Abstract
We report outcomes for a cohort of patients with multidrug-resistant tuberculosis who received high-dose isoniazid in Haiti. Patients who received high-dose isoniazid had a faster time to culture conversion and higher odds of successful outcome, despite high-level isoniazid resistance. This suggests high-dose isoniazid may have effectiveness even with phenotypic resistance.
Keywords: tuberculosis, high-dose isoniazid, drug resistance, MDR-TB
Multidrug-resistant tuberculosis (MDR-TB), resistant to at least isoniazid and rifampin, is a serious global health concern. Treatment requires prolonged multidrug regimens that often include toxic and weakly effective medications. Isoniazid resistance is most often caused by inhA mutations, which confer low-level resistance, or katG mutations, which confer high-level resistance. Although high-dose isoniazid is generally considered ineffective in strains with the katG mutation [1], some evidence suggests that higher doses of isoniazid might have clinical efficacy [2, 3]. We retrospectively examined the effectiveness of high-dose isoniazid in the treatment of MDR-TB in human immunodeficiency virus (HIV)-negative persons in Haiti.
METHODS
Study Site and Population
Located in Port-au-Prince, the Haitian Group for the Study of Kaposi’s Sarcoma and Opportunistic Infections (GHESKIO) is 1 of 2 MDR-TB treatment programs in Haiti, a low-income country. Between January 2009 and December 2015, 187 HIV-negative adult patients were diagnosed with culture-positive MDR-TB. From October 2011 to December 2014, GHESKIO included high-dose isoniazid as part of the standard MDR-TB treatment regimen for HIV-negative individuals.
MDR-TB Diagnosis and Drug Susceptibility Testing
Patients were diagnosed with MDR-TB based on clinical evaluation, chest radiography, and molecular testing using Xpert MTB/RIF (Cepheid, Sunnyvale, CA, USA) or Genotype MDRTB Plus (Hain LifeScience, Nehren, Germany) assays. All samples with rifampin resistance detected by molecular testing were cultured using liquid media (BACTEC MGIT 960 tube [Becton Dickenson, Franklin Lakes, NJ, USA]) and solid media (Lowenstein-Jensen slant), and first- and second-line drug susceptibility testing (DST) was conducted per routine protocol. First-line DST was performed with BACTEC MGIT 960 SIRE and PZA kits in accordance with the manufacturer’s recommendations; isoniazid susceptibility was tested at 0.1 µg/mL. Second-line DST was performed using the standard proportion method on 7H10 medium agar; isoniazid susceptibility was repeated at 0.2 µg/mL and 1.0 µg/mL concentrations. Low-level resistance was defined as resistant at 0.1 µg/mL or 0.2 µg/mL but sensitive at 1.0 µg/mL. High-level resistance was defined as being resistant at 1.0 µg/mL.
Description of Treatment Regimens, Clinical Care, and Outcome Measures
MDR-TB treatment regimens changed between 2009 and 2015 based on World Health Organization (WHO) and Haitian national guidelines. Initially, patients were started on an empiric standardized regimen including a fluoroquinolone (moxifloxacin or levofloxacin) and second-line injectable (kanamycin) with p-aminosalicylic acid (PAS), cycloserine, ethionamide, and pyrazinamide for 8–10 months (intensive phase). During the continuation phase (14–16 months), the oral medications were adjusted based on second-line DST results. Due to budget constraints, in October 2011, PAS was removed from the national empiric MDR-TB treatment regimen for HIV-negative patients. High-dose isoniazid was added with the goal of overcoming low-level isoniazid resistance; high-dose isoniazid had not been previously used at GHESKIO. Its use was discontinued in December 2014 after a review of DST data revealed the large proportion of patients with high-level isoniazid resistance. The controls used in our analysis were HIV-negative patients who did not receive high-dose isoniazid as they initiated treatment either prior to October 2011 or after December 2014. High-dose isoniazid was dosed to achieve a goal of 16–18 mg/kg daily, using 300 mg tablets. Twenty-five patients (25%) received doses ranging from 18 to 21 mg/kg. Twenty patients (20%) received doses ranging from 12.1 to 15.9 mg/kg. Of note, standard-dose isoniazid is 5 mg/kg (usual dose of 300 mg) and is used for the treatment of drug-susceptible tuberculosis in Haiti.
Culture was obtained per protocol, monthly during the intensive phase and every other month during the continuation phase. Culture was again obtained monthly during the last 5 months of treatment (2009 to 2013) or the last 3 months of treatment (2013 to 2015). Culture conversion was defined as 2 consecutive negative cultures at least 30 days apart. Treatment outcomes were defined according to the 2014 WHO guidelines [4]. Cure was treatment completion without evidence of failure and at least 3 consecutive negative cultures taken at least 30 days apart after the intensive phase. Treatment completion was completion of drug regimen without failure or mycobacteriological evidence of cure. Failure was prematurely ending treatment or changing ≥2 drugs due to lack of culture conversion, bacteriological reversion in the continuation phase, adverse drug event, or newly acquired pre-XDR tuberculosis.
