Abstract
Panax ginseng, a medicinal plant, has been used as a blood-nourishing tonic for thousands of years in Asia, including Korea and China. P. ginseng exhibits adaptogen activity that maintains homeostasis by restoring general biological functions and non-specifically enhancing the body's resistance to external stress. Several P. ginseng effects have been reported. Korean Red Ginseng, in particular, has been reported in both basic and clinical studies to possess diverse effects such as enhanced immunity, fatigue relief, memory, blood circulation, and anti-oxidation. Moreover, it also protects against menopausal symptoms, cancer, cardiac diseases, and neurological disorders. The active components found in most Korean Red Ginseng varieties are known to include ginsenosides, polysaccharides, peptides, alkaloids, polyacetylene, and phenolic compounds. In this review, the identity and bioactivity of the non-saponin components of Korean Red Ginseng discovered to date are evaluated and the components are classified into polysaccharide and nitrogen compounds (protein, peptide, amino acid, nucleic acid, and alkaloid), as well as fat-soluble components such as polyacetylene, phenols, essential oils, and phytosterols. The distinct bioactivity of Korean Red Ginseng was found to originate from both saponin and non-saponin components rather than from only one or two specific components. Therefore, it is important to consider saponin and non-saponin elements together.
Keywords: Alkaloids, Fat-soluble component, Korean Red Ginseng, Non-saponins, Polysaccharides
1. Introduction
Ginseng is a medicinal herb that has long been used as a blood-enriching and tonifying agent in Korea, China, and other parts of East Asia. In 1843, ginseng was first named Panax ginseng C.A. Meyer, meaning ‘cure-all’, by the Russian scientist, C.A. Meyer. Ginseng and similar herbs are also used in the US, China, Japan, and Russia but they differ from P. ginseng in terms of their taxonomy, morphology, and constituents. Globally, there are 6–7 known species in the genus Panax; however, only three ginseng species are cultivated and commercially distributed globally [1]. These are P. ginseng (geographically distributed and cultivated in Far East Asia, including South Korea and China), American ginseng (P. quinquefolium L., cultivated in the US and Canada), and Chinese ginseng (P. notoginseng (Burk) F.H. Chen, produced in the Yunnan and Guangxi provinces in southern China) [2]. American ginseng and Chinese ginseng are different species from P. ginseng; thus, the term ‘ginseng’ is generally only used for P. ginseng.
The pharmacological histories of American ginseng and Chinese ginseng are much shorter than that of P. ginseng. Chinese ginseng has been used medicinally since the 16th century whereas American ginseng has only been used since the early 18th century when it was first discovered in Canada and became known to Chinese merchants [3]. At that time, P. ginseng was rare and very expensive in China, so Chinese ginseng and American ginseng were used as alternatives. The authentic ginseng, which has a long pharmacological history and is still recognized as one of the most important traditional medicines today, is the P. ginseng species [1]. Historically, after Korea became known internationally by its current name, the ginseng cultivated and produced in Korea has been called P. ginseng. It is a global product that is promoted on a backdrop of the mountainous scenery of South Korea and is currently considered the ‘ultimate’ ginseng [4,5]. P. ginseng is an intermediate shade-tolerant perennial plant in the family Araliaceae, genus Panax.
Depending on the processing method used, ginseng can be broadly categorized into fresh ginseng, white ginseng, and red ginseng. Fresh ginseng is a natural product that takes 4–6 years to mature and is sourced from the earth; the unique ginseng compounds are preserved, and given that the water content is 70–80%, the product can decay and be damaged easily during distribution, so long-term storage is difficult without specialized storage facilities or packaging. White ginseng is produced by sun-drying or hot air-drying of 4–6-year-old fresh ginseng either in its original state or after removing the outer layer; its water content accounts for ≤14% and it is milky-white or pale yellow in color. Red ginseng is produced by steam cooking and then drying the fresh ginseng; its water content accounts for ≤15.5% and it can be pale red or red-brown in color. Depending on its quality, red ginseng is divided into chonsam, jisam, and yangsam [6]. In the process of steaming and drying fresh ginseng to make red ginseng, there are changes in the types and concentrations of unique compounds found in ginseng known as ginsenosides. There are also physical and chemical changes in polysaccharides, the most abundant compounds in Korean Red Ginseng; notably, starch gelatinization enables long-term storage [4,5,[7], [8], [9], [10], [11], [12], [13], [14]]. Korean Red Ginseng contains a high concentration (60–70%) of carbohydrates, including starch, but also includes a number of specific compounds that are not found in other plants, including ginsenosides, proteins that are nitrogen-containing compounds, peptides, alkaloids, polyacetylenes that are liposoluble, and polysaccharides as well as flavonoids and fatty acids (Table 1) [4,7,15].
Table 1.
Saponin and non-saponin components existing in Korean Red Ginseng
| Common components | ||
|---|---|---|
| Saponin | Ginsenosides | Protopanaxadiol (27 types) |
| Protopanaxatriol (14 types) | ||
| Oleanane (2 types) | ||
| Non-saponin | Saccharides | Monosaccharide, disaccharide, trisaccharide, polysaccharides (including red ginseng polysaccharide, etc.), crude fiber, pectin |
| Nitrogen-containing compounds | Protein, peptide, amino acid, nucleic acids, alkaloid | |
| Fat-soluble components | Lipid, fatty acid, polyacetylenes, phenolic compounds, essential oils, phytosterols, organic acid, terpenoid | |
| Vitamin | Water-soluble vitamins | |
| Ash | Minerals | |
A Japanese team led by Shibata and Tanaka embarked on intensive research on the chemical structure of ginsenosides in 1962 and clarified the chemical structures of the ginsenoside aglycones, i.e., protopanaxadiol and protopanaxatriol [[16], [17], [18], [19], [20], [21]]. Subsequent research led to the purification of the ginsenosides, i.e., saponins, from P. ginseng and the chemical structures of the individual saponins were revealed in the late 1960s [19,22,23]. Since the early 1980s, research has focused on the saponin composition in red and white ginseng, classified according to the processing methods used, and each type of ginseng was found to contain unique saponins [24]. Since the 1960s, when the first studies on the chemical purification of ginsenosides were performed, the chemical structures of least 43 saponins have been isolated from P. ginseng [6]. Now that it is possible to purify individual saponins from Korean Red Ginseng, the effects of Korean Red Ginseng have been investigated at the compound level and various effects have been demonstrated for each saponin. Furthermore, several studies that used high-quality analytic devices and techniques have analyzed additional components in Korean Red Ginseng compounds including polysaccharides, polyacetylenes, phenol compounds, essential oils, peptides, alkaloids, and vitamins [6].
The non-saponin fraction has been demonstrated to have various types of pharmacological activity; this has led to multifaceted exploratory research on the pharmacology of the active fraction [25,26]. Although some physiologically active non-saponin contents of Korean Red Ginseng have been identified in other herbs and natural products, some contents are unique to Korean Red Ginseng and are contributing substantially to our understanding of the efficacy of ginsenosides and the diverse effects of Korean Red Ginseng [27]. The first research on the non-saponin compounds in Korean Red Ginseng was performed in Japan in 1914 by Sakai who isolated an aromatic compound from an ether extract of Korean Red Ginseng and named it panacene. Thereafter, chemical analyses of phytosterols [28] and polyacetylenes [29] were pursued, but since most of these compounds are also present in other herbal medicines, they received little attention as active substances in Korean Red Ginseng. At that time, ginsenosides were emphasized as unique compounds in Korean Red Ginseng, resulting in even less research on the effects of non-saponin compounds. However, from the late 1960s onwards, Korean researchers isolated maltol and several phenolic compounds from Korean Red Ginseng and tested their activity [30]. For instance, the alkaloid fraction in Korean Red Ginseng was reported to suppress the proliferation of cancer cells [31], as was a petroleum ether extract [32]. This was the starting point for considerable exploratory research on the active compounds in Korean Red Ginseng, focusing on non-saponin compounds. While there has been much research on the saponin content in Korean Red Ginseng, there has been relatively little research on its non-saponin content. There is a misperception that all the effects of Korean Red Ginseng are mediated by saponins; recently, there has been a surge of marketing claiming that a stronger saponin effect can be achieved by increasing the absorption rate of certain saponins. Korean Red Ginseng contains saponin but facilitates the best effects when ingested with non-saponin components evenly. In this review, various studies on the physiological effects of non-saponin compounds in Korean Red Ginseng are evaluated to demonstrate that these compounds also have a variety of actions.
2. Polysaccharides
Until the 1960s, there was almost no research on the chemistry or physiological effects of Korean Red Ginseng polysaccharides, which are macromolecules. With developments in the life sciences, it was discovered that carbohydrates have important functions as simple structural molecules or energy sources and as signal transmitters for biological functions, leading to renewed recognition of their importance [33]. Polysaccharides are the most abundant compounds in Korean Red Ginseng. Some polysaccharides are formed from chains of many simple sugars, including monosaccharides, such as glucose and fructose, and disaccharides, such as maltose and sucrose. Pectin is a polysaccharide that has been isolated from Korean Red Ginseng and is a major constituent of galacturonic acid, glucose, galactose, and arabinose. Pectin is also a minor constituent of xylose, rhamnose, and galacturonan [33].
Likely due to their structural complexity, research on Korean Red Ginseng polysaccharides and their activity started much later than similar research on saponins. In 1984, a research team led by Hikino and Tomoda first isolated panaxans A, B, C, D, and E from Korean Red Ginseng and subsequently isolated panaxans F to U, giving a total of 21 panaxan types [[34], [35], [36], [37], [38], [39], [40]]. Regarding physiological activity, these polysaccharides showed notable hypoglycemic activity in diabetic mice [36,41]. Yang et al also administered polysaccharides from Korean Red Ginseng to animals and reported that they lowered blood glucose, reduced glycogen levels in the liver, and promoted insulin secretion [42]. The water-soluble and alkali-soluble (0.5M sodium hydroxide) polysaccharide fractions from P. ginseng show anti-complementary activity that was strongest for the acidic polysaccharide fraction [43]. Over a period of 10 years, Okuda et al isolated acidic polysaccharides from the water extract of Korean Red Ginseng and found the chemical structure of these compounds to consist of an α-1, 4-polygalacturonan backbone similar to pectin with several acetoxy groups [44]. After this, two new acidic polysaccharides were isolated, ginsenan PA and ginsenan PB; these had molecular weights of 16,000 and 5,500, respectively, and their chemical structure consisted of α-arabino-β-3,6-galactan-type and rhamnogalacturonan-type structural units.
