Abstract
Background
It is well established that obesity is a disease of sustained low-grade inflammation. However, it is currently unknown if obesity plays a role in the clinical manifestations and prognosis of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infected patients. In this study, we aimed to investigate whether obesity played a role in clinical manifestations and prognosis in patients infected with SARS-CoV-2.
Methods
This is a retrospective multicenter clinical study. A total of 96 patients hospitalized with SARS-CoV-2 infection were enrolled from Dongguan People’s Hospital, Nanfang hospital and the First Affiliated Hospital of Xiamen University between 23 January and 14 February 2020. Demographic and clinical data were extracted from medical records. Acute respiratory distress syndrome (ARDS) was defined as oxygenation index (PaO2/FiO2) ≤ 300 mmHg. We grouped patients through the body mass index (BMI). Associations were examined using the t test, χ2 test and multivariate logistic forward regression test.
Results
Patients with BMI < 24 were significantly younger (P = 0.025) with lower creatine kinase (P = 0.013), lower diastolic pressure blood (P = 0.035), lower serum creatinine (P = 0.012), lower lactate dehydrogenase (P = 0.001) and higher platelet count (P = 0.002). The BMI level was 20.78 ± 3.15 in patients without pneumonia compared with the patients with pneumonia (23.81 ± 3.49, P = 0.001). For patients without ARDS, an average BMI level of 22.65 ± 3.53 was observed, significantly lower than patients with ARDS (24.57 ± 3.59, P = 0.022). The mean BMI was 22.35 ± 3.56 in patients experienced with relieving the clinical symptoms or stable condition by radiographic tests, lower than patients with disease exacerbation with 24.89 ± 3.17 (P = 0.001). In addition, lymphocyte count (r = − 0.23, P = 0.027) and platelet count (r = − 0.44, P < 0.001) were negatively correlated with BMI. While hemoglobin (r = 0.267, P = 0.008), creatine kinase (r = 0.331, P = 0.001), serum creatinine (r = 0.424, P < 0.001) and lactate dehydrogenase (r = 0.343, P = 0.001) were significantly positive correlated with BMI. Multivariate analysis showed that older age (OR = 1.046, P = 0.009) and BMI ≥ 24 (OR = 1.258, P = 0.005) were independent risk factors associated ICU admission while BMI ≥ 24 (OR = 4.219, P = 0.007) was independent risk factor associated with radiographic disease exacerbation.
Conclusions
Our study found BMI was significantly associated with clinical manifestations and prognosis of patients with SARS-CoV-2 infection. For patients with increased risk, clinicians should intervene promptly to avoid disease progression.
Keywords: SARS-CoV-2, Obesity, Body mass index, Prognosis, Pneumonia, Acute respiratory distress syndrome
Background
A series of unexplained viral pneumonia cases occurred worldwide recently [1]. Subsequent studies have shown that this series of pneumonia was associated with a new coronavirus infection (SARS-CoV-2) [1, 2]. This virus epidemic is posing a huge threat to global public health [3–5].
This sudden infectious disease is mainly manifested as fever, fatigue, and cough [6, 7]. Upper respiratory symptoms are relatively rare, which may be due to the fact that the virus infects cells through angiotensin converting enzyme 2, which is mainly expressed in cells of the lower respiratory tract [8, 9]. About one-half of the patients developed dyspnoea after 1 week [6]. In severe and critical cases, it progressed rapidly (average 9 days) to acute respiratory distress syndrome (ARDS) with only mild symptoms in early stage [6, 10]. This brings difficulties in managing the infectious diseases. In order to control the epidemic better and reduce the spread of the disease, early detection, quarantine and timely treatment are the keys to management. However, it is still no very clear which cohort of the population is at high risk.
Obesity is now a global health issue [11, 12]. It is well established that obesity is a disease of sustained low-grade inflammation [13, 14]. Such inflammation has been suggested to be associated with obesity related disease. A series of inflammatory markers have been proved related with both obesity and obesity associated disease. Previous research has confirmed a positive association between obesity and C-reactive protein level [15]. Similar associations have also been reported for erythrocyte sedimentation rate [16] and some other inflammatory cytokines [17, 18]. Those findings further support the potential association between obesity and inflammation. The interactions between obesity and infectious diseases have recently received increasing recognition. Previously published data have indicated an association between obesity and poor outcome in pandemic H1N1 influenza infection [19]. Obesity is an established risk factor for surgical-site infections, nosocomial infections, periodontitis and skin infections [20]. However, it is currently unknown if obesity plays a role in the clinical manifestations and prognosis of SARS-CoV-2 infected patients.