Ethics
This study was approved by the institutional review boards of all affiliated institutions.
Statistical Analysis
We evaluated time to culture conversion using Kaplan-Meier survival analysis and log-rank test for comparison. Logistic regression was used to determine predictors of successful outcome. Variables with P < .2 and an odds ratio (OR) of <0.8 or >1.2 in the univariate model were included in adjusted logistic regression.
RESULTS
Between January 2009 and December 2015, 187 individuals ≥18 years of age were diagnosed with MDR-TB and initiated treatment. Ninety-nine patients received high-dose isoniazid; 88 patients did not. Median age was 29 years (interquartile range: 23, 40), 51% (n = 96) were male, 98% (n = 183) had previous exposure to first-line anti-tuberculous medications, and 94% (n = 176) had high-level isoniazid resistance based on second-line DST. Specific isoniazid mutations were unable to be determined. One isolate was resistant to isoniazid only at 0.1 ug/mL; 6 isolates were resistant to isoniazid 0.2 ug/mL but sensitive to1.0 ug/mL; and data on the level of isoniazid resistance were missing for 4 patients. Six individuals with low-level isoniazid resistance received high-dose isoniazid; 1 patient did not. Further details are available in Supplementary Tables 1 and 2.
Among all 187 patients, 84% (n = 158) achieved successful outcome (treatment completion or cure), 9% (n = 16) died, and 7% (n = 13) were lost to follow-up. Adverse events were not systematically reported.
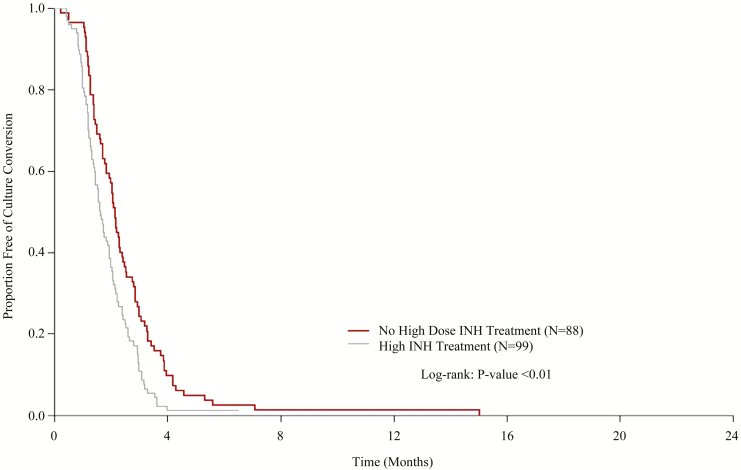
Time to culture conversion is shown in Figure 1. Patients who received high-dose isoniazid had faster time to culture conversion, with a median time of 7.0 weeks (95% confidence interval [CI]: 6.3, 8.3) compared to 9.1 weeks (95% CI: 7.9, 10.4) for those who did not receive high-dose isoniazid (P < .001). The difference in time to culture conversion remained statistically significant when patients with only low-level isoniazid resistance were excluded.
Figure 1.
Time to culture conversion stratified by high-dose isoniazid treatment among human immunodeficiency virus–negative patients. Abbreviation: INH, isoniazid.
In the univariate analysis, year of treatment, age, sex, education, income, relationship status, pyrazinamide, ethionamide, fluoroquinolone and/or second-line injectable resistance, and receipt of high-dose isoniazid were included. The following variables were included in the adjusted model, based on P-values and odd ratios in the univariate model: male sex (OR: 0.50; 95% CI: 0.21, 1.12; P = .101), pre-XDR resistance pattern (OR: 0.24; 95% CI: 0.04, 1.92; P = .132), and receipt of high-dose isoniazid (OR: 2.06; 95% CI: 0.92, 4.77; P = .082). In the adjusted regression, receipt of high-dose isoniazid was the only variable associated with successful outcome (adjusted OR: 2.53, 95% CI: 1.08, 6.28; P = .036). Univariate and multivariate models produced similar results when patients with only low-level isoniazid resistance were excluded.
DISCUSSION
In this brief report we demonstrate that a cohort of HIV-negative patients with MDR-TB treated with high-dose isoniazid achieved faster culture conversion and had greater odds of achieving successful outcome, compared with patients who did not receive high-dose isoniazid, despite a high prevalence of high-dose isoniazid resistance by phenotypic DST.
