Preface:
Combination anti-retroviral therapy (ART) has revolutionized the treatment and prevention of HIV-1 infection. Taken daily, ART prevents and suppresses the infection. However, ART interruption almost invariably leads to rebound viremia in infected individuals due to a long-lived latent reservoir of integrated proviruses. Therefore, ART must be administered on a lifelong basis. Here we review recent preclinical and clinical studies that suggest that immunotherapy may be an alternative or an adjuvant to ART because in addition to preventing new infections, anti-HIV-1 antibodies clear the virus, directly kill infected cells and produce immune complexes that can enhance host immunity to the virus.
Antibodies are uniquely attractive pharmaceutical agents because of their dual functionality. In addition to targeting a specific epitope with their variable domains, antibodies harness host effector functions by their constant domains which engage host Fc receptors. This class of receptors is found on a variety of different host immune cells that can mediate effector functions such as initiating phagocytosis or direct cellular cytotoxicity1. For example, antibody-based cancer therapies eliminate malignant cells, and induce adaptive T cell immunity. In addition, antibody-based checkpoint inhibitors further stimutate T cell immunity resulting in remarkable improvements in cancer therapy. The dual features of antibody-based therapies have led to a remarkable increase in the approval and use of monoclonal antibodies in the clinic. While immunotherapy was initially limited to a small number of antibody products, to date over 70 monoclonal antibodies are clinically used to treat a broad spectrum of diseases2 (Fig. 1).
Figure 1. Approved monoclonal antibodies and bNAbs tested in clinical trials.
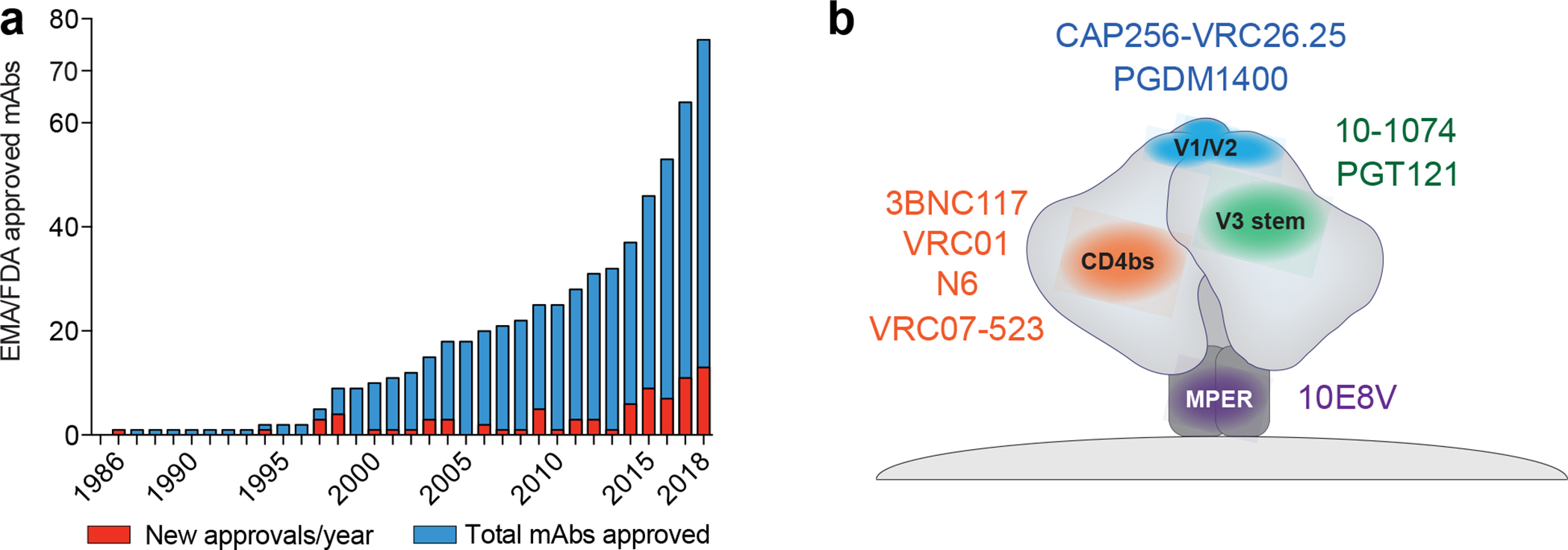
a.) Number of EMA (European Medicines Agency) and FDA (U.S. Food and Drug Administration) approved monoclonal antibodies up until the end of 2018. Red columns indicate the number of newly approved mAbs in a given year. Blue columns show the number of total mAbs approved2. b.) Second generation bNAbs target various epitopes on the HIV-1 envelope trimer (grey). Antibodies against the CD4bs (orange; 3BNC117, VRC01, VRC07–523, N6), the V1/V2 loop (blue; PDGM1400), the V3-stem (green; 10–1074, PGT121), and the Membrane Proximal External Region (MPER; purple; 10E8V) are currently being investigated in clinical studies.
Cancer and HIV infection share a number of features that complicate treatment and cure. Both are chronic diseases that display high degrees of genetic adaptation in response to therapeutic interventions. Latency is also a common feature as is the rare occurrence of spontaneous immune control. Moreover, in both cases combinations of drugs are required to control disease. The introduction of immunotherapy for cancer has dramatically increased the number of individuals that control and survive malignant diseases. Whether the same concepts are applicable to the treatment of HIV-1 infection is just beginning to be explored.
Here we review recent preclinical and clinical studies that indicate that monoclonal antibodies may also have a role in the prevention and therapy of HIV-1 infection.
Broadly neutralizing antibodies to HIV-1
The HIV-1 envelope spike (Env) is the only target on the surface of the virus. It is a trimer of gp41/gp120 heterodimers, that contains several sites of vulnerability targeted by antibodies. However, HIV-1 is enormously diverse and many of the antibodies that develop in infected individuals are strain specific3,4. Nevertheless, antibodies with neutralizing breadth were described early on in the epidemic starting with mouse hybridomas5. Subsequently, antibodies with increasing breadth and potency targeting distinct epitopes were obtained from humans6–9. Although these early antibodies displayed neutralizing breadth, their potency was limited resulting in little clinical benefit10,11. It was only after the introduction of single cell antibody cloning methods that highly potent broadly neutralizing antibodies (bNAbs) were obtained from infected individuals12,13. The properties of second generation bNAbs, engineered versions of these antibodies, and their target sites on the HIV-1 envelope spike have been reviewed extensively elsewhere14–17. Here we will focus on the clinical potential of bNAbs that have or will soon enter clinical trials (Table 1).
Table 1.
