Abstract
Introduction
The purpose of this study was to compare operative times, surgical outcomes, resource utilization, and hospital charges before and after the implementation of a sentinel lymph node (SLN) mapping algorithm in endometrial cancer.
Methods
All patients with clinical stage I endometrial cancer were identified pre- (2012) and post- (2017) implementation of the SLN algorithm. Clinical data were summarized and compared between groups. Total hospital charges incurred on the day of surgery were extracted from the hospital financial system for each patient and all charges were adjusted to 2017 US dollars.
Results
A total of 203 patients were included: 71 patients in 2012 and 130 patients in 2017. There was no difference in median age, body mass index, or stage. In 2012, 35/71 patients (49.3%) underwent a lymphadenectomy. In 2017, SLN mapping was attempted in 120/130 patients (92.3%) and at least one SLN was identified in 110/120 (91.7%). Median estimated blood loss was similar between groups (100 mL vs 75 mL, p=0.081). There was a significant decrease in both median operative time (210 vs 171 min, p=0.007) and utilization of intraoperative frozen section (63.4% vs 14.6%, p<0.0001). No significant differences were noted in intraoperative (p=1.00) or 30 day postoperative complication rates (p=0.30). The median total hospital charges decreased by 2.73% in 2017 as compared with 2012 (p=0.96).
Discussion
Implementation of an SLN mapping algorithm for high- and low-risk endometrial cancer resulted in a decrease in both operative time and intraoperative frozen section utilization with no change in surgical morbidity. While hospital charges did not significantly change, further studies are warranted to assess the true cost of SLN mapping.
INTRODUCTION
An estimated 63 230 women will be diagnosed with endometrial cancer in 2018, making it the most common gynecologic malignancy in the USA.1 Surgical staging to evaluate for metastatic disease has been the standard of care since 1988, when the International Federation of Gynecology and Obstetrics (FIGO) moved from a clinical to a surgical staging system. Current recommendations for endometrial cancer staging include a hysterectomy, bilateral salpingo-oophorectomy, lymph node assessment, and biopsy of any suspicious areas. Significant controversy remains regarding the best approach to lymph node sampling given the increased surgical morbidity of complete lymphadenectomy and two randomized trials demonstrating no survival benefit.2,3
A selective lymphadenectomy approach using intraoperative frozen section has been developed in an attempt to identify low-risk patients in whom a lymphadenectomy can be safely omitted. The Mayo Clinic reported that in patients with grade 1 or 2 endometrioid histology, with a tumor size ≤2 cm and <50% myometrial invasion on intraoperative frozen section, there was no risk of lymph node involvement.4 Another study from the Mayo Clinic determined intraoperative frozen section to be reliable; however, it is not universally available and it is unclear if it remains accurate outside of major academic medical centers with highly specialized pathologists.5,6
Sentinel lymph node (SLN) mapping has evolved as an alternative option to assess lymph node status and does not rely on the availability of intraoperative frozen section. It provides the diagnostic information necessary to guide adjuvant therapy while minimizing surgical morbidity. Through removal of only the first draining node(s), surgeons can accurately determine lymph node status while reducing risks associated with removal of more lymph nodes and decreasing operative time.7 It also helps to prevent understaging in those patients with discordant frozen and final pathology in whom a lymphadenectomy was inappropriately omitted.