In this study, the clinical manifestations and clinical outcomes of SARS-CoV-2 infected patients were evaluated. The purpose of this study was to determine the role of obesity in the prognoses of patients infected with SARS-CoV-2.
Subjects and methods
Subjects
This is a retrospective multicentre clinical study. This study was approved by the institutional ethics board of Nanfang Hospital, Southern Medical University. All consecutive patients with confirmed SARS-CoV-2 infection in Dongguan People’s Hospital, Nanfang hospital affiliated Southern Medical University and the First Affiliated Hospital of Xiamen University between 23 January and 14 February 2020 were enrolled. Oral consent was obtained from patients. All patients were diagnosed with SARS-CoV-2 by pharyngeal swab samples. The SARS-CoV-2 infection diagnostic standard is detection of two target genes of SARS-CoV-2 in pharyngeal swab samples using polymerase chain reaction (PCR) [10].
Data collection
We collected patient medical records and recorded patient demographic and clinical data. All data were reviewed by a team of experienced physicians. ARDS was defined as acute onset, oxygenation index (PaO2/FiO2) ≤ 300 mmHg, and a chest radiograph that showed patchy shadows [21]. We categorized patients based on body mass index (BMI, kg/m2). All BMIs were calculated based on the height and weight measured on admission.
Statistical analysis
Continuous variables were expressed as average values and compared with student t-test. The categorical variables were expressed as a number (percentage) and compared by chi-square test. A univariate and multivariate regression analysis was used, with the results presented as an odds ratio (OR) with a 95% confidence interval (CI). All analyses were performed using SPSS software package (version 13.0, SPSS Inc. Chicago, USA), alpha level was 0.05.
Results
Characteristics of patients infected SARS-CoV-2 grouped by BMI
A total of 96 patients infected with SARS-CoV-2 were enrolled. Among these, 59 had a BMI < 24 and 37 of them had a BMI ≥ 24. Demographic and clinical characteristics were compared and shown in Table 1. Patients with BMI < 24 were significantly younger than others (P = 0.025), while the creatine kinase (CK) (P = 0.013), diastolic pressure blood (DBP), serum creatinine (P = 0.012) and lactate dehydrogenase (LDH) (P = 0.001) level were significantly lower. Platelet counts (P = 0.002) were significantly higher in patients with BMI < 24.
Table 1.
The demographics and clinical characteristics between patients with COVID-19
| Characteristic | Group | P value | |
|---|---|---|---|
| BMI < 24 | BMI ≥ 24 | ||
| Sample size, n | 59 | 37 | – |
| Sex (male), n (%) | 31 (52.5) | 23 (62.2) | 0.355 |
| Age (years) | 35.41 ± 18.45 | 44.00 ± 17.23 | 0.025 |
| Systolic blood pressure | 124.84 ± 16.11 | 126.29 ± 15.51 | 0.666 |
| Diastolic blood pressure | 81.13 ± 9.61 | 85.57 ± 10.08 | 0.035 |
| Creatine kinase | 84.04 ± 112.12 | 169.37 ± 212.25 | 0.013 |
| Serum lactic acid | 1.64 ± 0.70 | 1.39 ± 0.65 | 0.123 |
| Neutrophil count | 3.33 ± 1.50 | 3.31 ± 1.41 | 0.927 |
| Lymphocyte count | 1.46 ± 1.04 | 1.19 ± 0.61 | 0.169 |
| Hemoglobin | 139.22 ± 15.07 | 142.51 ± 19.95 | 0.361 |
| Platelet count | 222.23 ± 66.42 | 180.89 ± 51.92 | 0.002 |
| Serum creatinine | 60.58 ± 19.09 | 70.19 ± 15.18 | 0.012 |
| ALT | 20.38 ± 16.65 | 26.22 ± 18.51 | 0.118 |
| AST | 21.70 ± 8.59 | 26.05 ± 13.88 | 0.065 |
| Lactate dehydrogenase | 177.82 ± 54.39 | 221.94 ± 73.21 | 0.001 |
| Smoking tobacco, n (%) | 5 (8.5) | 3 (8.1) | 0.950 |
ALT Alanine aminotransferase; AST Aspartate aminotransferase
We also analyzed the difference of clinical characteristics between patients with age < 18 and ≥ 18 years old. We found that patients younger than 18 years (n = 15) had lower systolic blood pressure (SBP) and DBP than adults (n = 81) (SBP: 114.67 ± 12.98 vs 127.01 ± 15.62 mmHg, P = 0.011; DBP: 75.25 ± 8.52 vs 84.02 ± 9.73 mmHg, P = 0.004). The average level of lymphocyte count in patients younger than 18 years was 2.29 ± 1.63, which was significantly higher than that of ≥ 18-year age group (1.18 ± 0.56, P < 0.001). However, no significantly different with CK level and lactate level was found between the two groups.
