, and basic research GCP knowledge e.g. role of IRB, process of informed consent, reporting of adverse events, data management, study audit, protocol deviation, source documentation, conflict of interest and patient privacy. Each correct response received 5 points for a maximum of 100 points.
We also assessed the relevance of the topic/content, effectiveness of the presenter and the learning environment of each of the sessions through a participant survey. Each question was scored 1–5, with 5 being the best and 1 being the worst. Faculty received feedback based upon survey results, which provided the impetus to improve the content and presentations for subsequent series.
At the 1-year mark following completion of CITP 4.0, we assessed outcome of the CITP course in terms of first time assumption of PI responsibilities, independent development of an investigator-initiated clinical trial (IIT), and participation in a formal training program or auditing of university courses relevant to clinical investigation. We independently assessed the involvement of CITP participants in clinical investigation using data derived from the institutional database (Oncore) and the Office of sponsored program (OSP). PI-ship or participation in additional investigator training and clinical trials initiated after attending CITP were all considered a positive outcome of CITP. As a comparable, we similarly assessed the progress of those candidates who had not been selected to participate in CITP.
2.4. Statistical analysis
This is a descriptive paper with quantitative analysis of metrics on participants’ characteristics and their performances during and following CITP. Categorical values were described as frequencies and percentages while continuous variables were described using means and ranges. The differences in means of pre-course and post-course test scores were compared by standard t-test at a confidence level of .05 and reported with 95% confidence interval. For categorical variables, we conducted chi-square test and reported p-values to check for significant associations.
3. Results
We received an average of 28 nominations and selected 15 participants for each CITP course from across various schools/departments/divisions (Fig. 1).
The majority of nominations were from the School of Medicine (84%) while the rest came from the Schools of Nursing (5%), Dentistry (5%), Health Professions (2%) and Engineering. Over a period of 2 years (2016–2018), we conducted 4 CITP courses with 15 participants in each and graduated a total of 60 participants. Of these, 30 (50%) were female. Thirty (50%) candidates were Caucasian, 5 (8.33%) were African American, and 25 (41.67%) were from other racial background. 46 participants (77%) were affiliated to the School of Medicine with from the rest were affiliated to the School of Nursing, Health Sciences, Dentistry, Optometry, and Engineering (Fig. 1). Of those who were affiliated to the School of Medicine, 63% were from department of medicine, 18% were from department of pediatrics, and 13% were from department of surgery, with 1 participant each (2%) from neurology, radiology and radiation oncology (Fig. 1). Fifty participants (83%) were practicing clinicians while 10 (17%) were non-MD with a doctoral degree in basic science and/or nursing.
3.1. Pre- and post-course test outcomes
Comparison between the mean pre- and post-course test for all 60 participants demonstrated an increase in mean scores following each of the four CITP courses respectively (74.3 vs 86 points; 71.4 vs 77.1 points; 72 vs 79.3 points; and 73.6 vs 87.5 points) for a statistically significant overall increase (difference in mean scores = 8.0 points, P < .01) (Table 2).
Table 2.
Performance of participants in the pre and post-test (CITP 1.0–4.0).
| CITP 1.0 (N = 15) |
CITP 2.0 (N = 15) |
CITP 3.0 (N = 15) |
CITP 4.0 (N = 15) |
Total (N = 60) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre- Course test | Post-course test | Pre- Course test | Post-course test | Pre- Course test | Post-course test | Pre- Course test | Post-course test | Pre- Course test | Post-course test | |
| Mean (SD) | 74.3 (8.1) | 86 (6.2) | 71.4 (5.4) | 77.1 (9.0) | 72 (12.1) | 79.3 (11.7) | 73.6 (6.1) | 87.5 (6.7) | 72.9 (8.5) | 80.9 (9.4) |
| Difference in mean scores | 11.7 | 5.7 | 7.3 | 13.9 | 8.0 | |||||
| p-value | <.01 | .05 | .10 | <.01 | <.01 | |||||
Scores for each participant was calculated from a total of 100. Mean of 15 students was then calculated to conduct statistical analyses across 4 courses of CITP.