Anti-HIV-1 bNAbs in clinical development
| Target site | bNAb | Geometric mean IC50 (μg/ml) * | Fold Δ in neutralizing IC50 Primary viral isolates vs. pseudoviruses** | Half-life (HIV-uninfected individuals) | Antiviral activity in humans Decline in viremia (log10 copies/ml) | References |
|---|---|---|---|---|---|---|
| CD4 binding site | 3BNC117 | 0.4 | 12.8 | 17.6 +/− 5.7 days | 0.8 – 2.5 (median: 1.48) | Scheid et al., 2011 (22); Caskey et al., 2015 (65) |
| LSmean: 1.48 | ||||||
| VRC01 | 0.83 | 9.4 | 15 +/− 3.9 days | 1.1 – 1.8 (median: 1.35) | Wu et al., 2010 (21); Ledgerwood et al., 2015 (64); Lynch et al., 2015 (63) | |
| LS variant: 71 +/− 18 days | Ko et al., 2014 (43); Gaudinski et al., 2018 (76) | |||||
| VRC07–523 | 0.08 | n.a. | LS variant: 33 +/− 10 days | n.a. | Rudicell et al., 2014 (23); Ledgerwood et al., 2018 (100) | |
| N6 | 0.07 | n.a. | n.a. | n.a. | Huang et al., 2016 (24) | |
| V3 loop | 10–1074 | 0.38 | 4.3 | 24 +/− 6.6 days | 0.9 – 2.06 (median: 1.45) | Mouquet et al, 2012 (22); Caskey et al., 2017 (65) |
| LSmean: 1.52 | ||||||
| PGT121 | 0.44 | n.a. | Walker et al., 2011 (23) | |||
| V1/V2 loop | PDGM1400 | 0.17 | 3.3 | n.a. | n.a. | Sok et al., 2014 (26) |
| CAP256 (VRC26.25) | 0.11 | n.a. | Phase 1 study not started | Daria-Rose et al., 2012 (27) | ||
| MPER | 10E8 | 0.42 | 92.2 | n.a. | n.a. | Huang et al., 2012 (24); Kwon et al., 2016 (25) |
Anti-HIV-1 antibodies currently in clinical development. The table presents the IC50s on pseudovirus panels, the difference in potency of the antibodies against pseudoviruses and T cell–derived primary isolates, antibody half-life in vivo and activity against HIV-1 in viremic individuals. LSmean, least-square mean.
In vitro neutralizing activity measured by TZM.bl assay against 316 common pseudovirus multi-clade strains (Antibody Database, West A.P et al., 2013 (99))
Fold difference between the geometric mean IC50 of original clade B pseudovirus panels and PBMC-derived isolates (Cohen et al., 2018 (76))
The most clinically advanced bNAbs target a subset of vulnerability sites on Env: VRC0118, 3BNC11719, VRC07–52320, N621 and their variants target the CD4 binding site (CD4bs); 10–107422, and PGT12123 recognize the base of the V3 loop and surrounding glycans; 10E824,25, binds to the membrane proximal region (MPER); PGDM140026, and CAP25627 recognize the V1–V2 region and associated glycans.
HIV-1 Prevention in Non-Human Primates
Protection against HIV-1 infection by monoclonal antibodies targeting Env was first demonstrated in chimpanzees nearly 3 decades ago28. However, systematic studies to understand the dose and mechanism of antibody protection only became practical after the development of chimeric simian/human immunodeficiency viruses (SHIVs)29–32. These chimeric viruses combine components of simian immunodeficiency virus (SIV) with HIV-1 Env which makes them susceptible to neutralization by anti-HIV-1 specific antibodies. Like HIV-1, different SHIV strains vary in their sensitivity to bNAbs, for example SHIVBAL33 is a relatively easy to neutralize strain equivalent to laboratory adapted HIV-1 strains, and SHIVAD8 is similar to a primary HIV-1 isolate34.
Dose response experiments in macaques using first generation anti-HIV-1 monoclonal antibodies or pooled sera from HIV-infected individuals (HIVIG) indicated that even relatively low doses of potent antibodies might be sufficient for protection against high dose mucosal challenge35,36. These results were confirmed and extended when 6 different antibodies targeting the CD4bs or the V3-glycan patch were compared in protection experiments against high dose mucosal challenge in 60 macaques. Protection was directly related to antibody concentration and neutralizing activity. Probit regression analysis of the combined data set revealed that plasma ID50 neutralization titers of 1:100 were sufficient to prevent acquisition in 50% of the macaques37,38.
In contrast to high dose challenge in macaques, mucosal infection with HIV-1 in humans typically requires numerous exposures39. Human infection was modeled in macaques by low dose weekly mucosal challenges with SHIVAD840–42. A single bNAb infusion protected for up to 23 weeks in this model. When different bNAbs were compared, protective activity was directly related to antibody potency. Whereas 3BNC117, a CD4bs antibody, protected for a median of 13 weeks, VRC01, which targets the same site but is less potent, only protected for 8 weeks41. The protective activity of all bNAbs tested was extended by increasing their half-life with the introduction of two amino acid substitutions in the antibody’s Fc domain (M428L and N434S, or the LS mutation). These substitutions increase antibody binding affinity to the neonatal Fc receptor (FcRn), a recycling receptor that plays an important role in regulating antibody half-life in vivo41,43–45. For example, protection from low dose infection with SHIVAD8 by a single injection of 10–1074 was extended from a median of 13 to 27 weeks by the LS mutation44. Thus, antibodies can be highly effective and provide protection against infection for long periods of time. Altogether, these results suggest that the clinical potential of a bNAb or combination of bNAbs for prophylaxis against highly diverse circulating HIV-1 strains will depend on their breadth, potency and half-life in vivo.
Therapy in Preclinical Animal Models
Humanized mice and macaques are the best available preclinical models for testing the efficacy of passive immunotherapy against HIV-1. The advantage of using humanized mouse models is that they are reconstituted with human cells that can be infected with authentic HIV-1. Although the humanized mouse is an important model, the potential disadvantages of this model organism are: 1. Infection is sustained for relatively short times due to limited life span or graft versus host reactions; 2. Absence of a robust adaptive immune response; 3. Relatively small number of infected cells and latent reservoir compared to humans. The advantage of macaques infected with SHIV is that they have an intact immune system, and they develop long-lasting infection that leads to AIDS-like disease with some but not all SHIVs. However, SHIV is a chimeric virus that is biologically and molecularly distinct from HIV-1 and infections with some SHIVs are far less robust than HIV-1 infection in humans. In addition, macaque anti-human antibody responses interfere with bNAb antiviral activity. Each of these caveats must be considered when evaluating the results obtained in either humanized mice or macaques.
Monoclonal antibodies were initially tested for their therapeutic activity against HIV-1 in humanized mice two decades ago46. The data showed little or no effect on viremia by individual antibodies or cocktails of antibodies leading to the conclusion that antibodies would not play a significant role in controlling HIV-1 infection in humans46. As a result, this line of preclinical research was nearly abandoned, and only re-initiated over a dozen years later when far more potent second generation bNAbs became available13,47. The new antibodies reversed the established dogma in the field by significantly reducing viral load in HIV-1-infected humanized mice47. The degree to which viremia was suppressed and the amount of time it remained suppressed was dependent on the potency of the antibody and the initial level of viremia. For example, when viremia was first controlled by ART, monotherapy with bNAbs maintained suppression48. In contrast, there was rapid viral rebound by selected escape mutants during monotherapy in animals with higher initial viral loads47,49–51.
Whereas bNAb monotherapy produced only transient effects on viremia, a combination of antibodies targeting non-overlapping sites on Env completely suppressed HIV-1YU2 infection in most of the animals. Due to the long half-life of antibodies, suppression was maintained for an average of 60 days after therapy was terminated47. Finally, when antibody levels were no longer therapeutic the rebounding virus remained bNAb susceptible. Therefore HIV-1YU2 was unable to escape combination antibody therapy in humanized mice.