Compared to white ginseng, red ginseng is reported to have far higher levels of pectin [8]. Heating causes an increase in galacturonic acid because esterified galacturonic acid is converted into non-esterified galacturonic acid [8]. Recently, there has been much research on the physiological actions of acidic polysaccharides. The red ginseng polysaccharide fraction inhibits immunotoxicity by increasing hemolytic plaque-forming cells and the white blood cell count; also, spleen weight is decreased by cyclophosphamide treatment [45]. Administering Korean Red Ginseng polysaccharides to sarcoma 180-bearing mice inhibited cancer development, increased the number of hemolytic plaque-forming cells that are involved in the production of antibodies, and increased phagocytic activity in the reticuloendothelial system; these effects were strongest in the acidic polysaccharide fraction found in red ginseng [45,46]. Park et al administered Korean Red Ginseng acidic polysaccharide (RGAP) to mice and reported a significant increase in the peritoneal macrophage count and a significantly increased production of antibodies against sheep red blood cells [47]. In a tumor graft mouse model, RGAP promoted the production of nitric oxide (NO) by macrophages and increased natural killer (NK) cell activation. Furthermore, tumorigenesis was greatly suppressed, and the lifespan of the mice was extended [48].
There have also been studies on suppressing the growth of solid cancers and lung cancer metastasis where RGAP significantly increased the phagocytic index of macrophages, significantly reduced the weight of solid tumors, and suppressed lung cancer metastasis because of a melanoma cell graft [49]. To investigate whether RGAP has a protective effect against the immunotoxicity of anticancer treatment and to explore its role as an adjuvant anticancer treatment, RGAP was administered with or without paclitaxel; the RGAP combination therapy group showed significantly increased NK cell activity in splenocytes and an increase in the cytotoxicity of macrophages from 15% to 45%, indicating a protective effect against immunotoxicity. Moreover, paclitaxel + RGAP combination therapy significantly increased the lifespan and reduced the weight of the solid tumors, demonstrating the potential of RGAP as an adjuvant anticancer therapy [50].
Kwak et al investigated the possibility of using RGAP in combination with cyclophosphamide or 5-fluorouracil as an immune-enhancing agent and to alleviate the adverse effects of immunotoxicity [51]. They reported an extended lifespan and significantly reduced tumor weight and size in mice grafted with sarcoma 180 or Lewis lung/2 lung carcinoma. RGAP inhibited the phagocytic activity of Brucella abortus by suppressing the levels of mitogen-activated protein kinase (MAPK) signaling proteins, extracellular signal-regulated kinase, Jun N-terminal kinase, and p38 and inhibited the intracellular replication of B. abortus by enhancing phagolysosome fusion. This indicates that RGAP could be used effectively for the control or treatment of Brucella spp. [52].
Byeon et al investigated the mechanisms of immune enhancement of Korean Red Ginseng via RGAP. Regular consumption of Korean Red Ginseng activated macrophages, promoting immune protein migration into the nucleus and inducing active secretion of factors (e.g., NO, reactive oxygen species, and tumor necrosis factors) that destroy cancer cells, various viruses, and bacteria [53]. In animals with acute hyperlipidemia, RGAP significantly suppressed levels of non-esterified fatty acids and triglycerides in the serum and liver and induced a significant dose-dependent increase, of up to 80%, in the activity of lipoprotein lipase, a major lipoprotein hydrolase. This demonstrates that RGAP is effective against hyperlipidemia [54].
When RGAP and pidotimod were administered in combination to immune-deficient mice to test the synergistic immune-enhancing effect, there were significant increases in concanavalin A-induced T-cell proliferation in the spleen and in lipopolysaccharide (LPS)-stimulated B-cell proliferation. Furthermore, NO production increased from peritoneal macrophages, NK cell activity increased, and interleukin (IL)-12 and interferon gamma (IFN-γ levels) increased, demonstrating heightened immune activity [55]. In another study, tumor cell death increased when murine B16 melanoma cells were exposed to a combination of RGAP + recombinant IFN-γ; moreover, IL-1, IL-6, tumor necrosis factor alpha (TNF-α), and NO productions were stimulated, showing a synergistic effect on macrophage activity [56]. Crude polysaccharides obtained after dividing water extract from red ginseng into α-amylase and amyloglucosidase significantly increased the intestinal immune system modulating activity and macrophage stimulating activity [57]. When whole red ginseng extract and Korean Red Ginseng polysaccharide and saponin fractions were administered orally for 14 days in mice infected with influenza A and uninfected controls, the survival rate in the group infected with influenza was 78% higher if they received the polysaccharide fraction, 67% if they received the water extract, and 56% if they received the saponin fraction, indicating that the polysaccharide fraction has the greatest survival benefits. Notably, the polysaccharide fraction was also the most effective at reducing the accumulation of TNF-α/inducible nitric oxide synthase (iNOS)-producing dendritic cells in the mouse lung [58]. Accordingly, the polysaccharide component is attracting attention as the main active ingredient of ginseng; more analyses and efficacy studies for physiologically active polysaccharide components are needed in the future. Table 2 summarizes the physiological activity of polysaccharides.
Table 2.
Physiological activity of polysaccharides
| Components | Physiological activity | Reference |
|---|---|---|
| Panaxans A–U | Hypoglycemic activity | [36,41] |
| Decrease in glycogen levels in the liver, promoting insulin secretion | [42] | |
| Water-soluble and alkali-soluble fraction | Anti-complementary activity | [43] |
| Korean Red Ginseng acidic polysaccharide | Immune activity | [[45], [46], [47], [48], [49], [50],53,55,56] |
| Anticancer activity | [[49], [50], [51], [52]] | |
| Hyperlipidemia inhibition | [54] | |
| Influenza defense | [58] | |
| Crude polysaccharides | Immune activity | [57] |
| Influenza defense | [58] |
3. Protein and peptide
The main nitrogen-containing compounds in Korean Red Ginseng are soluble proteins, peptides, and free amino acids; glycoproteins, amines, alkaloids, free nucleosides, and nucleic acid bases are also present in small quantities. In terms of research on the nitrogen-containing compounds in Korean Red Ginseng, although most of their chemical structures have been identified, there has been little research on their physiological activity. One reason for this is that most of the nitrogen-containing compounds in Korean Red Ginseng, such as amino acids, are primary metabolites that are commonly detected in other plants as well. Unlike saponins, which are secondary metabolites, the nitrogen-containing compounds are not unique to Korean Red Ginseng. Nevertheless, a correlation study on the traditional indicators of the quality of Korean Red Ginseng and on its chemical constituents has highlighted the importance of nitrogen-containing compounds in Korean Red Ginseng [59].
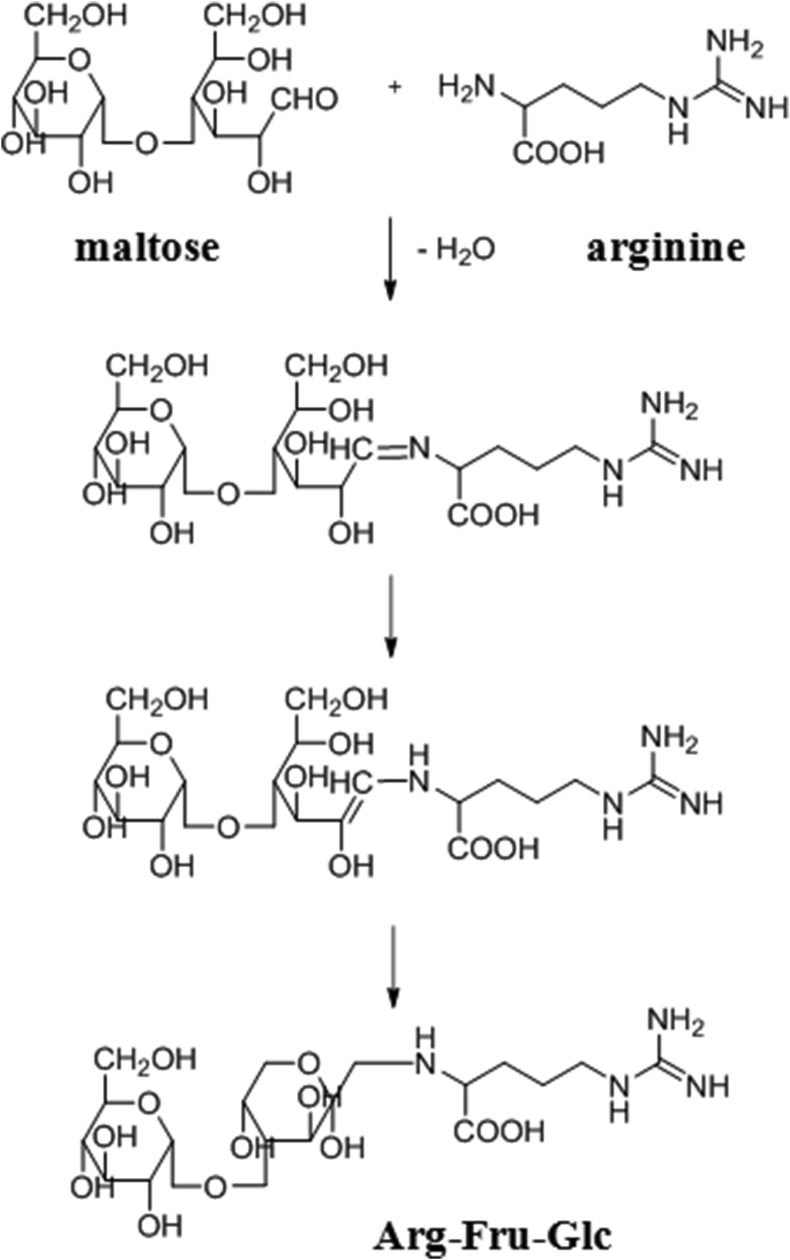
Around 60–90% of the nitrogen content in Korean Red Ginseng root (2–3% of the total content) is soluble nitrogen. Twenty percent of this is soluble protein that dissipates when the ginseng is boiled in water. Korean Red Ginseng protects against radiation, such as X-rays, and the active substance responsible for this is a non-saponin protein; specifically, it is reported to be a thermostable protein that is not denatured by heat, even when boiled in water [60]. Korean Red Ginseng proteins have been shown to provide strong protection against DNA injury caused by gamma-ray or ultraviolet irradiation [[61], [62], [63], [64]]. The initial research on Korean Red Ginseng peptides began in the 1960s when Gstirner et al obtained five peptide fractions from soluble Korean Red Ginseng extract and showed that these fractions had diverse amino acid compositions representing at least 20 different amino acids; however, these peptides were not purified and their physiological activity was not studied [65]. In the late 1980s, there was more focus on the soluble components of Korean Red Ginseng and more research was conducted to evaluate the chemistry and pharmacological activity of the peptides in Korean Red Ginseng. Okuda et al found that Korean Red Ginseng contains adenosine and an acidic substance that inhibits epinephrine-induced lipolysis in adipose cells and stimulates insulin-mediated lipogenesis [66]. This acidic substance was identified as pyroglutamic acid [67]. Adenosine and pyroglutamic acid were demonstrated to act as selective modulators that suppress lipolysis but promote lipid synthesis from glucose. Furthermore, a novel amino acid derivative was discovered to be a physiologically active substance in Korean Red Ginseng. The chemical structure of this substance with a molecular weight of 498 (C18H34N4O12) is arginine-fructose-glucose (AFG), in which an arginine molecule is linked to a fructose molecule that in turn binds to a glucose molecule. In manufacturing red ginseng, the Amadori rearrangement occurs between arginine and maltose or in glucose production including the amino sugars AFG and AF (Fig. 1) during the heating process. As the final product of the Maillard reaction, maltol is produced from these amino sugars [12,14,68].