Several prospective trials have validated the use of SLN mapping in lieu of lymphadenectomy in both low- and high-risk endometrial cancer.8,9 More recently, a multicenter study from Memorial Sloan Kettering and Mayo Clinic demonstrated that the use of SLN mapping in deeply invasive endometrioid endometrial cancer does not worsen oncologic outcomes.10 In response to the increasing evidence supporting SLN mapping, the National Comprehensive Cancer Network has added SLN mapping to its guidelines for the management of endometrial cancer in 2018.11
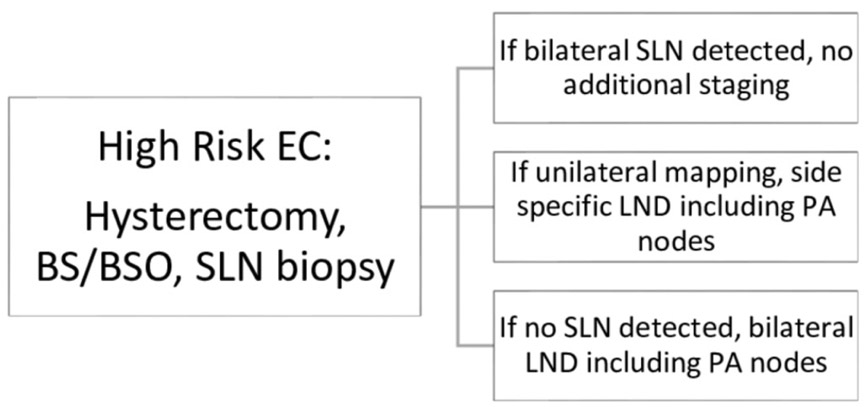
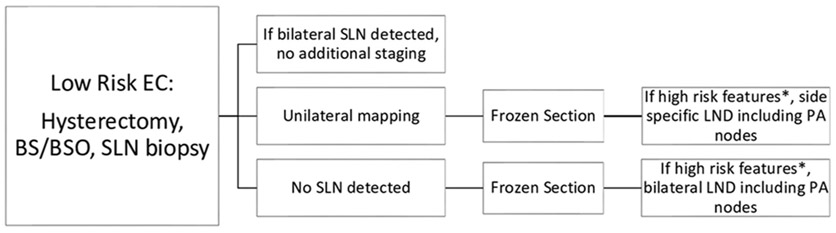
In November 2016, the Gynecologic Oncology and Reproductive Medicine Department at the University of Texas MD Anderson Cancer Center (MDACC) implemented a standardized SLN mapping algorithm for endometrial cancer (Figures 1-2). In this algorithm, all patients with apparent early stage endometrial cancer undergo SLN mapping at the commencement of the procedure. Intraoperative uterine frozen section is then only utilized if mapping fails in at least one hemipelvis in otherwise low-risk patients to determine the need for a complete pelvic and para-aortic lymphadenectomy.
Figure 1.
Sentinel lymph node mapping algorithm in high risk endometrial cancer. BS/BSO, bilateral salpingectomy or bilateral salpingo-oophorectomy; EC, endometrial cancer; LND, lymph node dissection; PA, para-aortic; SLN, sentinel lymph node,
Figure 2.
Sentinel lymph node mapping algorithm in low risk endometrial cancer. *High-risk features include: non-endometrioid histology, >50% myometrial invasion or endometrioid grade 3 with any evidence of invasion, tumor diameter >2 cm. BS/BSO, bilateral salpingectomy or bilateral salpingo-oophorectomy; EC, endometrial cancer; LND, lymph node dissection; PA, para-aortic; SLN, sentinel lymph node.
In the setting of continually rising healthcare costs, it is important to understand how clinical practice changes affect not only outcomes, but also the cost of care. A prior cost-effectiveness analysis found SLN mapping to be the most cost effective option when compared with routine and selective lymphadenectomy for low-risk endometrial cancer.12 The primary objective of this study was to compare resource utilization including operative time, use of intraoperative frozen section, and hospital charges, as well as surgical outcomes before and after the implementation of an SLN algorithm at a single large academic institution. We hypothesized that the application of a SLN mapping algorithm would decrease resource utilization while maintaining equivalent surgical outcomes.
METHODS
All patients with biopsy-proven, newly diagnosed clinical stage I endometrial cancer who underwent surgery at MDACC between January 1, 2012 and December 31, 2012 or between January 1, 2017 and December 31, 2017 and who had corresponding hospital charge data were identified. Patients were excluded if other procedures were performed at the time of surgery including tumor debulking, bowel surgery, or planned joint procedures with other services. Patients who received neoadjuvant chemotherapy or who had gross metastatic disease at the time of surgery were also excluded. In addition, patients were excluded from the 2012 cohort if SLN mapping was performed. The study was approved by the Institutional Review Board.