Similar experiments were subsequently performed in Rhesus macaques chronically infected with SHIVSF162.P3 or SHIVAD852,53. In both cases, monotherapy with bNAbs suppressed infection. However, the two SHIV strains differed in that bNAb monotherapy controlled SHIVSF162.P3 for longer periods of time than SHIVAD8. In addition, whereas SHIVSF162.P3 was unable to escape from monotherapy with PGT121, SHIVAD8 developed resistance to 10–1074 which targets the same site and is very closely related to PGT121. The discrepancy could be due to subtle differences in the mechanism of antigen binding by the two bNAbs, or simply that SHIVAD8 leads to a more robust infection than SHIVSF162.P3.
The combination of 3BNC117 and 10–1074 was tested in SHIVAD8- infected macaques52. In contrast to monotherapy, combination bNAb therapy led to prolonged control of SHIVAD8 infection with no evidence of escape from both of the antibodies. Thus, preclinical bNAb therapy experiments in SHIVAD8- infected macaques closely resembled the results of human clinical trials in which viremic individuals were administered the same bNAb combination (see below).
Remission in Preclinical Animal Models
Humanized mice and macaques have also been used to explore the possibility that bNAbs might induce prolonged HIV-1 remission or cure. In humanized mice infected with HIV-1YU2, combinations of bNAbs, latency reversal agents, and checkpoint blockade inhibitors led to remission in over 50% of the animals54. Important caveats to the mouse experiments are the small number of infected cells and the relatively short observation period.
An initial suggestion that passive antibody administration could alter the course of SHIV infection was obtained when prophylactic administration of a mixture of pooled HIV immune serum and b12, an early generation bNAb, led to attenuated SHIVSF162.P3 infection in animals that were not completely protected55. Conclusive evidence that passive immunotherapy changes the course of SHIV infection was obtained when a combination bNAbs was administered three days after SHIVAD8 infection56. Out of 13 macaques, 6 had undetectable viral loads after one year and another 4 showed low levels of viremia and normal CD4+ T cell counts equivalent to human controllers. Control was mediated by host CD8+ T cells because eliminating these cells led to recrudescent viremia in all 6 animals tested. Related results were subsequently obtained using SHIVSF162.P3 in animals initially treated with ART seven days after infection. The animals remained on ART and received a course of combination PGT121 and a TLR 7 agonist beginning two years after infection57. Animals with higher initial levels of SHIVSF162.P3 viremia controlled it by a CD8+ T cell mediated mechanism. In contrast, animals with the lowest initial viral loads appeared to clear the infection entirely. Whether or not CD8+ T cells contributed to clearing the infection could not be determined.
Can these preclinical studies be translated to humans? bNAb-therapy in macaques was initiated shortly after infection. Humans are not typically diagnosed and treated very early in infection, and therefore to be widely useful as immunotherapeutics, bNAbs would have to be effective in chronically infected individuals. Moreover, macaque infection with SHIV differs from human infection with HIV-1 in multiple respects. For example, the amount of SHIVSF162.P3 DNA present in the ART treated control animals in the combination PGT121 and TLR 7 agonist study was 1 copy per 106 cells which is 3 orders of magnitude less than in human HIV-1 infection57. Nevertheless, the results of two independent studies using different SHIVs and bNAbs establish that passive antibody therapy can help mediate host control of the infection. Thus, the preclinical studies support further testing of passive immunotherapy for HIV-1 in humans.
Human Trials
The idea that antibodies might be used to treat HIV-1 infection in humans was first tested in the clinic in 1992 by infusions of pooled polyclonal antibodies58 and the first monoclonal antibody was tested in viremic individuals in 199859. While antibody infusions were well tolerated, there were little or no antiviral effects. Over the next several years a number of groups tested combinations of first generation bNAbs for efficacy in viremic individuals, and for their ability to maintain viral suppression after ART discontinuation10,11,60,61. The antibodies were found to be generally safe, and one of the antibodies, 2G12, appeared to select for bNAb-resistant HIV-1 variants but showed little or no ability to control infection in either clinical setting. For example, a carefully controlled and well performed trial studied 8 individuals who initiated ART during chronic infection and 6 who were treated during acute infection. Despite harboring antibody-sensitive viruses, and receiving up to 13 infusions of a combination of 3 bNAbs over an 11-week period, viral suppression was only maintained in 1 of the 8 chronically-infected and 1 of the 6 acutely-infected individuals10. An independent study in which participants received weekly infusions of the same triple bNAb combination showed similar results11. The results of these trials led clinical investigators to abandon passive bNAb immunotherapy for HIV-1 infection.
Preclinical data obtained with second generation bNAbs in humanized mice and macaques re-invigorated this area of clinical investigation. Clinical studies were first reported for 3BNC117, followed by VRC01 and 10–107462–65. To date, over 4,000 uninfected individuals have received infusions of second generation bNAbs. Most of these individuals are participants in two ongoing HIV-1 prevention efficacy trials in which VRC01 is administered on a bi-monthly basis over 18 months (NCT02716675, NCT02568215). A smaller number of HIV-infected individuals on and off ART have received VRC01, VRC01LS, 3BNC117, 3BNC117-LS, 10–1074, 10–1074-LS, VRC07–523LS, PGT121 and PDGM-1400. The antibodies have been generally safe and well tolerated. However, subcutaneous administration of 10E8VLS, a bNAb that targets the membrane proximal external region of Env in conjunction with self-lipids present on the viral membrane24,25, was associated with the development of injection site reactions, including grade 3 erythema, which was associated with fever and malaise. The 10E8VLS clinical trial (NCT03565315) is currently on hold. Whether reactivity to self-lipids was the cause of these local injection reactions remains to be determined, but this is an important issue to clarify because other bNAbs have measurable affinity for self-including self-glycans22, and may also be prone to similar injection site reactions.
3BNC117 was initially tested for activity in viremic individuals. 10 of 11 participants receiving a single infusion of this monoclonal showed a mean drop in viral load of 1.48 log10 copies/ml. Viremia remained significantly suppressed for 28 days after the infusion62. Nearly identical results were obtained with 10–1074 which targets a non-overlapping site on the HIV-1 Env65. In addition to suppressing viremia, 3BNC117 infusion accelerated infected cell clearance and was associated with increased breadth and potency of host humoral immunity to HIV-166,67. Although resistant variants emerged in all individuals receiving 10–1074, 3 of the 11 receiving 3BNC117 had viruses that remained sensitive throughout the observation period of up to 24 weeks67. 3BNC117 monotherapy was also administered to 13 individuals undergoing analytical treatment interruption (ATI)68. In contrast to previous studies10,11, there was a significant delay in viral rebound and restriction of viral populations emerging from the reservoir. Moreover, a small number of the participants maintained viral suppression until 3BNC117 levels fell below 20 μg/ml suggesting failure to select pre-existing escape variants or to develop de novo resistance to 3BNC117. Administration of 3BNC117 in combination with ART over a period of 6 months before ATI did not alter the results69.