3.2. Progress report
We tracked clinical trial participation before and after CITP and assessed whether participants achieved PI status following CITP. We were especially interested in whether participants, none of whom had previously not participated in clinical trials as PI, achieved PI status following CITP. One year following CITP 4.0, we assessed achievement of PI-ship for all CITP participants (Table 3).
Table 3.
Participation in clinical trials/other clinical investigation training before and after CITP.
| CITP 1.0 |
CITP 2.0 |
CITP 3.0 |
CITP 4.0 |
Total Cumulative (at least 1yr follow up) |
Unselected candidates |
|
|---|---|---|---|---|---|---|
| n = 15 | n = 15 | n = 15 | n = 15 | n = 60 | N = 36 | |
| PI status: pre/post CITP | 3/6 | 3/9 | 7/10 | 4/8 | 17/33 | 13/15 |
| PI of IIT pre/post CITP | 0/4 | 0/3 | 0/1 | 0/5 | 0/13 | 8/9 |
| Joined Master's Program/Non-degree course work pre/post CITP | 0/3 | 0/3 | 0 | 0/2 | 0/8 | 0/0 |
IIT = Investigator Initiated Trial PI = Principal Investigator.
At the time of enrollment to the program, 17 of 60 participants (28.3%) reported having conducted clinical research as PI. At the time of analysis, 16 additional participants reported first time PI status bringing the total number of PI to 33 (55%) (Chi-square = 8.77, p-value = 0.0031). The number of PI writing their own (investigator initiated) clinical trial increased significantly from none to 13 following CITP. We similarly assessed achievement of PI-status of candidates who were nominated but not selected for CITP. Of the 36 candidates not selected for CITP, 13 were PI before nomination (36%), and only 2 additional candidates became first time PI during the evaluation period for a total of 15 (42%) (Chi-square = 0.2338, p-value = 0.6287). Out of these 36 candidates, 8 had written their own clinical trial before being nominated but only 1 additional candidate developed their own clinical trial during the same follow up period.
3.3. Course evaluations
Participants consistently ranked the speakers as well as the content with a score of 4 (out of 5), with 5 being excellent and 1 being poor. Of all the sessions in each CITP course, the panel discussion (session # 4) was ranked highest and received a mean score of 5. Consistent feedback expressed by two or more of the 15 participants are listed in Table 4.
Table 4.
Participant feedback following CITP.
| Positive - |
|---|
| Experienced Faculty members and their rich experience were encouraging for participants |
| Details on budget formulation helpful |
| Panel discussion in session 4 greatly enlightening |
| Actual cases discussed were helpful for understanding of concepts |
| Practical aspects of conducting research |
| Group based learning with peers from different background helpful |
| Need for Improvements - |
| Need to include behavioral, observational and non-drug trials for course to be all inclusive |
| More time for group discussions during lecture sessions |
| Ethical discussion to be made concise and case-oriented |
| Suggestion to include topics related to clinical research grant submissions |
4. Discussion
Clinical investigation is a critical component of drug development and an exciting career path for clinicians and translational scientists. However, clinical investigation is much more than a sidebar clinical activity and requires added rigor, compliance, and oversight beyond that required of being a general clinician.
For the clinicians, revenue is predominantly generated through patient care, while for the basic scientist revenue is derived from research grants and teaching. Clinical practice often involves the generation of revenue through patient care. As an example, an adult hematologist and oncologists at an academic center generated an average of 3,745 work relative value units (wRVUs) [27]. This leaves very little time or effort to dedicate to clinical research. Thus, when faced with the dual burden of fulfilling responsibilities of a clinical investigator along with patient care responsibilities, clinicians often give priority to patient care (and revenue generation) to the detriment of clinical investigation.
In 2019, the total number of interventional trials monitored by the FDA totaled more than 120,000 [28]. However, the pool of clinical investigators charged to conduct these studies continues to shrink as a result of increasing pressures at work, including clinical responsibilities and the generation of wRVU, the burden of clinical documentation in the electronic medical record era, the competition for limited peer-reviewed research funding, and the ever increasing compliance and regulatory requirements imposed by the FDA, IRB, and sponsors in the conduct of clinical trials [8]. With the growing burden of expectations and competing priorities, the number of young clinicians opting for a career in clinical investigation is shrinking [29,30]. The culmination of these pressures creates an urgent need to foster clinical investigation training that will inspire and motivate junior clinicians to taken on the role of a PI.