All patients underwent comprehensive surgical staging including hysterectomy and bilateral salpingectomy or bilateral salpingo-oophorectomy, and lymph node evaluation with selective biopsies of suspicious areas. The method of lymph node evaluation was the only surgical factor that differed between the two cohorts. In the 2012 cohort, uterine factors using the Mayo Criteria were used to determine the need for a complete lymphadenectomy. Lymphadenectomy was omitted if all of the following criteria were met on preoperative endometrial sampling and intraoperative frozen section: grade 1 or 2 endometrioid histology with <50% myometrial invasion or grade 3 endometrioid histology with no myometrial invasion, tumor diameter ≤2 cm, and no evidence of gross disease outside the uterine corpus. If all of these factors were not met, a complete lymphadenectomy was recommended which included a pelvic and para-aortic lymphadenectomy up to the level of the renal vessels.
In the 2017 cohort, SLN mapping and biopsy was performed according to our SLN mapping institutional algorithm (Figures 1-2). Preoperative endometrial biopsy was used to stratify patients into low- versus high-risk endometrial cancer to determine the appropriate algorithm to follow. High-risk endometrial cancer was defined as grade 3 endometrioid, serous, clear cell, carcinosarcoma, or any mixed tumor containing one of these cell types. In high-risk endometrial cancer patients, if bilateral SLNs were detected, no further staging was performed. If a unilateral SLN was detected, a contralateral pelvic and para-aortic lymphadenectomy was performed. If no SLNs were detected, then a complete bilateral pelvic and para-aortic lymphadectomy was performed (Figure 1). Intraoperative frozen section was not used in patients with known high risk endometrial cancer. In contrast, in low risk endometrial cancer patients, intraoperative frozen section was utilized to further triage patients only if bilateral SLN mapping was unsuccessful. If a unilateral or no SLN was detected, intraoperative frozen section was sent to pathology. A complete pelvic and para-aortic lymphadenectomy was then performed on the side or sides that failed to map only if the frozen section pathology demonstrated high risk features based on Mayo Criteria (Figure 2). All lymph nodes from pelvic and para-aortic lymphadenectomy underwent traditional pathologic assessment via sectioning and hematoxylin and eosin (H&E) staining to assess for metastatic tumor cells. All SLNs were surgically removed and underwent ultrastaging techniques as previously described.8 In brief, ultrastaging involved sectioning followed by H&E staining. If no tumor cells were identified, additional sectioning was performed for another H&E stain and immunohistochemistry for pan-cytokeratin. Macroscopic (>2 mm) and microscopic (0.2–2 mm) nodal metastasis as well as isolated tumor cells (<0.2 mm) were all considered node-positive for staging purposes. All final pathologic specimens were reviewed by a gynecologic pathologist.
Demographic and clinical characteristics were collected for each patient and all study data were managed in Research Electronic Data Capture (REDCap) at MDACC. REDCap is a secure, web-based application designed to support data capture for research studies, providing: (1) an intuitive interface for validated data entry; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages; and (4) procedures for importing data from external sources.13 Postoperative complications were categorized according to the Accordion Severity Grading System and only complications ≥grade 3 were included.14 Line-item hospital and professional charges were extracted from the hospital financial system for each patient. Charges incurred on the day of surgery, where charge service date equaled surgery date, were then categorized based on line-item charge descriptions and revenue classes. All charges were adjusted to 2017 US dollars. The Fisher’s exact test was used to compare all categorical variables. The Wilcoxon rank sum test was used to compare medians of continuous variables including charge data. All reported p values were two-tailed and values of p<0.05 were considered significant. All statistical analyses were performed using SAS version 9.3 for Windows (Copyright 2002–2010 by SAS Institute Inc, Cary, NC) and StatXact-7 for Windows (Copyright 2005, 1989–2005, Cytel Software Corporation, Cambridge, MA).
RESULTS
There were a total of 71 patients in 2012 and 130 patients in 2017 who met the inclusion criteria. The clinical and pathologic characteristics are summarized in Table 1. Median age (p=0.19) and body mass index (p=0.60) were not significantly different between the two groups. FIGO stage (2010) also did not differ between the groups (p=0.32). There was a significantly greater proportion of endometrioid histology in the 2012 group (85.9% vs 70.0%, p=0.02).