The 2 most commonly reported mutations associated with isoniazid resistance are inhA and katG. Because the katG mutation inhibits the conversion of isoniazid into its active form, a higher dose may not overcome high-level resistance, adding toxicity without benefit. However, this inhibition may be incomplete, yielding variable levels of resistance [5, 6]. An intermediate susceptibility dose-dependent breakpoint, accounting for the variability of minimum inhibitory concentrations for isoniazid, was recently proposed. Using this breakpoint, most MDR-TB patients would have intermediate susceptibility, thereby potentially benefiting from high-dose isoniazid [7].
Differences in the rate of hepatic acetylation of isoniazid also contribute to pharmacokinetic variability, with higher rates of failure reported in fast acetylators [8]. There are no data on isoniazid acetylator status in Haitian populations. If a large proportion of our cohort were slow acetylators, isoniazid levels may have been sufficiently high to provide clinical benefit. This corresponds with previous studies suggesting that isoniazid-resistant tuberculosis strains may be susceptible to higher doses of isoniazid [9]. This is a critical point because the WHO recently removed high-dose isoniazid from the list of recommended MDR-TB medications [10].
Few studies have evaluated the effectiveness of high-dose isoniazid in patients with drug-resistant tuberculosis, but the limited data available suggest clinical benefit. Among a South African cohort of individuals with isoniazid-resistant tuberculosis, those with katG mutations treated with high-dose isoniazid had greater odds of successful outcome [3]. A randomized clinical trial among HIV-negative patients with MDR-TB in India compared high-dose isoniazid (16–18 mg/kg/day), standard-dose isoniazid, and placebo when added to a fluoroquinolone and injectable-based regimen. Receipt of high-dose isoniazid was associated with faster time to achievement of sputum negativity and likelihood of 6-month sputum negativity [2]. Neither study reported higher toxicity in patients who received high-dose isoniazid.
Further research is needed regarding the effectiveness and potential toxicity of high-dose isoniazid. Although our results suggest clinical benefit, they are limited by the retrospective and observational nature of our data. Prospective clinical trials are needed to better elucidate the value of high-dose isoniazid in patients with MDR-TB.
In conclusion, high-dose isoniazid may provide clinical benefit in the treatment of MDR-TB, despite high-level isoniazid resistance by phenotypic testing.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgements. The authors thank Dr. Jennifer Furin and Dr. Michael Rich for their support and advice in the clinical care of our patients.
Financial support. This work was supported by the Fogarty International Center, National Institutes of Health (grant number R25TW009337; 5D43TW010062-04).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Technical report on the pharmacokinetics and pharmacodynamics (PK/PD) of medicines used in the treatment of drug-resistant tuberculosis. Geneva, Switzerland: World Health Organization, 2018. [Google Scholar]
- 2. Katiyar SK, Bihari S, Prakash S, Mamtani M, Kulkarni H. A randomised controlled trial of high-dose isoniazid adjuvant therapy for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2008; 12:139–45. [PubMed] [Google Scholar]
- 3. Jacobson KR, Theron D, Victor TC, Streicher EM, Warren RM, Murray MB. Treatment outcomes of isoniazid-resistant tuberculosis patients, Western Cape Province, South Africa. Clin Infect Dis 2011; 53:369–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis. Geneva, Switzerland: World Health Organization, 2014. [PubMed] [Google Scholar]
- 5. Rieder HL, Van Deun A. Rationale for high-dose isoniazid in the treatment of multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2017; 21:123–4. [DOI] [PubMed] [Google Scholar]
- 6. Springer B, Calligaris-Maibach RC, Ritter C, Böttger EC. Tuberculosis drug resistance in an area of low endemicity in 2004 to 2006: semiquantitative drug susceptibility testing and genotyping. J Clin Microbiol 2008; 46:4064–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zuur MA, Pasipanodya JG, van Soolingen D, van der Werf TS, Gumbo T, Alffenaar JC. intermediate susceptibility dose-dependent breakpoints for high-dose rifampin, isoniazid, and pyrazinamide treatment in multidrug-resistant tuberculosis programs. Clin Infect Dis 2018; 67:1743–9. [DOI] [PubMed] [Google Scholar]
- 8. Pasipanodya JG, Srivastava S, Gumbo T. Meta-analysis of clinical studies supports the pharmacokinetic variability hypothesis for acquired drug resistance and failure of antituberculosis therapy. Clin Infect Dis 2012; 55:169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dooley KE, Mitnick CD, Ann DeGroote M, et al. ; Efficacy Subgroup, RESIST-TB Old drugs, new purpose: retooling existing drugs for optimized treatment of resistant tuberculosis. Clin Infect Dis 2012; 55:572–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization. Rapid communication: key changes to treatment of multidrug- and rifampicin-resistant tuberculosis (MDR/RR-TB). Geneva, Switzerland: World Health Organization, 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.