Fig. 1.
The process by which AFG is produced in the red ginseng manufacturing process. AFG, arginine-fructose-glucose; Arg, arginine; Fru, fructose; Glc, glucose; H, hydrogen, N, nitrogen, OH, hydroxide; CH2OH, hydroxymethyl; O, oxygen; CHO, aldehyde; H2N, amidogen-d2; COOH, carboxylic acid; H2O, water.
The decrease in free sugar and amino acid content in Korean Red Ginseng is due to the production of amino sugars followed by the formation of caramel coloring as a product of thermolysis [14]. In terms of physiological activity, these substances have been shown to exert anti-obesity effects via inhibiting the enzymatic activity of sucrase and maltase in the gastric mucosa, consequently suppressing the absorption of starch in the gastrointestinal tract [69]. These physiologically active substances constitute about 5% of Korean Red Ginseng, which is a higher concentration than that of white ginseng; this is because of the heating process used to create red ginseng and the fact that the concentration of these substances is higher in thicker roots as they have more starch content than thinner roots [69].
Hyun et al reported that AFG-enriched extracts significantly increased the LPS content, concanavalin A-induced splenocyte proliferation, and both the immune cell count and antibody activity in cyclophosphamide-induced immune-deficient animals [70]. Shao et al also found that AFG had strong immune activity in cyclophosphamide-induced immune-deficient animals, promoting splenic lymphocyte transformation and increasing IL-2 production [71]. Moreover, it has been reported that AF and AFG may have anti-diabetic effects by inhibiting carbohydrate absorption in the stomach, thereby restricting the postprandial elevation of blood glucose [72]. After long-term AF administration to diabetic mice, Lee et al observed inhibited glucose absorption and a reduced hemoglobin A1c level, indicating improved postprandial hyperglycemia [73]. In a double-blind, placebo-controlled study of 60 Koreans with prediabetes or type 2 diabetes, blood glucose was measured after consuming an AF dose of 1500 mg/day for 6 weeks; there was a significant decrease in postprandial blood glucose [74]. When the antioxidant activity of AF and AFG was measured, both showed antioxidant effects by increasing the permeability of cell membranes and boosting the scavenging ability of peroxyl and hydroxyl radicals [12,75]. AFG has also been reported to be effective in the treatment of renal dysfunction [76]. Following the oral administration of 40 mg/kg or 80 mg/kg of AFG in animals, there was a decrease in serum creatinine and blood urea nitrogen levels and a considerable improvement of cisplatin-induced renal dysfunction. Interestingly, after pretreatment with AFG, all oxidative stress indices showed sustained improvement for 10 days reflecting that AFG attenuates the cascade initiation steps for nuclear factor kappa B signals and modulates the participation of the phosphatidylinositol 3-kinase/protein kinase B signal pathway, thereby reducing cisplatin-induced inflammation and apoptosis.
4. Amino acids
There are 24 amino acids in P. ginseng, and the composition of amino acids in Korean Red Ginseng varies slightly from report to report, but a characteristically high arginine content is a ubiquitous finding. The major amino acids found in Korean Red Ginseng are shown in Table 3.
Table 3.
The major amino acids found in Korean Red Ginseng
| Amino acids | |
|---|---|
| Aspartic acid | Methionine |
| Threonine | Isoleucine |
| Serine | Leucine |
| Glutamic acid | Tyrosine |
| Glycine | Lysine |
| Alanine | Histidine |
| Valine | Arginine |
| Cystine | Proline |
The free amino acid content in Korean Red Ginseng is around 2% with arginine accounting for over 50% of the total amino acid content [77]. Arginine has been reported to have immune-enhancing effects. When arginine becomes a precursor of NO or iNO, it can promote the secretion of Th1 or Th2 cytokines [78,79]. Arginine plays an important role in the synthesis of proline (involved in wound healing and collagen synthesis) and polyamine, both of which are important for cell growth and proliferation. Arginine is an essential amino acid for angiogenesis, hemodynamic maintenance, spermatogenesis, embryo survival, and fetal and neonatal growth. Moreover, dietary supplementation or intravenous administration of arginine enhances genital, cardiovascular, pulmonary, renal, gastric, hepatic, and immune function while also promoting wound healing and improving insulin sensitivity [80]. Pyroglutamic acid selectively modulated opposing metabolic pathways in rat adipocytes, inhibiting lipolysis and stimulating lipogenesis [67]. Given that Korean Red Ginseng contains a high concentration of arginine, which plays an important role in the body, and glutamic acid, which acts as a selective modulator, we anticipate more research on the physiological activity of amino acid derivatives from Korean Red Ginseng.
5. Nucleic acids
Korean Red Ginseng contains nucleic acids such as uracil, guanine, adenine, uridine, adenosine, cytidine, cytosine, thymine, and orotic acid [81]. Ando et al demonstrated the presence of adenosine in Korean Red Ginseng powder, which inhibits epinephrine-induced lipolysis and stimulates insulin-mediated lipogenesis from glucose [66]. Takaku et al found that adenosine inhibited lipolysis in rat adipocytes but stimulated lipogenesis [67]. In view of these results, adenosine was described as a “selective modulator”. Table 4 summarizes the major physiological activity of red ginseng proteins, peptides, amino acids, and nucleic acids.
Table 4.
Physiological activity of proteins, peptides, amino acids, and nucleic acids
| Components | Physiological activity | Reference |
|---|---|---|
| Protein fraction | Protection against DNA injury | [[61], [62], [63], [64]] |
| Peptide fraction | Lipolysis inhibition | [66,67] |
| Arginine-fructose-glucose | Anti-obesity effects | [69] |
| Immune activity | [70,71] | |
| Anti-diabetic effects | [12,[72], [73], [74], [75]] | |
| Kidney injury protection | [76] | |
| Arginine | Immune activity | [78,79] |
| Genital, cardiovascular, pulmonary, renal, gastric, hepatic, wound healing, and insulin sensitivity enhancement | [80] | |
| Adenosine | Lipolysis inhibition and lipogenesis stimulation | [66,67,81] |
6. Alkaloids
Given that alkaloids tend to show physiological activity, even in small quantities, researchers have long been interested in knowing the presence of alkaloids in Korean Red Ginseng. In 1963, to identify anti-hypertensive agents in Korean Red Ginseng, choline was first isolated and identified from Korean Red Ginseng alcohol extract [82]. The alkaloids in Korean Red Ginseng are shown in Table 5.
Table 5.
The alkaloids found in Korean Red Ginseng
| Components | |
|---|---|
| N-Formyl-1-methyl-β-carboline 1-Carbobutoxy-β-carboline 1-Carboethoxy-β-carboline 1-Carbomethoxy-β-carboline 1-(5-Hydroxymethyl-2-furyl)-β-carboline Norharman |
Harman 4-Methyl-5-thiazoleethanol Spinacine Choline α-Pyrrolidone |
Choline is a precursor of the cell membrane component lecithin and shows physiological activity in terms of suppressing fat accumulation, lowering blood pressure, and improving memory [83]. In 1986, as part of research on the non-saponins in Korean Red Ginseng, Han et al became interested in trace alkaloids in Korean Red Ginseng. They obtained a crude alkaloid fraction from the ether-soluble fraction, identified positive Dragendorff spots on thin-layer chromatograms, and, using column chromatography, purified 3 β-carboline alkaloids for the first time [32]. When alkaloids were added to cell culture medium, they inhibited DNA and total protein synthesis, thereby limiting cancer cell proliferation [84]. The alkaloid fraction showed protective effects against radiation by suppressing radiation-induced chromosome damage and promoting the repair and regeneration of injured cells [85,86]. The alkaloid fraction also mitigated the chronic mutagenic effects of radiation by reducing the rate of double-stranded breaks in DNA, preventing cellular injury, and delaying the repair time of the slow component [87]. Table 6 summarizes the major physiological activity of Korean Red Ginseng alkaloids.
Table 6.