Table 1.
Clinical and pathologic characteristics
| Surgery year |
|||
|---|---|---|---|
| Characteristic | 2012 (n=71) | 2017 (n=130) | P value |
| Age (years), median (range) | 61.4 (30.7–84.5) | 64.1 (27.6–87.1) | 0.19 |
| BMI (kg/m2), median (range) | 33.9 (19.2–58.0) | 33.9 (18.4–58.1) | 0.60 |
| Final histology, N (%) | 0.016 | ||
| Endometrioid | 61 (85.9) | 91 (70.0) | |
| Non-endometrioid* | 10 (14.1) | 39 (30.0) | |
| FIGO stage (2010), N (%) | 0.32 | ||
| I | 60 (84.5) | 109 (83.8) | |
| II | 4 (5.6) | 2 (1.5) | |
| III | 7 (9.9) | 17 (13.1) | |
| IV | 0 (0.0) | 2 (1.5) | |
Non-endometrioid group included two patients demonstrating high grade, difficult to subtype on final pathology.
BMI, body mass index; FIGO, International Federation of Gynecology and Obstetrics.
Surgical characteristics are demonstrated in Table 2. There was no difference in the utilization of minimally invasive surgery between the two groups (88.7% vs 93.1%, p=0.30). The use of robotic surgery was also similar (23.9% vs 25.4%, p=0.87). The rate of conversion to open surgery was 6.3% vs 7.4% (p=1.00). Median estimated blood loss was not significantly different between the two groups (100 mL vs 75 mL, p=0.08). There was a significant decrease in median operative time in 2017 (210 vs 170.5 min, p=0.007). The rate of intraoperative complications was similar between the two groups (1.4% vs 2.3%, p=1.0). There was one intraoperative complication in 2012, specifically intraoperative bleeding requiring a blood transfusion. There were three complications in 2017 which included intraoperative bleeding requiring a blood transfusion and two cystotomy repairs. The 30 day postoperative complication rate including those categorized as ≥grade 3 was 1/71 (1.4%) and 3/130 (3.1%) in the 2012 and 2017 groups, respectively (p=0.30). In 2012, there was one postoperative complication which included a pelvic abscess that required drainage by interventional radiology. In 2017, the four postoperative complications included a gastric trocar injury and a large bowel perforation, which both presented postoperatively and required re-operation, as well as two pelvic abscesses that required drainage by interventional radiology.
Table 2.
Surgical outcomes
| Surgery year |
|||
|---|---|---|---|
| Characteristic | 2012 (n=71) | 2017 (n=130) | P value |
| Total operative time (minutes), median (range) | 210 (92–366) | 171 (96–416) | 0.007 |
| Estimated blood loss (mL), median (range) | 100 (20–2630) | 75 (10–1500) | 0.081 |
| Surgical approach, N (%) | 0.30 | ||
| MIS | 63 (88.7%) | 121 (93.1%) | |
| Open | 8 (11.3%) | 9 (6.9%) | |
| Conversion rate, N (%) | 4 (6.3%) | 9 (7.4%) | 1.00 |
| Intraoperative complications, N (%) | 1 (1.4%) | 3 (2.3%) | 1.00 |
| Postoperative complications*, N (%) | 1 (1.4%) | 4 (3.1%) | 0.30 |
| Frozen section utilization, N (%) | 45 (63.4%) | 19 (14.6%) | <0.0001 |
| SLN mapping attempted, N (%) | NA | 120 (92.3%) | |
| At least 1 SLN identified, N (%) | NA | 110 (91.7%) | |
Postoperative complications included ≥grade 3 as categorized by the Accordion Severity Grading System.
MIS, minimally invasive surgery; SLN, sentinel lymph node.
In 2012, 35/71 patients (49.3%) underwent a lymphadenectomy. The use of intraoperative frozen section decreased from 63.4% in 2012 to 14.6% in 2017 (p<0.0001). In 2017, SLN mapping was attempted in 120/130 patients (92.3%). Ten patients did not undergo SLN mapping due to the following reasons: extensive prior abdominal surgery (three patients); obesity/poor performance status/did not tolerate Trendelenburg well (four patients); clinically enlarged lymph node positive for carcinoma on frozen section (one patient); grade 1 endometrial cancer previously treated with levonorgestrel intrauterine device and surgeon decided not to do SLN evaluation (one patient); not noted in operative report (one patient).