Association between BMI and clinical outcomes of SARS-CoV-2 infected patients
To further evaluate the association between BMI and the clinical outcomes of SARS-CoV-2 infected patients, we measured the proportion of patients with different outcomes (see Table 2). Among 96 patients infected with SARS-CoV-2, 21 of them were without pneumonia based on the finding of computerized tomography (CT) scan, while 75 patients were diagnosed with pneumonia. The proportions of patients with negative CT results were 85.7, 14.3 and 0% for patients with BMI < 24, 24–27.9 and ≥ 28, respectively; however, in pneumonia group, the proportions were 54.7, 32.0 and 13.3% in respective BMI groups (P = 0.027). Mean BMI value was 20.78 ± 3.15 in patients without pneumonia, compared with 23.81 ± 3.49 with pneumonia (P = 0.001).
Table 2.
Proportion of viral pneumonia by groups
| Variable | Patients with COVID-19 | P value | |
|---|---|---|---|
| Without pneumonia n = 21, n (%) |
With pneumonia n = 75, n (%) |
||
| BMI stage | 0.027 | ||
| < 24 | 18 (85.7) | 41 (54.7) | |
| 24–27.9 | 3 (14.3) | 24 (32.0) | |
| ≥ 28 | 0 (0) | 10 (13.3) | |
| BMI level | 20.78 ± 3.15 | 23.81 ± 3.49 | 0.001 |
Among the whole cohort, 25 patients were diagnosed with ARDS. The proportions of patients with ARDS with a BMI < 24, 24–27.9, and ≥ 28 were 52.0, 24.0, and 24.0%, respectively, which were significantly different from the proportions of 64.8, 29.6 and 5.6% in patients without ARDS (P = 0.035). Average BMI was 22.65 ± 3.53 in patients without ARDS, which was significantly lower than that of patients with ARDS (24.57 ± 3.59, P = 0.022, Table 3).
Table 3.
Proportion of ARDS by groups
| Variable | Patients with COVID-19 | P value | |
|---|---|---|---|
| Without ARDS n = 71, n (%) |
With ARDS n = 25, n (%) |
||
| BMI stage | 0.035 | ||
| < 24 | 46 (64.8) | 13 (52.0) | |
| 24–27.9 | 21 (29.6) | 6 (24.0) | |
| ≥ 28 | 4 (5.6) | 6 (24.0) | |
| BMI level | 22.65 ± 3.53 | 24.57 ± 3.59 | 0.022 |
Patients enrolled were received at least one repeat CT scan within 1 month. After treatment, 66 patients showed improved or stable disease, while 30 showed exacerbated disease. The mean BMI was 22.35 ± 3.56 in patients with disease stable or relief, which was lower than the mean BMI of 24.89 ± 3.17, present in patients with disease exacerbation (P = 0.001), as shown in Table 4.
Table 4.