Clinical trials testing VRC01, which targets the same site as 3BNC117, produced similar but somewhat less pronounced results63,70. Viremic individuals who received a single infusion of VRC01 experienced a 1.1 to 1.8 log10 copies/ml decline in viremia (median 1.35 log10 copies/ml), and in all but 2 out of 8 individuals, viremia returned to baseline levels within 20 days, and increased resistance was seen in all individuals tested63,70. In the ATI setting, repeated infusions of VRC01 to 24 individuals maintained viral suppression for a median of approximately 4 weeks. The authors concluded that the effects of the antibody were limited by extensive pre-existing resistance to VRC0170. Thus, the efficacy of VRC01 and 3BNC117 in clinical trials is a reflection of their potency against primary HIV-1 isolates65,68,70,71, and is also directly related to their efficacy in preclinical models37,41.
The bNAb monotherapy trials led to the overall conclusion that these agents resemble small molecule drugs in that monotherapy with either modality selects for HIV-1 resistant variants. ART is effective because it combines drugs that target independent sites making it more difficult for resistant variants to emerge. The idea that bNAb combinations might be more effective against HIV-1 than monotherapy was tested in 2 Phase 1 clinical trials using the combination of 3BNC117 and 10–1074 that target non-overlapping sites on the HIV-1 Env.
The combination of 3BNC117 and 10–1074 was administered to 7 viremic individuals in a small clinical trial. The 4 individuals harboring viruses sensitive to both antibodies at baseline showed a 2.05 log10 copies/ml decrease in viremia that remained significantly reduced for 3 months. Complete suppression was only seen in the individual with the lowest starting viral load, but none developed de novo resistance to both antibodies despite persistent low-level viremia for several weeks. In keeping with the shorter half-life of 3BNC117, there was a period of 10–1074 monotherapy at the end of the observation period which coincided with the emergence of 10–1074 resistant variants. Thus, combination therapy was far more effective than monotherapy in viremic individuals, but it did not completely suppress viremia in participants with high baseline viral loads72.
The same bNAb combination was also administered to HIV-infected individuals who had achieved viral suppression on ART and underwent ART interruption. The antibodies were infused 2 days before ART interruption, and 2 more times after 3 and 6 weeks. Of the 9 individuals sensitive to both antibodies at baseline, 7 maintained viral suppression for a median of 21 weeks and the additional 2 continued to maintain suppression for months after the end of the 30-week study observation period. Similar to the viremic participants, there was no emergence of double-resistant viral variants. Thus, the combination of 3BNC117 and 10–1074 is effective in maintaining suppression for extended periods of time in individuals harboring HIV-1 strains sensitive to the antibodies73.
Future Clinical Applications
To date, the available clinical information on bNAbs is limited to Phase 1 trials with a small number of bNAbs, and therefore any discussion of their future clinical potential is highly speculative.
Prophylaxis:
Although there is no available clinical data, a large number of preclinical studies strongly suggest that bNAbs will be effective for prophylaxis because even relatively low concentrations of anti-HIV-1 neutralizing antibodies can block infection37,38,74,75. In addition, the relatively long half-life of bNAbs can be further extended by up to 4-fold by the LS mutation making them attractive candidates for use in prophylaxis43,76. This concept is currently being tested in 2 parallel phase 2b trials wherein VRC01 is being administered intravenously over 18 months at doses of 10 or 30 mg/Kg every 8 weeks77. VRC01 shows breadth and potency against large panels of pseudoviruses in vitro, however, its activity is significantly lower against primary HIV-1 isolates produced in CD4+ T cells65,68,70–73. Should the primary isolate data correlate with protective activity, as it appears to do for antibody therapy in infected individuals68,70, VRC01 will have only limited prophylactic efficacy. Moreover, intravenous delivery is impractical in resource poor areas of the world. Nevertheless, these proof of concept trials will provide important information on the relationship between potency, defined as neutralizing activity against pseudovirus panels or primary isolates in in vitro TZM/bl neutralization assays, and prophylactic efficacy.
Effective protection is likely to require a combination of antibodies to achieve adequate coverage (potency and breadth) against diverse circulating viral strains78–80 (Fig. 2). To become clinically useful, the antibodies will also need to have a long half-life, and be formulated at high concentrations so they can be delivered subcutaneously once every 3–4 months. All of these requirements can be met with existing technologies and it is likely that a long-acting subcutaneous antibody prophylactic will be tested for efficacy in the near future.
Figure 2. bNAb characteristics and virus/host factors determine efficacy of passive immunotherapy for HIV-1 prevention and therapy.
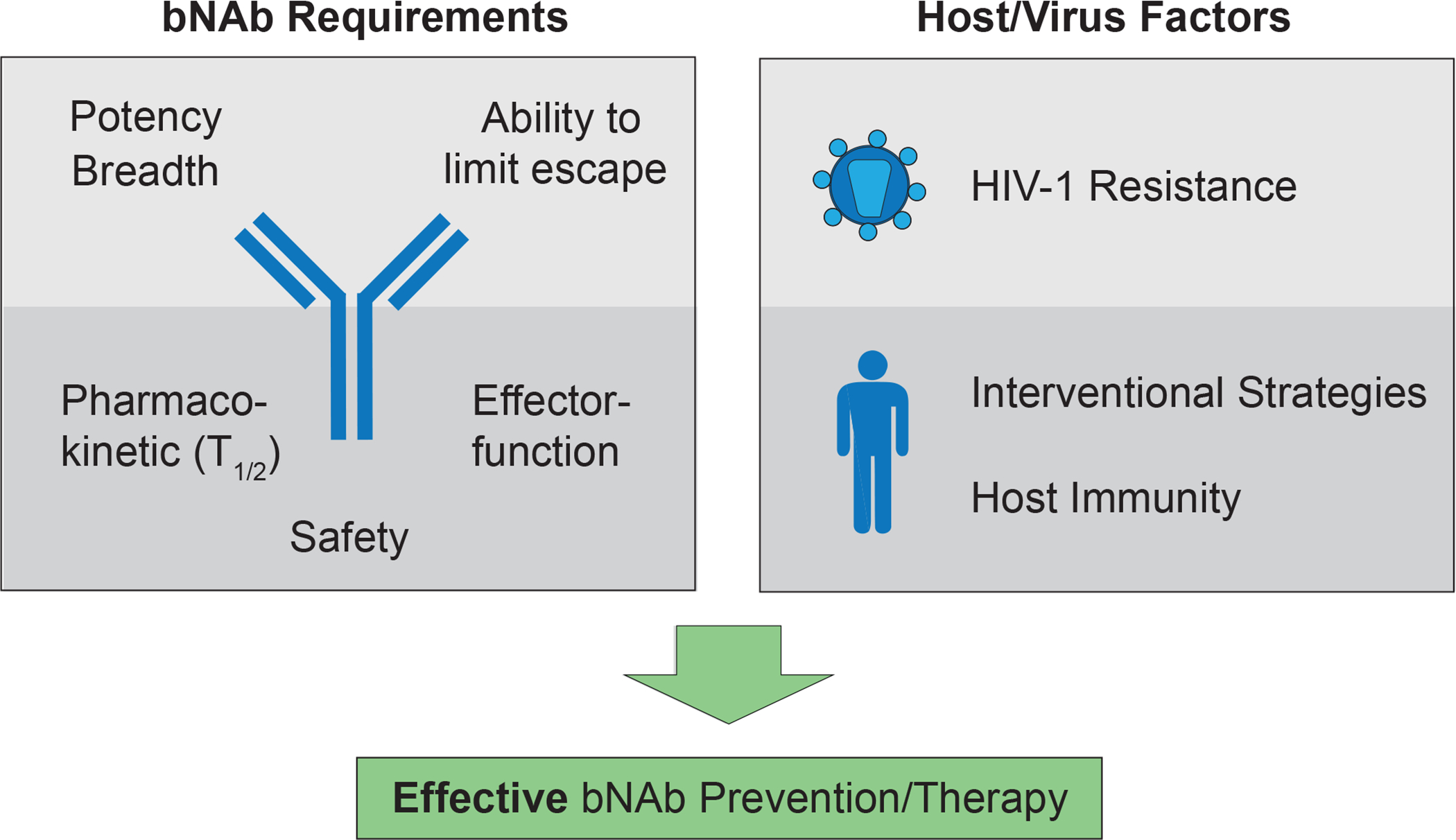
Potency (bNAb concentration required to prevent infection), breadth (neutralization coverage of HIV-1 strains), and the ability to restrict HIV-1 escape pathways are mainly determined by the antigen binding site of the bNAb. In addition, antibody Fc domains interact with host immune cells to mediate antiviral effector functions and can also be modified to increase bNAb half-life. Finally, tolerability and safety are prerequisites for the clinical application of bNAbs. Besides these antibody-intrinsic properties, viral features and host characteristics are critical for efficient bNAb-mediated interventions. Detailed characterization information of the viral quasi-species within an infected individual to identify pre-existing bNAb resistance is relevant for therapy, while the overall prevalence of antibody-sensitivity across circulating viral strains at a population-level is crucial for prevention. The contribution of host immunity and the selection of well-designed strategies will further determine the efficacy of bNAb-mediated interventions.