A number of academic institutions, including the University of Alabama at Birmingham (UAB), offer structured curricula (11,13. 14) to new investigators including degree programs in clinical investigations that require 50–60 credit hours towards a formal degree or certification. Other centers offer on-line, as well as, on-site training that focus on FDA regulations, ethical, and GCP guidelines [11,14,15]. Most of the formal training modalities available to clinical investigators are online with limited face-to-face training and most do not have a rigorous practical team science component that requires the participants to actually design a mock protocol, consent form and study budget. Those clinical investigator training programs that include on-site presence very often take the participant away from their workplace and home and involve travel and associated costs. There remains an unmet need to address practical aspects of clinical investigation without requiring a prolonged commitment of time away from personal and professional commitments yet adequately preparing the investigators for the PI role. CITP overcomes nearly all of these limitations, thereby making it ideal for replication at most academic centers. In CITP, we developed a practical and pragmatic approach to clinical trials training through a 16-h mixed method course that combined didactic training, hands-on activities, and team participatory projects over a 2-month period. The course was also born out of the need to inspire and motivate and train more clinicians to become involved in clinical investigation [31]. The course was designed to fit into the busy clinic schedule of most junior clinicians, and we encouraged department/division directors to provide protected time for the participants.
CITP included real case scenarios of the informed consent process, the ethical dilemmas encountered by investigators, for example excluding patients because of minor (clinically insignificant) deviation in laboratory parameters, ambiguities in the interpretations of the eligibility criteria amongst others. Few, if any, clinical investigator training platforms dedicate significant portion of the session to practical real life experiences as a PI, such as reviewing an actual FDA Form 483 issued to a PI and lessons learned in terms of GCP and investigator mandated study oversight. Formal programs granting degrees in clinical research are predominantly focused on theoretical concepts of clinical trials, often taught by faculty not directly or actively involved in clinical investigation. Case studies used in these courses tend to be generic and often include sound theoretical constructs, but little in the way of practical real life scenarios. For example, what does the study team do when they only have 1 slot on a promising study and two equally eligible candidates - one a 93 year old patient who has lived a fulfilling life and the other patient, a young mother of a 1 year old child. CITP was interactive and built up on the real-life experiences of the clinical faculty that helped young faculty assimilate information while sensitizing them to the complexities associated with clinical trials and the importance of compliance and research integrity.
CITP selected participants from various Schools, including some without a clinical background but potential to contribute to translational research. As an example, a CITP participant from the school of biomedical engineering subsequently developed a patented mini-dynamic flow chamber to support the growth of fresh human tumor biopsy sections. He collaborated with a clinical colleague during CITP and was able to translate his discovery into a funded companion pilot chemosensitivity study. The ongoing support from department/division across campus (Fig. 1) reaffirmed the unmet need for CITP and a desire by the leadership to encourage junior faculty to pursue a career in clinical investigation. All of the selected participants completed the entire course. The exercise to develop a mock clinical trial that included the protocol, a recruitment plan, sample size justification and statistical design, budget, and an informed consent provided a tangible outcome to the didactic curriculum and contributed to collaboration by participants from diverse backgrounds which has continued beyond CITP and resulted in a number of collaborative inter-departmental clinical projects.
Of the 60 participants, including 42 clinicians, 78% have continued to be involved in clinical trials. In addition to the 17 participants who had served as a principal investigator prior to CITP, 16 additional participants became first time principal investigators following CITP, and a total of 13 were first time PI of an investigator-initiated clinical trial. Additionally, of the 10 non-clinician participants, 8 are currently part of a clinical trials team in support of a translation research protocols they were involved in designing. In comparison, of the 36 nominees who were not selected for CITP, only two more nominees achieved PI status during the evaluation period. While the improvements and progress documented over the 1-year follow-up period may not be completely ascribed to CITP, the increase in first time PI status including first time PI of investigator initiated trial is an encouraging trend. In comparison, those not selected for CITP did not demonstrate comparable gains in PI-ship. The involvement of non-clinicians in clinical investigation also was a positive finding. Long term follow up will be required to determine the success since the course may have self-selected motivated clinicians interested in clinical investigation and their progress more an outcome of personal aspiration as opposed to CITP.