Of those patients who underwent sentinel lymph node mapping, at least one SLN was identified in 110 patients (91.7%), with bilateral mapping and unilateral mapping in 93/120 (78%) and 17/120 (14.2%) patients, respectively.
Median professional charges decreased by 8.67% (p=0.12), while median hospital charges increased by 0.37% (p=017). Median total hospital charges, which included professional and hospital charges, were 2.73% less in 2017 compared with 2012, but this did not reach statistical significance (p=0.96). Within the hospital charges, the post-anesthesia care unit (PACU) charges increased by 40.95% (p<0.001) and pathology charges increased by 63.38% (p<0.001). The pharmacy and supply charges decreased by 80.36% (p<0.001) and 11.85% (p=0.05), respectively. There was no significant difference in laboratory services, anesthesia, or operating room charges. Hospital and professional charge data are shown in Table 3.
Table 3.
Hospital and professional charge data
| Precent change, median |
P value | |
|---|---|---|
| Total charges | −2.73% | 0.96 |
| Hospital charges | 0.37% | 0.17 |
| Anesthesia | 0.79% | 0.08 |
| Labs | −86.63% | 0.62 |
| Recovery/PACU | 40.95% | <0.001 |
| Pathology | 63.38% | <0.001 |
| Operating room | −2.69% | 0.58 |
| Pharmacy | −80.36% | <0.001 |
| Supplies | −11.85% | 0.05 |
| Professional charges | −8.67% | 0.12 |
Median percent change comparing 2012 and 2017. Total charges equal hospital plus professional charges. All charges adjusted to 2017 US$.
PACU, post-anesthesia care unit.
DISCUSSION
The implementation of an SLN algorithm for the surgical staging of both high- and low-risk endometrial cancer demonstrated a reduction in resource utilization through a decrease in operative time and a more prudent use of intraoperative frozen section. There was no significant difference in total hospital charges with no change in the incidence of intraoperative or postoperative complications.
There are several reasons our study may not demonstrate a significant decrease in total hospital charges. First, with the introduction of SLN mapping, more advanced pathologic examination is essential including ultrastaging and immunohistochemistry techniques. These changes led to the 63% increase in pathology hospital charges. The PACU charges also significantly increased in 2017. We suspect this is secondary to the increase in same day discharges, thus a longer time spent in the PACU. In 2012, our minimally invasive surgery patients were often kept overnight for observation; however, same day discharge, when appropriate, was our standard practice in 2017. In 2012, 51% of the patients were discharged on the day of surgery as compared with 68% in 2017. Conversely, the charges for pharmacy and supplies significantly decreased by 80% and 12%, respectively. This decreased utilization of pharmacy and supplies corresponds with the implementation of enhanced recovery after surgery (ERAS) in our department in 2014. These resultant increases and decreases in hospital charge categories resulted in a net neutral effect on total hospital charges.
The optimal lymphatic assessment strategy in endometrial cancer remains controversial. A large retrospective study from Japan reported a survival benefit with lymphadenectomy in early-stage patients with intermediate- or high-risk features.15 In contrast, two randomized clinical trials failed to demonstrate a survival benefit in routine lymphadenectomy when compared with hysterectomy, primarily in low-grade endometrial cancer.2,3 Critics argue that there were significant design flaws in these trials including the omission of para-aortic lymphadenectomy, diverse adjuvant therapy regimens, and too many low-risk patients.16,17 SLN mapping has since emerged as an alternative strategy to provide the diagnostic benefit of lymphadenectomy without the morbidity. The clinical efficacy of SLN mapping techniques has been validated in several retrospective and prospective studies.8,9,18,19 In addition, the use of pathologic ultrastaging has been shown to improve the sensitivity in the detection of lymph node metastasis.20 With this rapid increase in supporting evidence, the practice is now gaining acceptance worldwide. A recent international survey in 2019 reported that 50.3% of respondents have adopted SLN techniques in endometrial cancer.21
With rising healthcare costs and a continued emphasis on value-based healthcare, however, it is important to understand the financial impact of our clinical decisions. The cost of care associated with the use of SLN mapping in endometrial cancer is less clear. A recent cost-utility analysis demonstrated that SLN mapping was the most cost effective strategy when compared with complete pelvic and para-aortic or selective lymphadenectomy for women with low risk endometrial cancer.12 For endometrial intraepithelial neoplasia in particular, another cost-effectiveness analysis found SLN mapping with or without frozen section to be more costly than hysterectomy with frozen section.22 Our study demonstrates hospital charges to be equivalent.