Physiological activity of alkaloids
7. Polyacetylenes
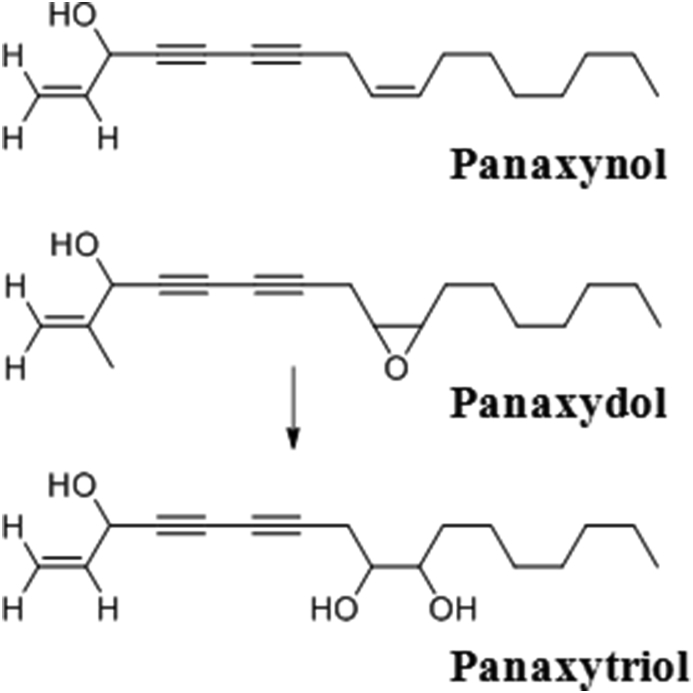
The first study on the polyacetylene content in Korean Red Ginseng was performed in 1964 in Japan by Takahashi et al who obtained a fraction with a high boiling point (120–152°C) from Korean Red Ginseng ether extract and isolated the yellow oil panaxynol [29]. In 1980, Poplawski et al manufactured an alcohol extract of Korean Red Ginseng in Poland and identified panaxynol in the petroleum ether-soluble fraction of the extract [88], while Dabrowski et al (1980) isolated heptadeca-1-ene-4,6-diyn-3,9-diol [89]. Kitagawa et al subsequently isolated panaxyol, panaxynol, and panaxytriol from the ether-soluble fractions of white ginseng and Korean Red Ginseng in Japan and identified panaxytriol as a unique constituent of Korean Red Ginseng (Fig. 2) [90]. So far, more than 20 polyacetylene components have been reported from Korean Red Ginseng (Table 7).
Fig. 2.
The process by which panaxytriol is produced in the red ginseng manufacturing process. H, hydrogen, HO, hydroxide; O, oxygen.
Table 7.
The major polyacetylenes found in Korean Red Ginseng
| Components | |
|---|---|
| Panaxydol Panaxynol Panaxytriol Panaxyne Panaxyne epoxide Acetylpanaxydol |
Heptadeca-1,8-t-diene-4,6-diyn-3,10-diol heptadeca-1-ene-4,6-diyn-3,9-diol 10-Acetylpanaxytriol Panaxydol chlorohydrin Ginsenoyne A ~ K |
Panaxydol, panaxynol, and panaxytriol are the best-known polyacetylenes; panaxydol and panaxynol account for over 90% of the total polyacetylene content. There have been several studies on their physiological activity, including research on inhibiting cancer cell proliferation and the mechanisms involved [31,[91], [92], [93], [94], [95]] as well as on inhibiting lipid peroxidation [96]. Panaxydol shows the strongest cytotoxicity against cancer cells [97]. Polyacetylenes have been shown to inhibit NO production in Raw 264.7 cells by blocking LPS-induced iNOS expression [98]. Panaxydol was reported to inhibit hypercholesterolemia in rats and mice fed a high-cholesterol diet [99], whereas panaxynol was shown to improve memory in a scopolamine-induced animal model of memory impairment [100]. Choi et al reported that polyacetylene (9R,10S)-epoxyheptadecan-4,6-diyn-3-one had an analgesic effect by inhibiting the sodium current in primary sensory neurons [101]. In another study, panaxydol was administered to mice to investigate whether it suppressed the formation of benzo(a)pyrene metabolite–DNA adducts in the liver; a clear decrease was noted in benzo(a)pyrene binding. This indicates that panaxynol and panaxydol are effective in suppressing genetic mutations and oncogenesis [102].
These polyacetylenes show inhibitory effects on the proliferation of cancer cells at low concentrations but show cytotoxicity in healthy cells at much higher concentrations, indicating that they have cancer-specific cytotoxicity [102]. Panaxytriol, which is unique to Korean Red Ginseng, has been reported to inhibit cancer cell proliferation and to have antitumor effects in animal studies [[103], [104], [105]]. Lee et al observed that polyacetylenes were involved in the selective expression of enzymes that take part in benzo(a)pyrene (a chemical carcinogen) metabolism in the body [106]. Park et al administered polyacetylene compounds (20–40 μM/kg body weight) intraperitoneally to healthy mice for 3 days, consecutively, to assess toxicity; compared with a control group, the group that received the highest dose (40 μM/kg) showed a 17% decrease in body weight but no major changes in organ mass or liver tissue, whereas the group that received the lowest dose (20 μM/kg) showed a 10% decrease in body weight with an increase in the weight loss rate in the order of panaxydol < panaxynol < panaxytriol [107]. Choi et al found that the polyacetylene compounds in Korean Red Ginseng had an inhibitory effect on the micronucleus formation induced by various carcinogens [108]. Table 8 summarizes the major physiological activity of Korean Red Ginseng polyacetylenes.
Table 8.
Physiological activity of polyacetylenes
| Components | Physiological activity | Reference |
|---|---|---|
| Panaxydol | Cancer cell proliferation inhibition | [31,91,[93], [94], [95],97] |
| Lipid peroxidation inhibition | [96] | |
| NO production inhibition | [98] | |
| Hypercholesterolemia inhibition | [99] | |
| Analgesic effect | [101] | |
| Suppressing genetic mutation and oncogenesis | [102] | |
| Toxicity relief | [[106], [107], [108]] | |
| Panaxytriol | Cancer cell proliferation inhibition | [31,[92], [93], [94], [95],[103], [104], [105]] |
| Lipid peroxidation inhibition | [96] | |
| NO production inhibition | [98] | |
| Toxicity relief | [[106], [107], [108]] | |
| Panaxynol | Memory improvement | [100] |
| Suppressing genetic mutation and oncogenesis | [102] | |
| Toxicity relief | [[106], [107], [108]] |
8. Phenolic compounds
Han et al revealed that the phenolic compounds, in the non-saponin fraction, such as maltol, vanillic acid, salicylic acid, ferulic acid, and caffeic acid have strong antioxidant and anti-fatigue effects [109,110]. Furthermore, although these phenolic substances show strong antioxidant activity, purified ginsenosides show no such activity. Maltol is not detected in fresh ginseng; it is unique to Korean Red Ginseng, from which it is produced by heat during the steaming process [111]. During steaming, maltose reacts with amino acids to produce unstable 4-0-α-D-glucosyl-deoxy-2,3-diketosaccharide, with further cyclization of a 2-ketone group, and a C-6-hydroxyl group produces glycoside B; this substance undergoes further rearrangement and deglycosylation to produce the aglycone compound maltol [112]. This reaction is known to follow the Maillard reaction.
Maltol has neuroprotective effects in hypoxia-induced neuroretinal cells; this effect is activated by nuclear factor kappa B and MAPK signaling pathways [113]. In a mouse model of carbon tetrachloride-induced liver injury, maltol showed a hepatoprotective effect by suppressing inflammation, reducing serum alanine transaminase and aspartate transaminase levels, and inhibiting cell apoptosis [114]. Similarly, in an animal model of alcohol-induced liver oxidative injury, maltol showed a hepatoprotective effect by increasing the activity of antioxidant enzymes [115]. Maltol has also been reported to have a protective effect against diabetic kidney injuries [116,117] as well as antioxidant effects [[118], [119], [120], [121], [122]]. Maltol ameliorated liver fibrosis by inhibiting the activation of hepatic stellate cells and inducing the apoptosis of activated hepatic stellate cells via the transforming growth factor-β1-mediated phosphoinositide 3-kinase/Akt signaling pathway [123].
In addition, p-coumaric acid, another phenolic compound in Korean Red Ginseng, inhibits platelet aggregation and suppresses the production of prostaglandins by modulating the metabolism of arachidonic acid [59]. p-Coumaric acid shows strong activity in terms of scavenging NO, hydroxide, and peroxynitrite [[120], [121], [122]]. It also increases cellular reactive oxygen species generation, superoxide dismutase activity, and glutathione levels [124]. Moreover, p-coumaric acid significantly lowers melanin levels, inhibits cellular tyrosinase activity, and reduces tyrosinase protein expression, thereby suppressing production of melanin [124]. Phenolic substances other than this component are not unique to red ginseng; thus, they cannot be considered distinct active ingredients that can represent the physiological activity of red ginseng. However, phenol is a meaningful component of the physiological activities of red ginseng. Table 9 summarizes the major phenolic compounds found in Korean Red Ginseng and Table 10 summarizes the major physiological activity of Korean Red Ginseng phenolic compounds.
Table 9.
The major phenolic compounds found in Korean Red Ginseng
| Components | |
|---|---|
| Maltol | Gentisic acid |
| Salicylic acid | Cinnamic acid |
| Vanillic acid | Protocatechuic acid |
| p-Coumaric acid | m-Coumaric acid |
| Ferulic acid | Polyphenol |
| p-Hydroxybenzoic acid | Salicyl alcohol |
| Caffeic acid | p-Hydroxybenzyl alcohol |
Table 10.