Maintenance Therapy:
Maintenance therapy is a higher bar than prevention because whereas a single antibody can block infection, long term therapy with individual bNAbs leads to resistance. Therefore, maintenance therapy requires combination bNAbs with redundant coverage that target non-overlapping sites on Env (Fig 2.).
Small clinical trials indicate that bNAb combinations can maintain viral suppression in individuals that have controlled replication on ART and that are infected with antibody sensitive viruses73. To date, these trials have been limited to unmodified bNAbs infused 3 times over a period of 6 weeks. On average, suppression was maintained for 15 weeks after the last bNAb infusion. Ongoing and soon to be initiated trials will extend the dosing regimens and test bNAbs that carry LS half-life extension mutations. Should the LS antibodies with extended half-life maintain suppression 3 to 4-fold longer than the parental antibodies, it may be possible to dose them intravenously on a bi-annual or yearly basis, or by subcutaneous self-injection on a monthly basis. This type of therapy would be particularly useful for adolescents and other populations for whom lifelong daily medications continue to pose a challenge. However, a significant number of individuals harbor HIV-1 variants that are resistant to individual bNAbs and this is an important potential limitation to this type of therapy. Moreover, while available data in chronically infected individuals suggest that escape from CD4bs antibodies may be harder to achieve than escape from antibodies targeting the V3 loop62,65,67,69,72,73, it is not yet known if antibody combinations targeting a specific subset of Env vulnerability sites will show superior efficacy. Therefore, reliable clinical assays for antibody resistance, and additional antibody combinations or antibody plus long-acting ARV combinations will be required for this type of therapy to become generally applicable.
Prolonged Remission or Cure:
HIV-1 integrates into the host genome and in rare instances it becomes latent in CD4+ T cells81. Whereas productive infection leads to cell death, CD4+ T cells containing latent viruses, are long-lived82. Moreover, latently-infected cells can undergo clonal expansion leading to a significant reservoir of latent viruses69,83–85. Stochastic activation of these reservoir viruses produces rebound viremia when ART is interrupted leading to a lifelong requirement for ART. Controlling or eliminating the reservoir is the most speculative of all of the potential uses for bNAbs. Nevertheless, preclinical and anecdotal clinical observations suggest that this idea should be further explored in the clinic54,56,57,66.
Rare individuals (< 1%) naturally control HIV-1 replication in the absence of therapy for prolonged periods of time by CD8+ T cell-mediated mechanisms86. These elite HIV-1 controllers are the counterparts of the occasional melanoma patient that also controls disease spontaneously87. More generally, individuals infected with HIV-1 partially control their disease with CD8+ T cells for long periods as evidenced by viral setpoints that are 1–3 orders of magnitude below initial peak viremia, and by the evolution of CD8+ T cell escape variants88–90. These CD8+ T cell responses can be enhanced in macaques treated early in infection with bNAbs such that they can control or eliminate SHIV infection56,57. How this happens is not entirely clear but may be due to formation of bNAb-HIV-1 immune complexes that activate dendritic cells leading to auto-vaccination by potent cross-presentation of HIV-1 antigens to CD8+ T cells91.
Whether bNAbs can also enhance CD8+ T cell responses in humans remains to be determined. If so, can it be done in the presence of ART or will it require treatment interruption to allow for increased antigen production? Moreover, even if broader and more functional CD8+ T cell responses are induced by bNAbs in humans, will these be sufficient to produce prolonged drug-free control or will control require additional immune modulation by CD8+ T cell-based vaccines or molecules such as checkpoint blockade inhibitors, TLR agonists or cytokine cocktails, as in cancer therapies? Despite the enormous success of checkpoint blockage in cancer therapy, response rates are highly variable and are, at least in part, related to the levels of preexisting anti-tumor T cell responses. Combinations of different immune-based strategies are being considered in cancer therapy as means to overcome low preexisting levels of anti-tumor T cells and acquired deficits in signaling pathways that may develop during therapy92. Similar approaches may be necessary for HIV-1 immunotherapy.
Another non-exclusive possibility is to use bNAbs to target the elimination of latent cells that are activated to transcribe the virus. Since productive infection leads to cell death, there has been a significant effort to find ways of activating latent viruses in the presence of ART in the hopes of killing cells containing latent viruses. Indeed, there are a number of inducers that can activate viral transcription in vitro. However, to date the “kick and kill” approach has not been effective in vivo perhaps because the level of viral transcription that can be safely achieved by the available inducers is insufficient to kill latent cells81,93. bNAbs could be valuable additions to “kick and kill” protocols if the level of viral transcription activated by inducers were sufficient to direct enough cell surface viral protein expression for bNAb recognition and cytotoxic cell targeting94,95.
Individuals who control HIV-1 replication in the absence of therapy after achieving ART-mediated viral suppression have also been described96–98. These post-treatment controllers are typically individuals diagnosed and treated during early HIV-1 infection. The mechanisms underlying long-term control in these individuals remain poorly understood. Unlike elite controllers, post-treatment controllers generally have low levels of CD8+ T cell responses and may control infection by other mechanisms, such as NK cell mediated killing99. Irrespective of the immune mechanism that underlies control, both elite and post-treatment controllers provide support to the idea that the immune system and therapies aimed at modulating its activities will play a role in viral remission or cure (Fig. 2).