Proportion of clinical outcome by groups
| Variables | COVID-19 patients with disease | P value | |
|---|---|---|---|
| Stable-relieve n = 66, n (%) |
Exacerbation n = 30, n (%) |
||
| BMI stage | 0.001 | ||
| < 24 | 49 (74.2) | 10 (33.3) | |
| 24–27.9 | 13 (19.7) | 14 (46.7) | |
| ≥ 28 | 4 (6.1) | 6 (20.0) | |
| BMI level | 22.35 ± 3.56 | 24.89 ± 3.17 | 0.001 |
Correlation between BMI and clinical variables
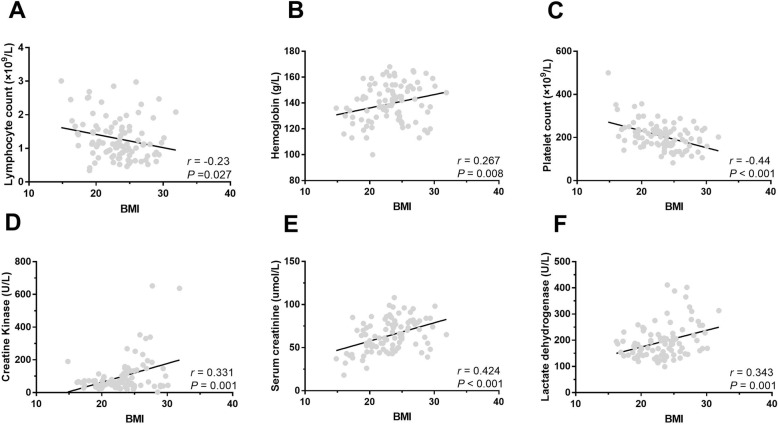
Interestingly, we found several unexpected clinical variables that were significantly correlated with BMI in all enrolled SARS-CoV-2 infected patients (Fig. 1). Lymphocyte count (r = − 0.23, P = 0.027) and platelet count (r = − 0.44, P < 0.001) were negatively correlated with BMI, while haemoglobin (r = 0.267, P = 0.008), CK (r = 0.331, P = 0.001), serum creatinine (r = 0.424, P < 0.001), and LDH (r = 0.343, P = 0.001) were significantly positive correlated with BMI.
Fig. 1.
Correlation between BMI and clinical variables in SARS-CoV-2 infected patients. a Correlation between lymphocyte and BMI (r = − 0.23, P = 0.027). b Correlation between haemoglobin and BMI (r = 0.267, P = 0.008). c Correlation between platelet count and BMI (r = − 0.44, P < 0.001). d Correlation between CK level and BMI (r = 0.331, P = 0.001). e Correlation between serum creatinine and BMI (r = 0.424, P < 0.001). f Correlation between LDH and BMI (r = 0.343, P = 0.001). BMI, Body mass index. CK, creatine kinase. LDH, lactate dehydrogenase
Univariate and multivariate analysis of factors associated with ICU admission
Logistic regression was performed to identify factors that were significantly associated with ICU admission in SARS-CoV-2-infected patients. The results of this multivariate analysis indicated that older age (OR = 1.046, P = 0.009) and BMI ≥ 24 (OR = 1.258, P = 0.005) were independent risk factors associated with ICU admission among patients with SARS-CoV-2 infection (Table 5).
Table 5.
Factors associated with ICU admission
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Sex (male vs female) | 1.361 | 0.595–3.112 | 0.465 | |||
| Age | 1.043 | 1.017–1.070 | 0.001 | 1.046 | 1.011–1.081 | 0.009 |
| Systolic blood pressure | 1.026 | 0.998–1.055 | 0.065 | |||
| Diastolic blood pressure | 1.023 | 0.980–1.068 | 0.295 | |||
| Creatine kinase | 1.002 | 0.999–1.004 | 0.256 | |||
| Serum lactic acid | 0.956 | 0.496–1.843 | 0.894 | |||
| Neutrophil count | 0.919 | 0.689–1.227 | 0.569 | |||
| Lymphocyte count | 0.532 | 0.274–1.032 | 0.062 | |||
| Hemoglobin | 1.016 | 0.992–1.040 | 0.198 | |||
| Platelet count | 0.992 | 0.985–1.000 | 0.041 | |||
| Serum creatinine | 1.021 | 0.998–1.045 | 0.073 | |||
| ALT | 1.019 | 0.995–1.044 | 0.117 | |||
| AST | 1.042 | 1.002–1.084 | 0.042 | |||
| Lactate dehydrogenase | 1.009 | 1.002–1.016 | 0.016 | |||
| Smoking tobacco (yes vs no) | 1.041 | 0.234–4.630 | 0.958 | |||
| BMI level (< 24 vs ≥ 24) | 5.190 | 2.110–12.763 | < 0.001 | 1.258 | 1.071–1.478 | 0.005 |
OR Odds ratio; CI Confidence interval; ALT Alanine aminotransferase; AST Aspartate aminotransferase
Univariate and multivariate analysis of factors associated with radiographic disease exacerbation
We also conducted logistic regression to identify factors that were associated with radiographic disease exacerbation in SARS-CoV-2 infected patients. Multivariate analyses showed that BMI ≥ 24 (OR = 4.219, P = 0.007) was independent risk factors associated with SARS-CoV-2 infected patients who experienced radiographic disease exacerbation (Table 6).