Physiological activity of phenolic compounds
| Components | Physiological activity | Reference |
|---|---|---|
| Maltol | Antioxidant activity | [111,[118], [119], [120], [121], [122]] |
| Neuroprotective effects | [113] | |
| Hepatoprotective effect | [114,115,123] | |
| Kidney injury protection | [116,117] | |
| p-Coumaric acid | Platelet aggregation inhibition Antioxidant activity |
[59] [[120], [121], [122],124] |
9. Essential oils
Korean Red Ginseng contains several volatile compounds with low or high boiling points. The compounds with a low boiling point (71–110°C) include panacene, contributing to the distinctive aroma of Korean Red Ginseng, and β-elemene, a type of β-sesquiterpenoid [29]. To date, there have been almost no studies on the physiological activity of the compounds that produce the distinctive aroma of Korean Red Ginseng; however, they are an important element in terms of sensory quality [125]. These compounds can be broadly divided into earthy, woody, hay-like, toast-like, and sweet; moreover, there are differences between fresh, white, and red ginseng depending on the processing methods used. The aroma of fresh ginseng is attributable to panacene, discovered in the initial studies of aroma compounds, as well as a complex mixture of several aroma-related compounds including methoxypyrazine and panasino A and B, which are sesquiterpene alcohols, and ginsenol [126]. Sensory evaluation of the Korean Red Ginseng aroma from different regions in the overseas Korean Red Ginseng market found that Korean Red Ginseng had a stronger aroma than Chinese red ginseng. Son et al investigated these differences by performing a pattern analysis of the major aroma-related compounds and a sensory evaluation of the fractions within these compounds using gas chromatography of the major headspace components in Korean and Chinese ginseng (both red and white) [127]. Specifically regarding characteristic patterns, among the aroma-related compounds identified, the mean β-patchoulene/ϒ- muurolene ratio was much higher in Korean Red Ginseng (≥1.0) than in Chinese ginseng (around 0.5) and Korean Red Ginseng had a stronger ginseng-like, toast-like, or sweet aroma. Park et al identified approximately 70 aroma-related compounds in fried red ginseng residue in distilled water and reported that the fragrant aromas, toasted rice, and refreshing aromas became stronger after frying whereas any unpleasant aroma was very weak [128].
10. Phytosterols
The main phytosterols in Korean Red Ginseng are stigmasterol [129] and β-sitosterol; these are mostly found in the unsaponifiable fraction [28]. There has been very little oil development from red ginseng because the compounds are present in such small quantities. However, Korean Red Ginseng oil (RGO) has recently been extracted and research is ongoing. Unlike typical plant oils, RGO has a very high linoleic acid concentration in fatty acids (18:2). The phytosterols in RGO include β-sitosterol, stigmasterol, and campesterol; approximately 87.0% of the phytosterol content is β-sitosterol, differentiating RGO from other plant oils.
RGO promotes the production of peroxyl radicals, improves the scavenging ability of free radicals, and inhibits oxidative stress in HepG2 cells [130]. Bak et al administered RGO to HepG2 cells under hydrogen peroxide-mediated oxidative stress and in an animal model of carbon tetrachloride-induced liver injury; the restoration of activity and the expression of antioxidant enzymes such as peroxide dismutase, catalase, and glutathione peroxidase were observed [131]. RGO suppresses lipid peroxidation by directly eliminating reactive oxygen species and simultaneously inducing cellular antioxidant enzyme activity and expression via the inhibition of MAPK, thereby protecting cells and tissues from oxidative injury [131]. RGO was found to induce nuclear factor erythroid 2-related factor 2/antioxidant response element-mediated phase II enzymes via the apoptosis signal-regulating kinase 1-MAPK 4/7–Jun N-terminal kinase and p38 MAPK signaling pathways, suggesting that RGO has potential as a natural chemopreventive and cellular defensive agent [132].
RGO has also been demonstrated to have anti-inflammatory effects [133]. In a study on the protective effects of RGO against Aβ25–35-induced oxidative stress and neuroinflammation, RGO protected against Aβ-induced cellular injury in an in vitro model of Alzheimer's disease [134]. RGO protects against B. abortus infection in vitro and in vivo and was found to be effective in the prevention and treatment of brucellosis [135]. RGO has shown a strong beneficial effect in terms of hair growth, so it demonstrates potential as an effective treatment for androgenetic alopecia [136]. Table 11 summarizes the major physiological activity of Korean Red Ginseng phytosterols.
Table 11.
Physiological activity of phytosterols
11. Other trace compounds
The vitamins in Korean Red Ginseng include B-complex vitamins (thiamine [B1], riboflavin [B2], cobalamin [B12], niacin, biotin, pantothenic acid, and folic acid. Minerals in Korean Red Ginseng include manganese, copper, vanadium, cobalt, arsenic, germanium, phosphorus, aluminum, and nickel. Germanium has been shown to have anti-cancer activity and to promote a shift from aged cells to new cells [137]. Furthermore, the lignans gomisin-A and gomisin-N have been isolated from red ginseng [138]. Gomisin-A and gomisin-N are the substances responsible for the adaptogenic and anti-hepatotoxic effects of Schisandra chinensis.
12. Conclusion
The current knowledge on the effects of non-saponins in Korean Red Ginseng has been discussed and evaluated. The distinct bioactivity of Korean Red Ginseng was found not to originate from only one or two specific components but from both saponin and non-saponin components, suggesting that both components should be studied collectively. Since Korean Red Ginseng contains various ingredients, it shows various effects. Therefore, if a variety of efficacy is desired via ingesting Korean Red Ginseng, it is important to eat all of its various ingredients. So far, research on non-saponin components is very poor compared to that of saponins. Until now, the importance of non-saponins in Korean Red Ginseng was overlooked due to its structural complexity, the separation into single components, and purification difficulties. In the future, it will be necessary to standardize the analysis method for the non-saponin components discussed above; also, it will be essential to upgrade the bioactivity verification by methods incorporating the latest science and technology. There are many non-saponin components other than the non-saponins discussed above. The search for new non-saponin components is also considered indispensable.
Conflicts of interest
All authors have no conflicts of interest to declare.
References
- 1.Hu S.Y. The genus Panax (Ginseng) in Chinese medicine. Econ Bot. 1976;30:11–28. [Google Scholar]
- 2.Hu S.Y. Proceedings of the 2nd international ginseng symposium. Korea Ginseng Research Institute; Seoul, Korea: 1978. The ecology, phytogeography and ethnobotany of ginseng; pp. 149–157. [Google Scholar]
- 3.Presons W.S. Bright Mountain Book, Inc.; Asheville, NC: 1994. American ginseng, Green gold; pp. 16–30. [Google Scholar]
- 4.Shin B.K., Kwon S.W., Park J.H. Chemical diversity of ginseng saponins from Panax ginseng. J Ginseng Res. 2015;39:287–298. doi: 10.1016/j.jgr.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y., Choi H.K., Brinckmann J.A., Jiang X., Huang L. Chemical analysis of Panax quinquefolius (North American ginseng): a review. J Chromatogr A. 2015;1426:1–15. doi: 10.1016/j.chroma.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 6.So S.H., Lee J.W., Kim Y.S., Hyun S.H., Han C.K. Red ginseng monograph. J Ginseng Res. 2018;42:549–561. doi: 10.1016/j.jgr.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christensen L.P. Ginsenosides: chemistry, biosynthesis, analysis, and potential health effects. Adv Food Nutr Res. 2009;55:1–99. doi: 10.1016/S1043-4526(08)00401-4. [DOI] [PubMed] [Google Scholar]
- 8.Jiaoa L., Zhanga X., Wanga M., Li B., Liua Z., Liu S. Chemical and antihyperglycemic activity changes of ginseng pectin induced by heat processing. Carbohydr Polym. 2014;114:567–573. doi: 10.1016/j.carbpol.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y.C., Li G., Jiang C., Yang B., Yang H.J., Xu H.Y., Huang L.Q. Tissue-specific distribution of ginsenosides in different aged ginseng and antioxidant activity of ginseng leaf. Molecules. 2014;19:1781–1799. doi: 10.3390/molecules191117381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blumenthal M., Goldberg A., Brinkmann J. Integrative Medicine Communications; Austin, TX: 2000. Herbal medicine: expanded commission E monographs; pp. 170–177. [Google Scholar]