Conclusions
Antibody based therapies have become essential clinical tools in treating chronic inflammatory and neoplastic disorders. Monoclonal antibodies are unique therapeutics because they are exquisitely specific, and can engage the host immune system to help fight disease. HIV-1 shares many of the features of these chronic diseases including rare instances of spontaneous control by the immune system. Immunotherapies enhance these responses and in cancer can produce prolonged remission or cure. Anti-HIV-1 immunotherapies have only recently entered the clinic but show promise for prevention, maintenance therapy and prolonged remission or cure. Engineered antibodies with dual or triple anti-HIV-1 specificities will soon enter clinical testing. In addition, different antibody delivery systems and bifunctional antibodies that bind HIV-1 Env while engaging a cellular target to enhance effector functions are also being developed17. Additional preclinical experiments and clinical trials aimed to discover methods to effectively prevent infection and enhance host immunity to HIV-1 will establish the role of bNAbs in the clinical armamentarium against HIV-1.
Acknowledgements
We thank members of the Klein and Nussenzweig laboratories for discussion. This work was supported by the NIH/National Institute of Allergy and Infectious Diseases Grant (U01AI129825), the Einstein-Rockefeller-CUNY Center for AIDS Research (1P30AI124414-01A1), BEAT-HIV Delaney grant UM1 AI126620 (M.C.); the Heisenberg-Program of the DFG (KL 2389/2-1), the European Research Council (ERC-StG639961), and the German Center for Infection Research (DZIF) (F.K.); Bill and Melinda Gates Foundation Collaboration for AIDS Vaccine Discovery (CAVD) grants OPP1092074, OPP1124068 and the NIH grants 1UM1 AI100663 and R01AI-129795 (M.C.N.); and the Robertson fund. M.C.N. is a Howard Hughes Medical Institute Investigator.
Footnotes
Competing Interests
There are patents on 3BNC117 and 10–1074 on which M.C.N is an inventor.
Bibliography
- 1.Bournazos S, Wang TT, Dahan R, Maamary J & Ravetch JV Signaling by Antibodies: Recent Progress. Annu Rev Immunol 35, 285–311, doi: 10.1146/annurev-immunol-051116-052433 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaplon H & Reichert JM Antibodies to watch in 2019. MAbs 11, 219–238, doi: 10.1080/19420862.2018.1556465 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei X et al. Antibody neutralization and escape by HIV-1. Nature 422, 307–312, doi: 10.1038/nature01470 (2003). [DOI] [PubMed] [Google Scholar]
- 4.Richman DD, Wrin T, Little SJ & Petropoulos CJ Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc Natl Acad Sci U S A 100, 4144–4149, doi: 10.1073/pnas.0630530100 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchacher A et al. Generation of human monoclonal antibodies against HIV-1 proteins; electrofusion and Epstein-Barr virus transformation for peripheral blood lymphocyte immortalization. AIDS Res Hum Retroviruses 10, 359–369, doi: 10.1089/aid.1994.10.359 (1994). [DOI] [PubMed] [Google Scholar]
- 6.Burton DR et al. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science 266, 1024–1027 (1994). [DOI] [PubMed] [Google Scholar]
- 7.Trkola A et al. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J Virol 70, 1100–1108 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zwick MB et al. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J Virol 75, 10892–10905, doi: 10.1128/JVI.75.22.10892-10905.2001 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorny MK et al. Neutralization of diverse human immunodeficiency virus type 1 variants by an anti-V3 human monoclonal antibody. J Virol 66, 7538–7542 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trkola A et al. Delay of HIV-1 rebound after cessation of antiretroviral therapy through passive transfer of human neutralizing antibodies. Nat Med 11, 615–622, doi: 10.1038/nm1244 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Mehandru S et al. Adjunctive passive immunotherapy in human immunodeficiency virus type 1-infected individuals treated with antiviral therapy during acute and early infection. J Virol 81, 11016–11031, doi: 10.1128/JVI.01340-07 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheid JF et al. A method for identification of HIV gp140 binding memory B cells in human blood. J Immunol Methods 343, 65–67, doi: 10.1016/j.jim.2008.11.012 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klein F et al. Antibodies in HIV-1 vaccine development and therapy. Science 341, 1199–1204 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.West AP Jr. et al. Structural insights on the role of antibodies in HIV-1 vaccine and therapy. Cell 156, 633–648, doi: 10.1016/j.cell.2014.01.052 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker LM & Burton DR Passive immunotherapy of viral infections: ‘super-antibodies’ enter the fray. Nat Rev Immunol 18, 297–308, doi: 10.1038/nri.2017.148 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mascola JR & Haynes BF HIV-1 neutralizing antibodies: understanding nature’s pathways. Immunol Rev 254, 225–244, doi: 10.1111/imr.12075 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gama L & Koup RA New-Generation High-Potency and Designer Antibodies: Role in HIV-1 Treatment. Annu Rev Med 69, 409–419, doi: 10.1146/annurev-med-061016-041032 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Wu X et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science 329, 856–861, doi: 10.1126/science.1187659 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scheid JF et al. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science 333, 1633–1637, doi: 10.1126/science.1207227 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rudicell RS et al. Enhanced potency of a broadly neutralizing HIV-1 antibody in vitro improves protection against lentiviral infection in vivo. J Virol 88, 12669–12682, doi: 10.1128/JVI.02213-14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang J et al. Identification of a CD4-Binding-Site Antibody to HIV that Evolved Near-Pan Neutralization Breadth. Immunity 45, 1108–1121, doi: 10.1016/j.immuni.2016.10.027 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mouquet H et al. Complex-type N-glycan recognition by potent broadly neutralizing HIV antibodies. Proc Natl Acad Sci U S A 109, E3268–3277, doi: 10.1073/pnas.1217207109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walker LM et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 477, 466–470, doi: 10.1038/nature10373 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang J et al. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature 491, 406–412, doi: 10.1038/nature11544 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwon YD et al. Optimization of the Solubility of HIV-1-Neutralizing Antibody 10E8 through Somatic Variation and Structure-Based Design. J Virol 90, 5899–5914, doi: 10.1128/JVI.03246-15 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sok D et al. Recombinant HIV envelope trimer selects for quaternary-dependent antibodies targeting the trimer apex. Proc Natl Acad Sci U S A 111, 17624–17629, doi: 10.1073/pnas.1415789111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doria-Rose NA et al. Developmental pathway for potent V1V2-directed HIV-neutralizing antibodies. Nature 509, 55–62, doi: 10.1038/nature13036 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Emini EA et al. Prevention of HIV-1 infection in chimpanzees by gp120 V3 domain-specific monoclonal antibody. Nature 355, 728–730, doi: 10.1038/355728a0 (1992). [DOI] [PubMed] [Google Scholar]
- 29.Mascola JR et al. Protection of Macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J Virol 73, 4009–4018 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mascola JR et al. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med 6, 207–210, doi: 10.1038/72318 (2000). [DOI] [PubMed] [Google Scholar]