Table 6.
Factors associated with radiographic exacerbation
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Sex (male vs female) | 1.025 | 0.429–2.448 | 0.956 | |||
| Age | 1.023 | 0.998–1.048 | 0.069 | |||
| Systolic blood pressure | 1.009 | 0.982–1.038 | 0.513 | |||
| Diastolic blood pressure | 1.001 | 0.958–1.045 | 0.978 | |||
| Creatine kinase | 1.002 | 1.000–1.005 | 0.097 | |||
| Serum lactic acid | 1.041 | 0.523–2.074 | 0.909 | |||
| Neutrophil count | 0.979 | 0.725–1.322 | 0.889 | |||
| Lymphocyte count | 0.684 | 0.350–1.338 | 0.267 | |||
| Hemoglobin | 1.006 | 0.981–1.032 | 0.630 | |||
| Platelet count | 0.997 | 0.990–1.004 | 0.398 | |||
| Serum creatinine | 1.008 | 0.984–1.033 | 0.503 | |||
| ALT | 1.017 | 0.992–1.042 | 0.190 | |||
| AST | 1.025 | 0.986–1.065 | 0.208 | |||
| Lactate dehydrogenase | 1.001 | 0.994–1.008 | 0.756 | |||
| Smoking tobacco (yes vs no) | 1.356 | 0.302–6.083 | 0.691 | |||
| BMI level (< 24 vs ≥ 24) | 5.765 | 2.255–14.734 | < 0.001 | 4.219 | 1.490–11.944 | 0.007 |
OR Odds ratio; CI Confidence interval; ALT Alanine aminotransferase; AST Aspartate aminotransferase
Discussion
In this study, we found that obesity played an important role in development of COVID-19. SARS-CoV-2 infected patients with a higher BMI were more likely to develop ARDS and to experience exacerbated disease. Moreover, we found that patients with higher BMIs had lower lymphocyte counts, lower platelet counts, and higher levels of haemoglobin, CK, creatinine, and LDH. Our results may help to stratify patients with SARS-CoV-2 infection: patients with high BMIs should receive prompt intervention to avoid disease progression. Most patients with SARS-CoV-2 infection will develop pneumonia [22]. However, a small number of patients have negative imaging findings for unclear reasons. As we know, the largest study to-date indicated that 3498 of the 3665 (95.5%) confirmed cases were diagnosed with pneumonia [23]. According to the 2019 New Coronavirus Pneumonia Diagnosis and Treatment Plan recommended by the National Health Committee of China, these patients without pneumonia showed only low fever and mild fatigue, and usually recovered after 1 week. Our research confirmed that, although SARS-CoV-2 infection was confirmed, some patients had negative CT results. In addition, our study found that this situation was more likely to occur in young patients with lower BMIs. Although the prognosis of these patients seems to be better than older patients with high BMIs, repeated CT examinations are still needed, particularly if the clinical symptoms worsen.
Obesity is a common worldwide epidemic. Obesity is usually accompanied by a low-grade chronic inflammatory state, which is characterized by an increase in systemic inflammation markers [13, 16]. This mild chronic inflammation and non-specific activation of the immune system are thought to induce obesity-related diseases [13]. The reasons why obesity causes inflammation have not yet been well described, but studies have revealed that adipocytes secrete a variety of cytokines that help initiate an inflammatory response [24, 25]. In long-term chronic inflammation, a series of changes occur in the body, including in blood glucose, lipid, and hormone levels [24]. These changes are accompanied by insulin and catecholamine resistance, abnormal tissue remodelling and fibrosis [24]. Some patients with SARS-CoV-2 infections rapidly developed into critically ill patients, in which case the disease usually manifests as ARDS [6, 26]. Current research showed that the mortality rate of SARS-CoV-2 infected patients was 4–15% [6, 26]. It is therefore important to identify this population early. However, the subpopulations who became severely ill may have had moderate to low fever during the early course of the disease, and these patients are still difficult to screen. Whether obesity plays a role here is unknown, and further researches are still needed.