- 11.Shan S.M., Luo J.G., Huang F., Kong L.Y. Chemical characteristics combined with bioactivity for comprehensive evaluation of Panax ginseng C.A. Meyer in different ages and seasons based on HPLC-DAD and chemometric methods. J Pharm Biomed Anal. 2014;89:76–82. doi: 10.1016/j.jpba.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 12.Kim G.N., Lee J.S., Song J.H., Oh Ch, Kwon Y.I., Jang H.D. Heat processing decreases Amadori products and increases total phenolic content and antioxidant activity of Korean Red ginseng. J Med Food. 2010;13:1478–1484. doi: 10.1089/jmf.2010.1076. [DOI] [PubMed] [Google Scholar]
- 13.Matsuura Y., Zheng Y., Takaku T., Kameda K., Okuda H. Isolation and physiological activities of new amino acid derivatives from Korean Red ginseng. J Ginseng Res. 1994;18:204–211. [Google Scholar]
- 14.Matsuura Y., Hirao Y., Yoshida S., Kunihiro K., Fuwa T., Kasai R., Tanaka O. Study on Red ginseng: new ginsenosides and a note on the occurrence of maltol. Chem Pharm Bull (Tokyo) 1984;32:4674–4677. [Google Scholar]
- 15.Lee S.M., Bae B.S., Park H.W., Ahn N.G., Cho B.G., Cho Y.L., Kwark Y.S. Characterization of Korean Red ginseng (Panax ginseng Meyer): history, preparation method, and chemical composition. J Ginseng Res. 2015;39:382–391. doi: 10.1016/j.jgr.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shibata S., Fujita M., Itowa H., Tanaka O., Ishii T. The structure of panaxadiol, a sapogenin of ginseng. Tetrahedron Lett. 1962;10:419–422. [Google Scholar]
- 17.Shibata S., Fujita M., Itokawa H., Tanaka O., Ishii T. Panaxadiol, a sapogenin of ginseng roots (1) Chem Pharm Bull (Tokyo) 1963;11:759–776. doi: 10.1248/cpb.11.759. [DOI] [PubMed] [Google Scholar]
- 18.Shibata S., Tanaka O., Nagai M., Ishii T. Studies of the constituents of Japanese and Chinese crude drugs, XII. a sapogenin of ginseng root (2) Chem Pharm Bull (Tokyo) 1963;11:762–765. doi: 10.1248/cpb.11.762. [DOI] [PubMed] [Google Scholar]
- 19.Shibata S., Tanaka O., Soma K., Iita Y., Ando T., Nakamura H. Studies on saponins and sapogenins of ginseng. The structure of panaxatriol. Tetrahedron Lett. 1965;3:207–213. doi: 10.1016/s0040-4039(01)99595-4. [DOI] [PubMed] [Google Scholar]
- 20.Shibata S., Tanaka T., Ando T., Sado M., Tsushima S., Ohsawa T. Chemical studies on oriental plant drugs (XIV). Protopanaxadiol, a genuine sapogenin of ginseng saponins. Chem Pharm Bull (Tokyo) 1966;14:595–600. doi: 10.1248/cpb.14.595. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka O., Nagai M., Ohsawa T., Tanaka N., Shibata S. Stereochemistry of protopanaxadiol. Tetrahedron Lett. 1967;5:391–396. [Google Scholar]
- 22.Nagai Y., Tanaka O., Shibata S. Structure of ginsenosede Rg1, a neutral saponin of ginseng root. Tetrahedron. 1971;27:881–892. [Google Scholar]
- 23.Sanada S., Konso N., Shoji J., Tanaka O., Shibata S. Studies on the saponins of ginseng I. Structures of ginsenosides Ro, Rb1, Rb2 Rc and Rd. Chem Pharm Bull (Tokyo) 1974;22:421–428. [Google Scholar]
- 24.Kitagawa I. Chemical investigation of naturally occurring drug materials. Elucidation of scientific basis for traditional medicines and exploitation of new naturally occurring drugs. Yakugaku Zasshi. 1992;112:1–4. doi: 10.1248/yakushi1947.112.1_1. [DOI] [PubMed] [Google Scholar]
- 25.Yuo C.R., Yong J.J., Popovich D.G. Isolation and characterization of bioactive polyacetylenes Panax ginseng Meyer roots. J Pharmaceut Biomed Anal. 2017;139:148–155. doi: 10.1016/j.jpba.2017.02.054. [DOI] [PubMed] [Google Scholar]
- 26.Kitagawa I., Yoshikawa M., Yoshihara M., Hayashi T., Taniyama T. Chemical studies on crude drug precession. I. On the constituents of ginseng radix rubra (1) Yakugaku Zasshi. 1983;103:612. [PubMed] [Google Scholar]
- 27.Hiromichi O., Lee S.D., Yukinaga M., Yinan Z., Takeshi T., Kenji K., Kumi H., Kazuhiro O., Osamu T., Toshiie S. Biological activities of non-saponin compounds isolated from Korean Red ginseng. J Ginseng Res. 1990;14:157–161. [Google Scholar]
- 28.Horhammer L., Wagner H., Lay B. Contents of Panax ginseng root, preliminary report. Pharm Ztg. 1961;106:1307–1312. [PubMed] [Google Scholar]
- 29.Takahashi M., Yoshikura M. [Studies on the components of Panax ginseng C.A. Meyer. V. On the structure of a new acetylene derivative "Panaxynol" (3). Synthesis of 1,9-(cis)-heptadecadiene-4,6-diyn-3-ol] Yakugaku Zasshi. 1966;86:1053–1056. doi: 10.1248/yakushi1947.86.11_1053. Article in Japanese. [DOI] [PubMed] [Google Scholar]
- 30.Woo L.K., Suh C.S., Chang J.J., Shin K.H. Presence of α-pyrrolidone in ginseng extracts. Yakhak Hoeji. 1969;13:121. [Google Scholar]
- 31.Shim S.C., Koh H.Y., Han B.H. Polyacetylene compounds from panax ginseng CA Meyer. Bull Korean Chem Soc. 1983;4:183–188. [Google Scholar]
- 32.Han B.H., Park M.H., Han Y.N., Woo L.K. Alkaloidal components of Panax ginseng. Arch Pharm Res. 1986;9:21. [Google Scholar]
- 33.Ovodov Y.S., Solov’eva T.F. Polysaccharides of panax ginseng. Chem Nat Compd. 1966;2:243–245. [Google Scholar]
- 34.Tomoda M., Shimada K., Konno C., Sugiyama K., Hikino H. Partial structure of panaxan A, a hypoglycaemic glycan of Panax ginseng roots. Planta Med. 1984;50:436–438. doi: 10.1055/s-2007-969758. [DOI] [PubMed] [Google Scholar]
- 35.Tomoda M., Shimada K., Konno C., Hikono H. Structure of panaxan B, A, hypoglycemic glycan of Panax ginseng roots. Phytochemistry. 1985;24 doi: 10.1055/s-2007-969758. 2431–23. [DOI] [PubMed] [Google Scholar]
- 36.Konno C., Sugiyama K., Kano M., Takahashi M., Hikino H. Isolation and hypoglycaemic activity of panaxans A, B, C, D and E, glycans of Panax ginseng roots. Planta Med. 1984;50:434–436. doi: 10.1055/s-2007-969757. [DOI] [PubMed] [Google Scholar]
- 37.Konno C., Murakami M., Oshima Y., Hikino H. Isolation and hypoglycemic activity of panaxans Q, R, S, T and U, glycans of Panax ginseng roots. J Ethnopharmacol. 1985;14:69–74. doi: 10.1016/0378-8741(85)90030-3. [DOI] [PubMed] [Google Scholar]
- 38.Konno C., Hikino H. Isolation and hypoglycemic activity of panaxans M, N, O and P, glycans of Panax ginseng roots. Int J Crude Drug Res. 1987;25:53–56. [Google Scholar]
- 39.Hikino H., Oshima Y., Suzuki Y., Konno C. Isolation and hypoglycemic activity of panaxans F, G and H, glycans of Panax ginseng roots. Jpn J Pharmacogn. 1985;39:331–333. [Google Scholar]
- 40.Oshima Y., Konno C., Hikino H. Isolation and hypoglycemic activity of panaxans I, J, K and L, glycans of Panax ginseng roots. J Ethnopharmacol. 1985;14:255–259. doi: 10.1016/0378-8741(85)90091-1. [DOI] [PubMed] [Google Scholar]
- 41.Ng T.B., Yeung H.W. Hypoglycemic constituents of Panax ginseng. Gen Pharmacol. 1985;16:549–552. doi: 10.1016/0306-3623(85)90140-5. [DOI] [PubMed] [Google Scholar]
- 42.Yang M., Wang B.X., Jin Y.L., Wang Y., Cui Z.Y. Effects of ginseng polysaccharides on reducing blood glucose and liver glycogen. Zhongguo Yao Li Xue Bao. 1990;11 520–4. Chinese. [PubMed] [Google Scholar]
- 43.Gao Q.P., Kiyohara H., Cyong J.C., Yamada H. Chemical properties and anti-complementary activities of polysaccharide fractions from roots and leaves of Panax ginseng. Planta Med. 1989;55:9–12. doi: 10.1055/s-2006-961765. [DOI] [PubMed] [Google Scholar]
- 44.Lee D.K., Kameda K., Takaku T., Keizo S., Kumi H.H., Kazuhiro O., Osamu T., Hiromichi O. Effect of acidic polysaccharide of red ginseng on lipolytic action of toxohormone-L, from cancerous ascites fluid. Korean J Ginseng Sci. 1990;14:1–5. [Google Scholar]
- 45.Kim Y.S., Kang K.S., Kim S.I. Study on antitumor and immunomodulating activities of polysaccharide fractions from Panax ginseng: comparison of effects of neutral and acidic polysaccharide fraction. Arch Pharm Res. 1990;13:330–337. [Google Scholar]
- 46.Kim Y.S., Kang K.S., Kim S.I. Effects of Ginseng components on immunotoxicity of cyclophosphamide. J Ginseng Res. 1991;15:13–20. [Google Scholar]
- 47.Park K.M., Kim Y.S., Jeong T.C., Joe C.O., Shin H.J., Lee Y.H., Nam K.Y., Park J.D. Nitric oxide is involved in the immunomodulating activities of acidic polysaccharide from Panax ginseng. Planta Medica. 2001;67:122–126. doi: 10.1055/s-2001-11508. [DOI] [PubMed] [Google Scholar]
- 48.Kim Y.S., Park K.M., Shin H.J., Song K.S., Nam K.Y., Park J.D. Anticancer activities of red ginseng acidic polysaccharide by activation of macrophages and natural killer cells. Yakhak Hoeji. 2002;46:113–119. [Google Scholar]
- 49.Shin H.J., Kim Y.S., Kwak Y.S., Song Y.B., Kyung J.S., Wee J.J., Park J.D. A further study on the inhibition of tumor growth and metastasis by Red ginseng acidic polysaccharide (RGAP) Natural Product Sciences. 2004;10:284–288. [Google Scholar]