- 31.Shibata R et al. Generation of a chimeric human and simian immunodeficiency virus infectious to monkey peripheral blood mononuclear cells. J Virol 65, 3514–3520 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li J, Lord CI, Haseltine W, Letvin NL & Sodroski J Infection of cynomolgus monkeys with a chimeric HIV-1/SIVmac virus that expresses the HIV-1 envelope glycoproteins. J Acquir Immune Defic Syndr 5, 639–646 (1992). [PubMed] [Google Scholar]
- 33.Pal R et al. Characterization of a simian human immunodeficiency virus encoding the envelope gene from the CCR5-tropic HIV-1 Ba-L. J Acquir Immune Defic Syndr 33, 300–307 (2003). [DOI] [PubMed] [Google Scholar]
- 34.Gautam R et al. Pathogenicity and mucosal transmissibility of the R5-tropic simian/human immunodeficiency virus SHIV(AD8) in rhesus macaques: implications for use in vaccine studies. J Virol 86, 8516–8526, doi: 10.1128/JVI.00644-12 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parren PW et al. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J Virol 75, 8340–8347 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishimura Y et al. Determination of a statistically valid neutralization titer in plasma that confers protection against simian-human immunodeficiency virus challenge following passive transfer of high-titered neutralizing antibodies. Journal of Virology 76, 2123–2130, doi: 10.1128/Jvi.76.5.2123.2130.2002 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shingai M et al. Passive transfer of modest titers of potent and broadly neutralizing anti-HIV monoclonal antibodies block SHIV infection in macaques. J Exp Med 211, 2061–2074, doi: 10.1084/jem.20132494 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang Y et al. Modeling cumulative overall prevention efficacy for the VRC01 phase 2b efficacy trials. Hum Vaccin Immunother 14, 2116–2127, doi: 10.1080/21645515.2018.1462640 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patel P et al. Estimating per-act HIV transmission risk: a systematic review. AIDS 28, 1509–1519, doi: 10.1097/QAD.0000000000000298 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hessell AJ et al. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat Med 15, 951–954, doi: 10.1038/nm.1974 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gautam R et al. A single injection of anti-HIV-1 antibodies protects against repeated SHIV challenges. Nature 533, 105–109, doi: 10.1038/nature17677 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moldt B et al. A nonfucosylated variant of the anti-HIV-1 monoclonal antibody b12 has enhanced FcgammaRIIIa-mediated antiviral activity in vitro but does not improve protection against mucosal SHIV challenge in macaques. J Virol 86, 6189–6196, doi: 10.1128/JVI.00491-12 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ko SY et al. Enhanced neonatal Fc receptor function improves protection against primate SHIV infection. Nature 514, 642–645, doi: 10.1038/nature13612 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gautam R et al. A single injection of crystallizable fragment domain-modified antibodies elicits durable protection from SHIV infection. Nat Med 24, 610–616, doi: 10.1038/s41591-018-0001-2 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hinton PR et al. An engineered human IgG1 antibody with longer serum half-life. J Immunol 176, 346–356 (2006). [DOI] [PubMed] [Google Scholar]
- 46.Poignard P et al. Neutralizing antibodies have limited effects on the control of established HIV-1 infection in vivo. Immunity 10, 431–438 (1999). [DOI] [PubMed] [Google Scholar]
- 47.Klein F et al. HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature 492, 118–122, doi: 10.1038/nature11604 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Horwitz JA et al. HIV-1 suppression and durable control by combining single broadly neutralizing antibodies and antiretroviral drugs in humanized mice. Proc Natl Acad Sci U S A 110, 16538–16543, doi: 10.1073/pnas.1315295110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diskin R et al. Restricting HIV-1 pathways for escape using rationally designed anti-HIV-1 antibodies. J Exp Med 210, 1235–1249, doi: 10.1084/jem.20130221 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klein F et al. Enhanced HIV-1 immunotherapy by commonly arising antibodies that target virus escape variants. J Exp Med 211, 2361–2372, doi: 10.1084/jem.20141050 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Freund NT et al. Coexistence of potent HIV-1 broadly neutralizing antibodies and antibody-sensitive viruses in a viremic controller. Sci Transl Med 9, doi: 10.1126/scitranslmed.aal2144 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shingai M et al. Antibody-mediated immunotherapy of macaques chronically infected with SHIV suppresses viraemia. Nature 503, 277–280, doi: 10.1038/nature12746 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barouch DH et al. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature 503, 224–228, doi: 10.1038/nature12744 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Halper-Stromberg A et al. Broadly neutralizing antibodies and viral inducers decrease rebound from HIV-1 latent reservoirs in humanized mice. Cell 158, 989–999, doi: 10.1016/j.cell.2014.07.043 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jaworski JP et al. Neutralizing polyclonal IgG present during acute infection prevents rapid disease onset in simian-human immunodeficiency virus SHIVSF162P3-infected infant rhesus macaques. J Virol 87, 10447–10459, doi: 10.1128/JVI.00049-13 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nishimura Y et al. Early antibody therapy can induce long-lasting immunity to SHIV. Nature 543, 559–563, doi: 10.1038/nature21435 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Borducchi EN et al. Antibody and TLR7 agonist delay viral rebound in SHIV-infected monkeys. Nature 563, 360–364, doi: 10.1038/s41586-018-0600-6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vittecoq D et al. Passive Immunotherapy in AIDS: A Randomized Trial of Serial Human Immunodeficiency Virus-Positive Transfusions of Plasma Rich in p24 Antibodies versus Transfusions of Seronegative Plasma. The Journal of Infectious Diseases 165, 364–368, doi: 10.1093/infdis/165.2.364 (1992). [DOI] [PubMed] [Google Scholar]
- 59.Cavacini LA et al. Phase I study of a human monoclonal antibody directed against the CD4-binding site of HIV type 1 glycoprotein 120. AIDS Res Hum Retroviruses 14, 545–550, doi: 10.1089/aid.1998.14.545 (1998). [DOI] [PubMed] [Google Scholar]
- 60.Armbruster C et al. A phase I trial with two human monoclonal antibodies (hMAb 2F5, 2G12) against HIV-1. AIDS 16, 227–233 (2002). [DOI] [PubMed] [Google Scholar]
- 61.Armbruster C et al. Passive immunization with the anti-HIV-1 human monoclonal antibody (hMAb) 4E10 and the hMAb combination 4E10/2F5/2G12. J Antimicrob Chemother 54, 915–920, doi: 10.1093/jac/dkh428 (2004). [DOI] [PubMed] [Google Scholar]