Our results suggest high BMI is closely related to patient disease progression. At the same time, our study showed that BMI was negatively correlated with lymphocytes and platelets, but positively correlated with CK, LDH, and creatinine. Patients with SARS-CoV-2-infections often experience lymphocytopenia [4, 7]. It is unknown whether SARS-CoV-2 infection will induce immune deficiency, and whether obesity and adipocytes play a role here. However, according to the results of our study, patients who with a high BMI, even if their symptoms are not obvious, should be given sufficient attention to avoid rapid deterioration.
The current study has several limitations. Firstly, this was a retrospective survey, with a relatively limited sample size. In addition, the role of obesity in the pathophysiology of SARS-CoV-2 infection requires further research to confirm. It is also an important topic to identify patients with SARS-CoV-2-associated pneumonia developing ARDS during the course of disease. This issue merits additional research.
Conclusions
Our study found that BMI was significantly related with clinical manifestations and clinical outcomes of patients with SARS-CoV-2 infections. Patients with higher BMIs were more likely to develop ARDS and to experience disease progression. Older age and high BMI are independent risk factors associated with ICU admission in SARS-CoV-2 infected patients, while patients with higher BMIa are more likely to experience disease exacerbation. For patients with risk factors, clinicians should intervene promptly to avoid disease progression.
Acknowledgements
We wish to thank Jian Zhang for his helpful assistance in the study.
Abbreviations
- ARDS
Acute respiratory distress syndrome
- BMI
Body mass index
- OR
Odds ratio
- CI
Confidence interval
- CK
Creatine kinase
- DBP
Diastolic pressure blood
- SBP
Systolic blood pressure
- CT
Computerized tomography
- COVID-19
Corona Virus Induced Disease 2019
Authors’ contributions
Shaohang Cai, Lili Liu, Wei Liao designed and guided this study, Shuwen Chen and Shaohang Cai wrote the main manuscript text. Zhidan Zheng and Siyao Liu prepared all tables and data analysis. All authors reviewed the manuscript. The author(s) read and approved the final manuscript.
Funding
This work was supported by the grants from the Major Science and Technology Special Project of China (2017ZX09304016, 2017ZX10302201004008), the National Natural Science Foundation of China (81971949) and the Clinical Research Startup Program of Southern Medical University by High-level University Construction Funding of Guangdong Provincial Department of Education (LC2016PY003)..
Availability of data and materials
Authors can confirm all relevant data are included in the article and materials are available on request from the authors. The original data of this study has been uploaded to the RDD platform (RDDA2020001502).
Ethics approval and consent to participate
The Institutional Review Board of Nanfang Hospital, Southern Medical University had approved this study. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. Oral consent was obtained from patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no financial or non-financial interests with other people or organizations that could inappropriately influence this work.
Footnotes
Shao-Hang Cai and Wei Liao contributed equally to this work.
Contributor Information
Shao-Hang Cai, Email: shaohangcai@foxmail.com.