This study has several limitations. The sample size was small due to strict inclusion criteria. It was carried out at a single academic institution and only hospital charge data were captured which often does not reflect the actual cost to an institution. In addition, due to institutional restrictions, we cannot directly report hospital charges. Prior cost-effectiveness studies used Medicare reimbursement rates, not hospital charges, in their modeling, so cross-study comparisons cannot be made. Reimbursement rates vary by type of insurance coverage and also may not always reflect the cost of providing care from a hospital perspective. Many items used in surgical procedures are non-chargeable items, which leads to wide variations in hospital charges. In addition, hospital charges are not the same across the country. Another limitation is that the exact difference in operating room cost could not be accounted for in the hospital charge data because operating room time is charged in time blocks rather than by the minute. However, we demonstrated a significant decrease in the operating room time, which decreases resource utilization. The major strength of this study is the fact that it does not rely on modeling and includes actual line-item hospital charge data from real patients pre- and post-implementation of an SLN practice change. In addition, it was obtained from a large academic center with 22 gynecologic oncology surgeons, thus reflecting the real world variation seen in billing practices of different providers.
In conclusion, the implementation of an SLN mapping algorithm for high- and low-risk endometrial cancer at our institution resulted in a decrease in both operative time and the utilization of intraoperative frozen section without affecting surgical morbidity. While total hospital charges were not significantly different, future studies are warranted to assess the true cost of SLN mapping as healthcare transparency expands.
HIGHLIGHTS.
The implementation of a SLN mapping algorithm for endometrial cancer decreased operative time
The use of intraoperative frozen section decreased with the use of SLN mapping
Total hospital charges did not significantly change after SLN mapping was introduced
Acknowledgments
Funding This research was supported in part by the NIH T32 Training Grant for Gynecologic Oncologists (T32CA101642), Cancer Center Support Grant (NCI Grant P30 CA016672), NCI SPORE in Uterine Cancer (2P50 CA098258-06), and Andrew Sabin Family Fellowship (NCI K07-CA201013).
Footnotes
Editor's note This paper will feature in a special issue on sentinel lymph node mapping in 2020.
Competing interests LAM reports research support from AstraZeneca, outside the submitted work. SW reports grants from the NIH during the conduct of the study; personal fees and other from AstraZeneca, personal fees and other from Clovis, personal fees and other from Tesaro, personal fees and other from Roche/Genentech, other from Cotinga Pharmaceuticals, personal fees from Merck, personal fees from Pfizer, other from Novartis all outside the submitted work. MF reports grants and personal fees from Novadaq/Stryker, grants from Navidea, personal fees from Johnson and Johnson, personal fees from Genetech, personal fees from Ipsen all outside the submitted work. PS reports grants from Novartis, personal fees from Clovis Oncology all outside the submitted work.
Patient consent for publication Not required.