- 62.Caskey M et al. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature 522, 487–491, doi: 10.1038/nature14411 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lynch RM et al. Virologic effects of broadly neutralizing antibody VRC01 administration during chronic HIV-1 infection. Sci Transl Med 7, 319ra206, doi: 10.1126/scitranslmed.aad5752 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ledgerwood JE et al. Safety, pharmacokinetics and neutralization of the broadly neutralizing HIV-1 human monoclonal antibody VRC01 in healthy adults. Clin Exp Immunol 182, 289–301, doi: 10.1111/cei.12692 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Caskey M et al. Antibody 10–1074 suppresses viremia in HIV-1-infected individuals. Nat Med, doi: 10.1038/nm.4268 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lu CL et al. Enhanced clearance of HIV-1-infected cells by anti-HIV-1 broadly neutralizing antibodies in vivo. Science (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schoofs T et al. HIV-1 therapy with monoclonal antibody 3BNC117 elicits host immune responses against HIV-1. Science 352, 997–1001, doi: 10.1126/science.aaf0972 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scheid JF et al. HIV-1 antibody 3BNC117 suppresses viral rebound in humans during treatment interruption. Nature 535, 556–560, doi: 10.1038/nature18929 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cohen YZ et al. Relationship between latent and rebound viruses in a clinical trial of anti-HIV-1 antibody 3BNC117. J Exp Med, doi: 10.1084/jem.20180936 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bar KJ et al. Effect of HIV Antibody VRC01 on Viral Rebound after Treatment Interruption. N Engl J Med 375, 2037–2050, doi: 10.1056/NEJMoa1608243 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cohen YZ et al. Neutralizing Activity of Broadly Neutralizing Anti-HIV-1 Antibodies against Clade B Clinical Isolates Produced in Peripheral Blood Mononuclear Cells. J Virol 92, doi: 10.1128/JVI.01883-17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bar-On Y et al. Safety and antiviral activity of combination HIV-1 broadly neutralizing antibodies in viremic individuals. Nat Med 10.1038/s41591-018-0186-4, doi: 10.1038/s41591-018-0186-4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mendoza P et al. Combination therapy with anti-HIV-1 antibodies maintains viral suppression. Nature 561, 479–484, doi: 10.1038/s41586-018-0531-2 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moldt B et al. Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proc Natl Acad Sci U S A 109, 18921–18925, doi: 10.1073/pnas.1214785109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Julg B et al. Broadly neutralizing antibodies targeting the HIV-1 envelope V2 apex confer protection against a clade C SHIV challenge. Sci Transl Med 9, doi: 10.1126/scitranslmed.aal1321 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gaudinski MR et al. Safety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: A Phase 1 open-label clinical trial in healthy adults. PLoS Med 15, e1002493, doi: 10.1371/journal.pmed.1002493 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gilbert PB et al. Basis and Statistical Design of the Passive HIV-1 Antibody Mediated Prevention (AMP) Test-of-Concept Efficacy Trials. Stat Commun Infect Dis 9, doi: 10.1515/scid-2016-0001 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wagh K et al. Potential of conventional & bispecific broadly neutralizing antibodies for prevention of HIV-1 subtype A, C & D infections. PLoS Pathog 14, e1006860, doi: 10.1371/journal.ppat.1006860 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Doria-Rose NA et al. HIV-1 neutralization coverage is improved by combining monoclonal antibodies that target independent epitopes. J Virol 86, 3393–3397, doi: 10.1128/JVI.06745-11 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kong R et al. Improving neutralization potency and breadth by combining broadly reactive HIV-1 antibodies targeting major neutralization epitopes. J Virol 89, 2659–2671, doi: 10.1128/JVI.03136-14 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sengupta S & Siliciano RF Targeting the Latent Reservoir for HIV-1. Immunity 48, 872–895, doi: 10.1016/j.immuni.2018.04.030 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chun TW et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature 387, 183–188, doi: 10.1038/387183a0 (1997). [DOI] [PubMed] [Google Scholar]
- 83.Lorenzi JC et al. Paired quantitative and qualitative assessment of the replication-competent HIV-1 reservoir and comparison with integrated proviral DNA. Proc Natl Acad Sci U S A 113, E7908–E7916, doi: 10.1073/pnas.1617789113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hosmane NN et al. Proliferation of latently infected CD4(+) T cells carrying replication-competent HIV-1: Potential role in latent reservoir dynamics. J Exp Med 214, 959–972, doi: 10.1084/jem.20170193 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang Z et al. Expanded cellular clones carrying replication-competent HIV-1 persist, wax, and wane. Proc Natl Acad Sci U S A 115, E2575–E2584, doi: 10.1073/pnas.1720665115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Walker BD & Yu XG Unravelling the mechanisms of durable control of HIV-1. Nat Rev Immunol 13, 487–498, doi: 10.1038/nri3478 (2013). [DOI] [PubMed] [Google Scholar]
- 87.Kalialis LV, Drzewiecki KT & Klyver H Spontaneous regression of metastases from melanoma: review of the literature. Melanoma Res 19, 275–282, doi: 10.1097/CMR.0b013e32832eabd5 (2009). [DOI] [PubMed] [Google Scholar]
- 88.Koup RA et al. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol 68, 4650–4655 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Walker BD et al. HIV-specific cytotoxic T lymphocytes in seropositive individuals. Nature 328, 345–348, doi: 10.1038/328345a0 (1987). [DOI] [PubMed] [Google Scholar]
- 90.Deng K et al. Broad CTL response is required to clear latent HIV-1 due to dominance of escape mutations. Nature 517, 381–385, doi: 10.1038/nature14053 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dhodapkar KM et al. Selective blockade of inhibitory Fcgamma receptor enables human dendritic cell maturation with IL-12p70 production and immunity to antibody-coated tumor cells. Proc Natl Acad Sci U S A 102, 2910–2915, doi: 10.1073/pnas.0500014102 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ribas A & Wolchok JD Cancer immunotherapy using checkpoint blockade. Science 359, 1350–1355, doi: 10.1126/science.aar4060 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Margolis DM & Archin NM Proviral Latency, Persistent Human Immunodeficiency Virus Infection, and the Development of Latency Reversing Agents. J Infect Dis 215, S111–S118, doi: 10.1093/infdis/jiw618 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cohn LB et al. Clonal CD4(+) T cells in the HIV-1 latent reservoir display a distinct gene profile upon reactivation. Nat Med 24, 604–609, doi: 10.1038/s41591-018-0017-7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shan L et al. Transcriptional Reprogramming during Effector-to-Memory Transition Renders CD4(+) T Cells Permissive for Latent HIV-1Infection. Immunity 47, 766–775 e763, doi: 10.1016/j.immuni.2017.09.014 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cockerham LR, Hatano H & Deeks SG Post-Treatment Controllers: Role in HIV “Cure” Research. Curr HIV/AIDS Rep 13, 1–9, doi: 10.1007/s11904-016-0296-x (2016). [DOI] [PubMed] [Google Scholar]
- 97.Namazi G et al. The Control of HIV After Antiretroviral Medication Pause (CHAMP) Study: Posttreatment Controllers Identified From 14 Clinical Studies. J Infect Dis 218, 1954–1963, doi: 10.1093/infdis/jiy479 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Saez-Cirion A et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog 9, e1003211, doi: 10.1371/journal.ppat.1003211 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Scott-Algaram D D. C; Arnold V; Cummings JS; Boufassa F; Lambotte O; Hocqueloux L; Saez-Cirion A; Rouzioux C Post-treatment controllers have particular NK cells with high anti-HIV capacity: VISCONTI study. Conference on Retroviruses and Opportunistic Infections Abstract 52 (2015). [Google Scholar]
- 100.West AP Jr. et al. Computational analysis of anti-HIV-1 antibody neutralization panel data to identify potential functional epitope residues. Proc Natl Acad Sci U S A 110, 10598–10603, doi: 10.1073/pnas.1309215110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]