Zhi-Dan Zheng, Email: 385338012@qq.com.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu XW, Wu XX, Jiang XG, Xu KJ, Ying LJ, Ma CL, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parry J. China coronavirus: cases surge as official admits human to human transmission. BMJ. 2020;368:m236. doi: 10.1136/bmj.m236. [DOI] [PubMed] [Google Scholar]
- 5.Silverstein WK, Stroud L, Cleghorn GE, Leis JA. First imported case of 2019 novel coronavirus in Canada, presenting as mild pneumonia. Lancet. 2020;395(10225):734. doi: 10.1016/S0140-6736(20)30370-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, et al. Clinical characteristics of 140 patients infected by SARS-CoV-2 in Wuhan, China. Allergy. 2020;10.1111/all.14238. [DOI] [PubMed]
- 8.Ren X, Glende J, Al-Falah M, de Vries V, Schwegmann-Wessels C, Qu X, et al. Analysis of ACE2 in polarized epithelial cells: surface expression and function as receptor for severe acute respiratory syndrome-associated coronavirus. J Gen Virol. 2006;87(Pt 6):1691–1695. doi: 10.1099/vir.0.81749-0. [DOI] [PubMed] [Google Scholar]
- 9.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin YH, Cai L, Cheng ZS, Cheng H, Deng T, Fan YP, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Mil Med Res. 2020;7(1):4. doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryan DH, Kahan S. Guideline recommendations for obesity management. Med Clin North Am. 2018;102(1):49–63. doi: 10.1016/j.mcna.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Ou H, Cai S, Liu Y, Xia M, Peng J. A noninvasive diagnostic model to assess nonalcoholic hepatic steatosis in patients with chronic hepatitis B. Ther Adv Gastroenterol. 2017;10(2):207–217. doi: 10.1177/1756283X16681707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cox AJ, West NP, Cripps AW. Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol. 2015;3(3):207–215. doi: 10.1016/S2213-8587(14)70134-2. [DOI] [PubMed] [Google Scholar]
- 14.Cai S, Ou Z, Liu D, Liu L, Liu Y, Wu X, et al. Risk factors associated with liver steatosis and fibrosis in chronic hepatitis B patient with component of metabolic syndrome. United European Gastroenterol J. 2018;6(4):558–566. doi: 10.1177/2050640617751252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi J, Joseph L, Pilote L. Obesity and C-reactive protein in various populations: a systematic review and meta-analysis. Obes Rev. 2013;14(3):232–244. doi: 10.1111/obr.12003. [DOI] [PubMed] [Google Scholar]
- 16.de Rooij SR, Nijpels G, Nilsson PM, Nolan JJ, Gabriel R, Bobbioni-Harsch E, et al. Low-grade chronic inflammation in the relationship between insulin sensitivity and cardiovascular disease (RISC) population: associations with insulin resistance and cardiometabolic risk profile. Diabetes Care. 2009;32(7):1295–1301. doi: 10.2337/dc08-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bahceci M, Gokalp D, Bahceci S, Tuzcu A, Atmaca S, Arikan S. The correlation between adiposity and adiponectin, tumor necrosis factor alpha, interleukin-6 and high sensitivity C-reactive protein levels. Is adipocyte size associated with inflammation in adults? J Endocrinol Investig. 2007;30(3):210–214. doi: 10.1007/BF03347427. [DOI] [PubMed] [Google Scholar]
- 18.Marques MA, Combes M, Roussel B, Vidal-Dupont L, Thalamas C, Lafontan M, et al. Impact of a mechanical massage on gene expression profile and lipid mobilization in female gluteofemoral adipose tissue. Obes Facts. 2011;4(2):121–129. doi: 10.1159/000327347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsatsanis C, Margioris AN, Kontoyiannis DP. Association between H1N1 infection severity and obesity-adiponectin as a potential etiologic factor. J Infect Dis. 2010;202(3):459–460. doi: 10.1086/653842. [DOI] [PubMed] [Google Scholar]
- 20.Huttunen R, Syrjanen J. Obesity and the risk and outcome of infection. Int J Obes. 2013;37(3):333–340. doi: 10.1038/ijo.2012.62. [DOI] [PubMed] [Google Scholar]
- 21.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 22.Zu ZY, Jiang MD, Xu PP, Chen W, Ni QQ, Lu GM, et al. Coronavirus disease 2019 (COVID-19): a perspective from China. Radiology. 2020:200490. 10.1148/radiol.2020200490. [DOI] [PMC free article] [PubMed]
- 23.Yang Y, Lu Q, Liu M, Wang Y, Zhang A, Jalali N, et al. Epidemiological and clinical features of the 2019 novel coronavirus outbreak in China. medRxiv. 2020. 10.1101/2020.02.10.20021675.
- 24.Karczewski J, Sledzinska E, Baturo A, Jonczyk I, Maleszko A, Samborski P, et al. Obesity and inflammation. Eur Cytokine Netw. 2018;29(3):83–94. doi: 10.1684/ecn.2018.0415. [DOI] [PubMed] [Google Scholar]
- 25.Reilly SM, Saltiel AR. Adapting to obesity with adipose tissue inflammation. Nat Rev Endocrinol. 2017;13(11):633–643. doi: 10.1038/nrendo.2017.90. [DOI] [PubMed] [Google Scholar]
- 26.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Authors can confirm all relevant data are included in the article and materials are available on request from the authors. The original data of this study has been uploaded to the RDD platform (RDDA2020001502).