Aside from disseminating core competencies and achieving a statistically significant improvement in knowledge acquisition as determined by the pre-post tests, the course discussions aptly conveyed the critical role and responsibilities of principal investigators. Thus, the session on FDA 483 (session # 3) was viewed as one of the most “eye opening” since it included discussion on audit preparedness and consequences of poor oversight of a clinical trial. Session # 4 involving discussion with mentors and seasoned investigator was judged as the most inspiring and motivating.
There is an ongoing interest amongst schools/department/division leadership to nominate participants to the CITP. The CITP in its current format has been assimilated into the educational portfolio of UAB's Center for Clinical and Translational Sciences (CCTS), which is funded by the National Center for Advancing Translational Sciences (NCATS). There are increasing requests to offer the course by way of on-line lectures to UAB's partner sites, as well as, candidates from outside UAB. We believe all these are value-added attributes that justify the need for and importance of the CITP.
In addition to increasing to the pool of inspired investigators, 13 of the participants wrote an investigator-initiated trial (IIT) during the follow-up period. While none of the participants signed up for a master's program in clinical investigation following CITP, a number enrolled in non-degree lecture series pertaining to clinical and translational research citing CITP as a motivating factor.
Our study did have some limitations. Our program was geared towards training physicians in an academic setting. We invited department chairs to allow selected candidates to participate and receive protected time to commit to CITP. We thus recruited select candidate who, in the opinion of their chairs, were being guided into a clinical investigator career. Their participation and commitment may well be different than if we randomly selected CITP participants. However, in the outcome/impact analysis we did compare the productivity of CITP participants to those who were not selected (due to limits on participants), and we were able to show enhanced uptake in clinical investigator activity in CITP participants. CITP was designed in the context of an academic setting and it received institutional peer reviewed grant support. Faculty volunteered their time without compensation as an additional undertaking. This support and/or resource may not be available in other settings e.g. community practice or smaller academic centers. Currently, there is no uniform tool to assess competency of investigators in their knowledge of and compliance with ICH-GCP and PI responsibilities, except outcome at a future study audit. Our use of pre- and post-test to assess the improvement in knowledge provided a narrow evaluation of the didactic competencies gained because of CITP modules. A much longer follow up would be required to determine if the gains observed at the end of CITP, and the follow-up over 2 years, did have lasting impact on the academic productivity of the participants. Participants indicated that the time commitment in the middle of a busy clinical schedule was a major limitation and thus expressed an interest in an on-line course. At the same time, though, a majority of the participants also were concerned about the loss of direct on-site interaction and group dynamic as a major drawback when contemplating an on-line course.
Our experience demonstrated a niche for a practical and pragmatic investigator training above and beyond the mandated CITI and institutional compliance requirements, which often lacked the connection to real life investigator experiences. CITP appeared to meet many of the needs of young clinical investigators and our data accumulated provides evidence of a positive outcome. Ongoing accumulation of experiences and refining the content would serve to reach more young clinicians, who can be inspired into a career in clinical investigation. The success of CITP, as evidenced by the dedication of the faculty and the experiences shared by panelists during session # 4, underscored the need for generous mentorship on part of experienced senior investigators as an important requisite to motivate and inspire a new generation of clinical investigators.
CRediT authorship contribution statement
Mansoor Saleh: Conceptualization, Methodology, Writing - original draft, Supervision, Funding acquisition. Gurudatta Naik: Data curation, Methodology, Writing - original draft, Project administration. Penelope Jester: Conceptualization, Writing - review & editing. Cynthia Joiner: Conceptualization, Writing - review & editing. Elizabeth Westfall: Conceptualization, Writing - review & editing. David W. Kimberlin: Conceptualization, Writing - review & editing. James Willig: Conceptualization, Writing - review & editing. David Redden: Conceptualization, Writing - review & editing. Juliette Southworth: Conceptualization, Project administration. Mark T. Dransfield: Conceptualization, Writing - review & editing.