- 50.Shin H.J., Kim Y.S., Kwak Y.S., Song Y.B., Kim Y.S., Park J.D. Enhancement of antitumor effects of paclitaxel (Taxol) in combination with Red ginseng acidic polysaccharide (RGAP) Planta Med. 2004;70:1–6. doi: 10.1055/s-2004-832643. [DOI] [PubMed] [Google Scholar]
- 51.Kwak Y.S., Kim S.K., Shin H.J., Song Y.B., Park J.D. Anticancer activities by combines treatment of red ginseng acidic polysaccharide (RGAP) and anticancer agents. J Ginseng Res. 2003;27:47–51. [Google Scholar]
- 52.Reyes A.W., Simborio H.L., Hop H.T., Arayan L.T., Min W.G., Lee H.J., Rhee M.H., Chang H.H., Kim S. Inhibitory effect of red ginseng acidic polysaccharide from Korean Red ginseng on phagocytic activity and intracellular replication of Brucella abortus in RAW 264.7 cells. J Vet Sci. 2016;17:315–321. doi: 10.4142/jvs.2016.17.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Byeon S.E., Lee J., Kim J.H., Yang W.S., Kwak Y.S., Kim S.Y., Choung E.S., Rhee M.H., Cho J.Y. Molecular mechanism of macrophage activation by red ginseng acidic polysaccharide from Korean Red ginseng. Mediators Inflamm. 2012;2012:732860. doi: 10.1155/2012/732860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kwak Y.S., Kyung J.S., Kim J.S., Cho J.Y., Rhee M.H. Anti-hyperlipidemic effects of red ginseng acidic polysaccharide from Korean Red ginseng. Biol Pharm Bull. 2010;33:468–472. doi: 10.1248/bpb.33.468. [DOI] [PubMed] [Google Scholar]
- 55.Du X.F., Jiang C.Z., Wu C.F., Won E.K., Choung S.Y. Synergistic immunostimulating activity of pidotimod and red ginseng acidic polysaccharide against cyclophosphamide-induced immunosuppression. Arch Pharm Res. 2008;31:1153–1159. doi: 10.1007/s12272-001-1282-6. [DOI] [PubMed] [Google Scholar]
- 56.Choi H.S., Kim K.H., Sohn E., Park J.D., Kim B.O., Moon E.Y., Rhee D.K., Pyo S. Red ginseng acidic polysaccharide (RGAP) in combination with IFN-gamma results in enhanced macrophage function through activation of the NF-kappaB pathway. Biosci Biotechnol Biochem. 2008;72:1817–1825. doi: 10.1271/bbb.80085. [DOI] [PubMed] [Google Scholar]
- 57.Kim H., Kim H.W., Yu K.W., Suh H.J. Polysaccharides fractionated from enzyme digests of Korean Red ginseng water extracts enhance the immunostimulatory activity. Int J Biol Macromol. 2019;121 doi: 10.1016/j.ijbiomac.2018.10.127. 913–20. Arch Pharm Res 2008;31:1153–1159. [DOI] [PubMed] [Google Scholar]
- 58.Yin S.Y., Kim H.J., Kim H.J. A comparative study of the effects of whole red ginseng extract and polysaccharide and saponin fractions on influenza A (H1N1) virus infection. Biol Pharm Bull. 2013;36:1002–1007. doi: 10.1248/bpb.b13-00123. [DOI] [PubMed] [Google Scholar]
- 59.Park H., Cho B.G., Lee M.K. Nitrogen compounds of Korea ginseng and their physiological significance. J Ginseng Res. 1990;14:317–331. [Google Scholar]
- 60.Yonezawa M., Katoh N., Takeda A. Restoration of radiation injury by ginseng. II. Some properties of the radioprotective substances. J Radiat Res. 1981;22:336–343. doi: 10.1269/jrr.22.336. [DOI] [PubMed] [Google Scholar]
- 61.Kim C.M., Han G.S. Radioprotective effects of ginseng proteins. Yakhak Hoeji. 1985;29:246–252. [Google Scholar]
- 62.Kim C.M., Choi J.E. Effects of radioprotective ginseng protein on UV induced sister chromatid exchanges. Arch Pharm Res. 1988;11:93–98. [Google Scholar]
- 63.Kim C.M., Choi M.K. DNA repair enhancement by radioprotective ginseng protein fraction. Yakhak Hoeji. 1992;36:449–454. [Google Scholar]
- 64.Kim C.M. Mechanisms of the radioprotective activity of ginseng protein fraction. J Ginseng Res. 1990;14:279–283. [Google Scholar]
- 65.Gstirner F., Vogt H.J. On peptides in White Korean ginseng. Arch Pharm Ber Dtsch Pharm Ges. 1966;299:936–944. doi: 10.1002/ardp.19662991108. [DOI] [PubMed] [Google Scholar]
- 66.Ando T., Muraoka T., Yamasaki N., Okuda H. Preparation of anti-lipolytic substance from Panax ginseng. Planta Med. 1980;38:18–23. doi: 10.1055/s-2008-1074832. [DOI] [PubMed] [Google Scholar]
- 67.Takaku T., Kameda K., Matsuura Y., Sekiya K., Okuda H. Studies on insulin-like substances in Korean Red ginseng. Planta Med. 1990;56:27–30. doi: 10.1055/s-2006-960877. [DOI] [PubMed] [Google Scholar]
- 68.Matsuura Y., Zheng Y., Takaku T., Kameda K., Okuda H. Isolation and physiological activities of new amino acid derivatives from Korean Red ginseng. Korean J Ginseng Sci. 1994;18:204–211. [Google Scholar]
- 69.Matsuura Y., Zheng Y., Takaku T., Kameda K., Okuda H. Isolation and physiological activities of a new amino acid derivative from Korean Red ginseng. J Trad Med. 1994;11:256–263. [Google Scholar]
- 70.Hyun S.H., Kim Y.S., Lee J.W., Han C.K., Park M.S., So S.H. Immunomodulatory effects of arginine-fructose-glucose enriched extracts of Red ginseng. Korean Soc J Food Nutr. 2018;47:1–6. [Google Scholar]
- 71.Shao Y., Sun R.H., Wang D., Li W., Zheng Y.N., Zhang J. The protective effect of arginyl-fructosyl-glucose against cyclophosphamide-induced immunosuppression in mice. Acta Nutr Sin. 2015;3 022. [Google Scholar]
- 72.Ha K.S., Jo S.H., Kang B.H., Apostolidis E., Lee M.S., Jang H.D., Kwon Y.I. In vitro and in vivo antihyperglycemic effect of 2 Amadori rearrangement compounds, arginyl-fructose and arginyl-fructosyl-glucose. J Food Sci. 2011;76:H188–H193. doi: 10.1111/j.1750-3841.2011.02361.x. [DOI] [PubMed] [Google Scholar]
- 73.Lee K.H., Ha K.S., Jo S.H., Lee C.M., Kim Y.C., Chung K.H., Kwon Y.I. Effect of long-term dietary arginyl-fructose (AF) on hyperglycemia and HbA1c in diabetic db/db mice. Int J Mol Sci. 2014;12(15):8352–8359. doi: 10.3390/ijms15058352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Park S.E., Kim O.H., Kwak J.H., Lee K.H., Kwon Y.I., Chung K.H., Lee J.H. Antihyperglycemic effect of short-term arginyl-fructose supplementation in subjects with prediabetes and newly diagnosed type 2 diabetes: randomized, double-blinded, placebo-controlled trial. Trials. 2015;16:521. doi: 10.1186/s13063-015-1036-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee J.S., Kim G.N., Lee S.H., Kim E.S., Ha K.S., Kwon Y.I., Jeong H.S., Jang H.D. In vitro and cellular antioxidant activity of arginyl-fructose and arginyl-fructosyl-glucose. Food Sci Biotechnol. 2009;18:1505–1510. [Google Scholar]
- 76.Li R.Y., Zhang W.Z., Yan X.T., Hou J.G., Wang Z., Ding C.B., Liu W.C., Zheng Y.N., Chen C. Arginyl-fructosyl-glucose, a major Maillard reaction product of Red ginseng, attenuates cisplatin-induced acute kidney injury by regulating nuclear factor κB and phosphatidylinositol 3-kinase/protein kinase B signaling pathways. J Agric Food Chem. 2019;67:5754–5763. doi: 10.1021/acs.jafc.9b00540. [DOI] [PubMed] [Google Scholar]
- 77.Cho E.J., Piao X.L., Jang M.H., Baek S.H., Kim H.Y., Kang K.S., Kwon S.W., Park J.H. The effect of steaming on the free amino acid contents and antioxidant activity of Panax ginseng. Food Chem. 2008;107:876–882. [Google Scholar]
- 78.Popovic P.J., Zeh H.J., 3rd, Ochoa J.B. Arginine and immunity. J Nutr. 2007;137 doi: 10.1093/jn/137.6.1681S. 1681S–6S. [DOI] [PubMed] [Google Scholar]
- 79.Bronte V., Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 80.Wu G., Bazer F.W., Davis T.A., Kim S.W., Li P., Rhoads J.M., Satterfield M.C., Smith S.B., Spencer T.E., Yin Y. Arginine metabolism and nutrition in growth, health and disease. Amino Acids. 2009;37:153–160. doi: 10.1007/s00726-008-0210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hiyama C., Miyai S., Yoshida H., Yamasaki K., Tanaka O. Application of high-speed liquid chromatography and dual wave-length thin-layer chromatograph-densitometry to analysis of crude drugs: nucleosides and free bases of nucleic acids in Ginseng roots. Yakugaku Zasshi. 1978;98:1132–1137. doi: 10.1248/yakushi1947.98.8_1132. [DOI] [PubMed] [Google Scholar]
- 82.Takatori K., Kato T., Ozaki M., Nakashima T. Choline in panax ginseng C. A. Meyer. Chem Pharm Bull (Tokyo) 1963;11:1342–1343. doi: 10.1248/cpb.11.1342. [DOI] [PubMed] [Google Scholar]
- 83.Hou J.P. The chemical constituents of Ginseng plants. Comp Med East West. 1977;5:123–145. doi: 10.1142/s0147291777000209. [DOI] [PubMed] [Google Scholar]
- 84.Woo L.K., Nakamura Y., Donati L. Effect of Korean ginseng on the growth rate of cells. Arch Ital Patol Clin Tumori. 1965;8:53–61. [PubMed] [Google Scholar]
- 85.Cho C.K., Kim T.H., Yoo S.Y., Koh K.H., Kim M.S., Kim J.H., Kim S.H., Yoon H.K., Ji Y.H. The effects of alkaloid fraction of Korean ginseng on the radiation-induced DNA strand breaks. J Korean Soc Ther Radiol. 1995;13:113–120. [Google Scholar]
- 86.Kim S.H., Cho C.K., Yoo S.Y., Koh K.H., Yun H.G., Kim T.H. In vivo radioprotective activity of Panax ginseng and diethyldithiocarbamate. Vivo. 1993;7:467–470. [PubMed] [Google Scholar]
- 87.Yoo S.Y., Cho C.K., Kim M.S., Yoo H.J., Kim S.H., Kim T.H. An experimental study of radioprotective effect of ginseng alkaloid fraction on cellular damage. J Radiat Protection Res. 1997;22:195. [Google Scholar]