Ethics approval The University of Texas MD Anderson Institutional Review Board, Protocol DR10-0987.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Data are available upon reasonable request.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Benedetti Panici P Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst 2008;100:1707–16. [DOI] [PubMed] [Google Scholar]
- 3.Kitchener H, Swart AMC, Qian Q, et al. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet 2009;373:125–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mariani A, Webb MJ, Keeney GL, et al. Low-risk corpus cancer: is lymphadenectomy or radiotherapy necessary? Am J Obstet Gynecol 2000;182:1506–19. [DOI] [PubMed] [Google Scholar]
- 5.Frumovitz M, Slomovitz BM, Singh DK, et al. Frozen section analyses as predictors of lymphatic spread in patients with early-stage uterine cancer. J Am Coll Surg 2004;199:388–93. [DOI] [PubMed] [Google Scholar]
- 6.Kumar S, Medeiros F, Dowdy SC, et al. A prospective assessment of the reliability of frozen section to direct intraoperative decision making in endometrial cancer. Gynecol Oncol 2012;127:525–31. [DOI] [PubMed] [Google Scholar]
- 7.Bodurtha Smith AJ, Fader AN, Tanner EJ. Sentinel lymph node assessment in endometrial cancer: a systematic review and meta-analysis. Am J Obstet Gynecol 2017;216:459–76. [DOI] [PubMed] [Google Scholar]
- 8.Soliman PT, Westin SN, Dioun S, et al. A prospective validation study of sentinel lymph node mapping for high-risk endometrial cancer. Gynecol Oncol 2017;146:234–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossi EC, Kowalski LD, Scalici J, et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. Lancet Oncol 2017;18:384–92. [DOI] [PubMed] [Google Scholar]
- 10.Schlappe BA, Weaver AL, Ducie JA, et al. Multicenter study comparing oncologic outcomes between two nodal assessment methods in patients with deeply invasive endometrioid endometrial carcinoma: a sentinel lymph node algorithm versus a comprehensive pelvic and paraaortic lymphadenectomy. Gynecol Oncol 2018;151:235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koh W-J, Abu-Rustum NR, Bean S, et al. Uterine neoplasms, version 1.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2018;16:170–99. [DOI] [PubMed] [Google Scholar]
- 12.Suidan RS, Sun CC, Cantor SB, et al. Three lymphadenectomy strategies in low-risk endometrial carcinoma: a cost-effectiveness analysis. Obstet Gynecol 2018;132:52–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strasberg SM, Linehan DC, Hawkins WG. The accordion severity grading system of surgical complications. Ann Surg 2009;250:177–86. [DOI] [PubMed] [Google Scholar]
- 15.Todo Y, Kato H, Kaneuchi M, et al. Survival effect of para-aortic lymphadenectomy in endometrial cancer (SEPAL study): a retrospective cohort analysis. Lancet 2010;375:1165–72. [DOI] [PubMed] [Google Scholar]
- 16.Uccella S, Podratz KC, Aletti GD, et al. Re: systematic pelvic lymphadenectomy vs no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst 2009;101:897–8. [DOI] [PubMed] [Google Scholar]
- 17.Naumann RW. The role of lymphadenectomy in endometrial cancer: was the ASTEC trial doomed by design and are we destined to repeat that mistake? Gynecol Oncol 2012;126:5–11. [DOI] [PubMed] [Google Scholar]
- 18.Sinno AK, Peijnenburg E, Fader AN, et al. Reducing overtreatment: a comparison of lymph node assessment strategies for endometrial cancer. Gynecol Oncol 2016;143:281–6. [DOI] [PubMed] [Google Scholar]
- 19.Ducie JA, Eriksson AGZ, Ali N, et al. Comparison of a sentinel lymph node mapping algorithm and comprehensive lymphadenectomy in the detection of stage IIIC endometrial carcinoma at higher risk for nodal disease. Gynecol Oncol 2017;147:541–8. [DOI] [PubMed] [Google Scholar]
- 20.Kim CH, Soslow RA, Park KJ, et al. Pathologic ultrastaging improves micrometastasis detection in sentinel lymph nodes during endometrial cancer staging. Int J Gynecol Cancer 2013;23:964–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Casarin J, Multinu F, Abu-Rustum N, et al. Factors influencing the adoption of the sentinel lymph node technique for endometrial cancer staging: an international survey of gynecologic oncologists. Int J Gynecol Cancer 2019;29:60–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim SL, Moss HA, Secord AA, et al. Hysterectomy with sentinel lymph node biopsy in the setting of pre-operative diagnosis of endometrial intraepithelial neoplasia: a cost-effectiveness analysis. Gynecol Oncol 2018;151:506–12. [DOI] [PubMed] [Google Scholar]