Declaration of competing interest
None of the authors had any conflicts or financial disclosures.
Acknowledgements
University of Alabama at BirminghamHealth Services Foundation–General Endowment Funding (UAB-HSF) grant.
UAB Center for Clinical and Translational Sciences (CCTS) for providing platform to conduct this training program across the university.
Anupam Agarwal, MD & Robert Kimberly, MD for their valuable inputs and insight through the course of this program and development of manuscript.
Alfreda Lewis, CCRP for the logistical inputs and support to program.
References
- 1.Miller F.G., Brody H. A critique of clinical equipoise. Therapeutic misconception in the ethics of clinical trials. Hastings Cent. Rep. 2003;33(3):19–28. [PubMed] [Google Scholar]
- 2.Moskowitz J., Thompson J.N. Enhancing the clinical research pipeline: training approaches for a new century. Acad. Med: J. Assoc. Am. Med. Colg. 2001;76(4):307–315. doi: 10.1097/00001888-200104000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Sambunjak D., Straus S.E., Marusic A. Mentoring in academic medicine: a systematic review. Jama. 2006;296(9):1103–1115. doi: 10.1001/jama.296.9.1103. [DOI] [PubMed] [Google Scholar]
- 4.Daley S.P., Palermo A.G., Nivet M., Soto-Greene M.L., Taylor V.S., Butts G.C. Diversity in academic medicine no. 6 successful programs in minority faculty development: ingredients of success. MSJM (Mt. Sinai J. Med.) 2008;75(6):533–551. doi: 10.1002/msj.20084. New York. [DOI] [PubMed] [Google Scholar]
- 5.Kosoko-Lasaki O., Sonnino R.E., Voytko M.L. Mentoring for women and underrepresented minority faculty and students: experience at two institutions of higher education. J. Natl. Med. Assoc. 2006;98(9):1449–1459. [PMC free article] [PubMed] [Google Scholar]
- 6.Lewellen-Williams C., Johnson V.A., Deloney L.A., Thomas B.R., Goyol A., Henry-Tillman R. The POD: a new model for mentoring underrepresented minority faculty. Acad. Med: J. Assoc. Am. Med. Colg. 2006;81(3):275–279. doi: 10.1097/00001888-200603000-00020. [DOI] [PubMed] [Google Scholar]
- 7.Robinson G.F., Schwartz L.S., DiMeglio L.A., Ahluwalia J.S., Gabrilove J.L. Understanding career success and its contributing factors for clinical and translational investigators. Academic medicine. J. Assoc. Am. Med. Colg. 2016;91(4):570–582. doi: 10.1097/ACM.0000000000000979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson C., Young P.A., Berenbaum A. Food and Drug Administration guidance: supervisory responsibilities of investigators. J. Dia. Sci. Technol. 2011;5(2):433–438. doi: 10.1177/193229681100500234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bechtel J., Chuck T., Forrest A., Hildebrand C., Panhuis J., Pattee S.R. Improving the quality conduct and efficiency of clinical trials with training: recommendations for preparedness and qualification of investigators and delegates. Contemp. Clin. Trials. 2020;89:105918. doi: 10.1016/j.cct.2019.105918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Center WCMCaTS https://ctscweb.weill.cornell.edu/education-training/programs/advanced-certificate-program-clinical-translational-investigation Available from:
- 11.CERSI. University of Maryland https://cersi.umd.edu/clinical-investigator-training-course Available from:
- 12.Health UoAaBSoP https://www.soph.uab.edu/master-science-public-health-clinical-and-translational-science-ctsb-0 Available from:
- 13.Innovation IoHPa. University of Michigan. https://ihpi.umich.edu/about/education-training/programs/ihpi-clinician-scholars-program/about-program Available from:
- 14.Louis WUSoMiS Graduate certificate in clinical investigation (CI) https://crtc.wustl.edu/programs/certificates/ci/ Available from:
- 15.Medicine J.H. https://www.hopkinsmedicine.org/clinicalresearchscholars/index.html Available from:
- 16.Bonham A. National Academy of Sciences; 2012. Developing a Robust Clinical Trials Workforce. [Google Scholar]
- 17.Getz K, Brown C, Stergiopoulos S, Beltre C. Baseline assessment of a global clinical investigator landscape poised for structural change. Ther Innov Regul Sc.51(5):575-581. [DOI] [PubMed]
- 18.Morgan-Linnell SK, Stewart DJ, Kurzrock R. U.S. Food and Drug Administration inspections of clinical investigators: overview of results from 1977 to 2009. Clin. Canc. Res..20(13):3364-3370. [DOI] [PubMed]
- 19.Donato B.J., Gibson T.R. Does your clinical investigator understand the consequences of non-compliance? Qual. Assur. 1999;7(3):135–145. doi: 10.1080/105294100750035116. [DOI] [PubMed] [Google Scholar]
- 20.Florence Top 5 trial site FDA inspection failures, and how to avoid them Florencehc.com. https://florencehc.com/top-5-trial-site-fda-inspection-failures-and-how-to-avoid-them/ Available from:
- 21.Morgan C. Medcity News; 2017. Is Clinical Trial Design Complexity behind the High Turnover Rate for Principal Investigators?https://medcitynews.com/2017/04/high-turnover-rate-for-principal-investigators/ updated 4/24/2017. Available from: [Google Scholar]
- 22.Corneli A., Pierre C., Hinkley T., Lin L., Fordyce C.B., Hamre G. One and done: reasons principal investigators conduct only one FDA-regulated drug trial. Contemp. Clinc. Trial. Commun. 2017;6:31–38. doi: 10.1016/j.conctc.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Norman G.A. Drugs, devices, and the FDA: Part 1: an overview of approval processes for drugs. JACC (J. Am. Coll. Cardiol.): Basic. Trans. Sci. 2016;1(3):170–179. doi: 10.1016/j.jacbts.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sonstein S.A., Jones C.T. Joint Task Force for clinical trial competency and clinical research professional workforce development. Front. Pharmacol. 2018;9(1148) doi: 10.3389/fphar.2018.01148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calvin-Naylor N.A., Jones C.T., Wartak M.M., Blackwell K., Davis J.M., Divecha R. Education and training of clinical and translational study investigators and research coordinators: a competency-based approach. J. Clinc. Trans. Sci. 2017;1(1):16–25. doi: 10.1017/cts.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaizen Center for Clinical and Translational Sciences University of Alabama at Birmingham. https://www.uab.edu/ccts/training-academy/innovation/kaizen Available from:
- 27.Stewart FM, Wasserman Rl Fau - Bloomfield CD, Bloomfield Cd Fau - Petersdorf S, Petersdorf S Fau - Witherspoon RP, Witherspoon Rp Fau - Appelbaum FR, Appelbaum Fr Fau - Ziskind A, et al. Benchmarks in clinical productivity: a national comprehensive cancer network survey. J Oncol Pract 3(1):2-8. [DOI] [PMC free article] [PubMed]
- 28.Trends, charts and maps U.S. National library of medicine, ClinicalTrials.gov. https://clinicaltrials.gov/ct2/resources/trends updated 3/14/2019. Available from:
- 29.Cohen J.M. In response to “future supply and demand for oncologists: challenges to assuring access to oncology Services. J. Onco. Pract. 2007;3(3):179. doi: 10.1200/JOP.0739002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang W., Williams J.H., Hogan P.F., Bruinooge S.S., Rodriguez G.I., Kosty M.P. Projected supply of and demand for oncologists and radiation oncologists through 2025: an aging, better-insured population will result in shortage. J. Onco. Pract. 2014;10(1):39–45. doi: 10.1200/JOP.2013.001319. [DOI] [PubMed] [Google Scholar]
- 31.Saleh M., Naik G. So you want to Be a principal investigator. J. Onco. Pract. 2018;14(6):e384–e392. doi: 10.1200/JOP.18.00011. [DOI] [PubMed] [Google Scholar]