- 88.Poplawski Z., Wrobel J.T., Glinka T. Panaxydol, a new polyacetyleneic epoxide from Panax ginseng roots. Phytochemistry. 1980;19:1539. [Google Scholar]
- 89.Dabrowski Z., Wrobel J.T., Wojtasiewicz K. Structure of an acetylenic compound from Panax ginseng. Phytochemistry. 1980;19:2464. [Google Scholar]
- 90.Kitagawa I., Taniyama T., Shibuya H., Noda T., Yoshikawa M. Chemical studies on crude drug processing. V. On the constituents of ginseng radix rubra (2): comparison of the constituents of white ginseng and red ginseng prepared from the same Panax ginseng root. Yakugaku Zasshi. 1987;107:495–505. doi: 10.1248/yakushi1947.107.7_495. [DOI] [PubMed] [Google Scholar]
- 91.Ahn B.Z., Kim S.I. Relationship between structure and cytotoxic activity of panaxydol analogs against L1210 cells. Arch Pharm (Weinheim) 1988;321:61–63. doi: 10.1002/ardp.19883210203. Article in Germany. [DOI] [PubMed] [Google Scholar]
- 92.Kim S.I., Lee Y.H., Kang K.S. 10-Acetyl panaxytriol, a new cytotoxic polyacetylene from Panax ginseng. Yakhak Hoeji. 1989;33:118–123. [Google Scholar]
- 93.Kim Y.S., Shin, Kim S.I., Hahn D.R. Effect of polyacetylene compounds from Panax ginseng on macromolecule synthesis of lymphoid leukemia L1210. Yakhak Hoeji. 1989;32:137–140. [Google Scholar]
- 94.Kim Y.S., Jin S.H., Kim S.I., Hahn D.R. Studies on the mechanism of cytotoxicities of polyacetylenes against L1210 cell. Arch Pharm Res. 1989;12:207. [Google Scholar]
- 95.Kim D.C., Lee J.Y., In M.J., Chae H.J., Hwang Y.K., Hwang W.I. Effects of polyacetylenes in ginseng on activity of enzymes related to post-translational modification of ras protein and effects of petroleum ether extract of ginseng on progression of cell cycle. J Ginseng Res. 2001;25:156–161. [Google Scholar]
- 96.Kim H.Y., Lee Y.H., Kim S.I. Effects of polyacetylene compounds from Panax ginseng CA Meyer on CCl4-induced lipid peroxidation in mouse liver. Toxicol Res. 1988;4:13–22. [Google Scholar]
- 97.Ahn B.Z., Kim S.I. [Relationship between structure and cytotoxic activity of panaxydol analogs against L1210 cells] Arch Pharm (Weinheim) 1988;321:61–63. doi: 10.1002/ardp.19883210203. Article in German. [DOI] [PubMed] [Google Scholar]
- 98.Ryu J.H., Jang S.R., Lee S.Y., Lee H.J., Han Y.N. Inhibitors of nitric oxide synthesis from ginseng in activated macrophages. J Ginseng Res. 1998;22:181–187. [Google Scholar]
- 99.Hyun H.C., Park J.K., Nam K.Y., Park K.H. Hypocholesterolemic effect of panaxydol in high cholesterol diet fed rats and mice. J Ginseng Res. 2001;25:162–166. [Google Scholar]
- 100.Yamazaki M., Hirakura K., Miyaichi Y., Imakura K., Kita M., Chiba K., Mohri T. Effect of polyacetylenes on the neurite outgrowth of neuronal culture cells and scopolamine-induced memory impairment in mice. Biol Pharm Bull. 2001;24:1434–1436. doi: 10.1248/bpb.24.1434. [DOI] [PubMed] [Google Scholar]
- 101.Choi S.J., Kim T.H., Shin Y.K., Lee C.S., Park M., Lee H.S., Song J.H. Effects of a polyacetylene from Panax ginseng on Na+ currents in rat dorsal root ganglion neurons. Brain Res. 2008;1191:75–83. doi: 10.1016/j.brainres.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 102.Park J.K., Kim S.I. Inhibition of the formation of adducts between metabolites of benzo(a)pyrene and DNA by Panaxydol in vivo and in vitro. J Ginseng Res. 1989;13:42–48. [Google Scholar]
- 103.Matsunaga H., Katano M., Yamamoto H., Mori M., Takata K. Studies on the panaxytriol of Panax ginseng C. A. Meyer. Isolation, determination and antitumor activity. Chem Pharm Bull (Tokyo) 1989;37:1279–1281. doi: 10.1248/cpb.37.1279. [DOI] [PubMed] [Google Scholar]
- 104.Matsunaga H., Katano M., Yamamoto H., Fujito H., Mori M., Takata K. Cytotoxic activity of polyacetylene compounds in Panax ginseng C. A. Meyer. Chem Pharm Bull (Tokyo) 1990;38:3480–3482. doi: 10.1248/cpb.38.3480. [DOI] [PubMed] [Google Scholar]
- 105.Matsunaga H., Katano M., Saita T., Yamamoto H., Mori M. Potentiation of cytotoxicity of mitomycin C by a polyacetylenic alcohol, panaxytriol. Cancer Chemother Pharmacol. 1994;33:291–297. doi: 10.1007/BF00685902. [DOI] [PubMed] [Google Scholar]
- 106.Lee F.C., Park J.K., Ko J.H., Lee J.S., Kim K.Y., Kim C.K. Effects of panax ginseng extract on the benzo(a)pyrene metabolizing enzyme system. Drug Chem Toxicol. 1987;10:227. doi: 10.3109/01480548709042984. [DOI] [PubMed] [Google Scholar]
- 107.Park J.K., Jin S.H. The toxicological parameter assessment in experimental animals for various dosages of polyacetylene compounds. J Ginseng Res. 1989;13:49–55. [Google Scholar]
- 108.Choi S.G., Heo M.Y. Anticlastogenic effect of petroleum ether extract of Panax ginseng against carcinogen-induced micromuclei in mice. Yakhak Hoeji. 1992;36:334–340. [Google Scholar]
- 109.Han B.H., Park M.H., Han Y.N. Studies on the antioxidant components of Korean ginseng (III) Arch Pharm Res. 1981;4:53–58. [Google Scholar]
- 110.Han B.H., Park M.H., Han Y.N. Chemical and biochemical studies on non-saponin constituents of Korean ginseng. J Ginseng Res. 1992;16:228–234. [Google Scholar]
- 111.Matsuura H., Hirao Y., Yoshida S., Kunihiro K., Fuwa T., Kasai R., Tanaka O. Study of red ginseng: new glucosides and a note on the occurrence of maltol. Chem Pharm Bull (Tokyo) 1984;32:4674–4677. [Google Scholar]
- 112.Li X.G. Studies on the transforming mechanism of amino acid components in the course of ginseng processing. Korean J Ginseng Sci. 1992;16:64–67. [Google Scholar]
- 113.Song Y., Hong S., Iizuka Y., Kim C.Y., Seong G.J. The neuroprotective effect of maltol against oxidative stress on rat retinal neuronal cells. Korean J Ophthalmol. 2015;29:58–65. doi: 10.3341/kjo.2015.29.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu W., Wang Z., Hou J.G., Zhou Y.D., He Y.F., Jiang S., Wang Y.P., Ren S., Li W. The liver protection effects of maltol, a flavoring agent, on carbon tetrachloride-induced acute liver injury in mice via inhibiting apoptosis and inflammatory response. Molecules. 2018;23(9) doi: 10.3390/molecules23092120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Han Y., Xu Q., Hu J.N., Han X.Y., Li W., Zhao L.C. Maltol, a food flavoring agent, attenuates acute alcohol-induced oxidative damage in mice. Nutrients. 2015;7:682–696. doi: 10.3390/nu7010682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kang K.S., Ham J., Kim Y.J., Park J.H., Cho E.J., Yamabe N. Heat-processed Panax ginseng and diabetic renal damage: active components and action mechanism. J Ginseng Res. 2013;37:379–388. doi: 10.5142/jgr.2013.37.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kang K.S., Yamabe N., Kim H.Y., Yokozawa T. Role of maltol in advanced glycation end products and free radicals: in-vitro and in-vivo studies. J Pharm Pharmacol. 2008;60:445–452. doi: 10.1211/jpp.60.4.0006. [DOI] [PubMed] [Google Scholar]
- 118.Yokozawa T., Kang K.S., Yamabe N., Kim H.Y. Therapeutic potential of heat-processed Panax ginseng with respect to oxidative tissue damage. Drug Discov Ther. 2007;1:30–44. [PubMed] [Google Scholar]
- 119.Kang K.S., Kim H.Y., Baek S.H., Yoo H.H., Park J.H., Yokozawa T. Study on the hydroxyl radical scavenging activity changes of ginseng and ginsenoside-Rb2 by heat processing. Biol Pharm Bull. 2007;30:724–728. doi: 10.1248/bpb.30.724. [DOI] [PubMed] [Google Scholar]
- 120.Kang K.S., Yokozawa T., Kim H.Y., Park J.H. Study on the nitric oxide scavenging effects of ginseng and its compounds. J Agric Food Chem. 2006;54:2558–2562. doi: 10.1021/jf0529520. [DOI] [PubMed] [Google Scholar]
- 121.Kang K.S., Kim H.Y., Pyo J.S., Yokozawa T. Increase in the free radical scavenging activity of ginseng by heat-processing. Biol Pharm Bull. 2006;29:750–754. doi: 10.1248/bpb.29.750. [DOI] [PubMed] [Google Scholar]
- 122.Kang K.S., Tanaka T., Cho E.J., Yokozawa T. Evaluation of the peroxynitrite scavenging activity of heat-processed ginseng. J Med Food. 2009;12:124–130. doi: 10.1089/jmf.2007.0646. [DOI] [PubMed] [Google Scholar]
- 123.Mi X.J., Hou J.G., Jiang Liu Z., Tang S., Liu X.X., Wang Y.P., Chen C., Wang Z., Li W. Maltol mitigates thioacetamide-induced liver fibrosis through TGF-β1-mediated activation of PI3K/Akt signaling pathway. J Agric Food Chem. 2019;67:1392–1401. doi: 10.1021/acs.jafc.8b05943. [DOI] [PubMed] [Google Scholar]
- 124.Jiang R., Xu X.H., Wang K., Yang X.Z., Bi Y.F., Yan Y., Liu J.Z., Chen X.N., Wang Z.Z., Guo Xl. Ethyl acetate extract from Panax ginseng C.A. Meyer and its main constituents inhibit α-melanocyte-stimulating hormone-induced melanogenesis by suppressing oxidative stress in B16 mouse melanoma cells. J Ethnopharmacol. 2017;208:149–156. doi: 10.1016/j.jep.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 125.Kim M.W., Park J.D. Studies on the volatile flavor components of fresh ginseng. J Ginseng Res. 1984;8:22–31. [Google Scholar]
- 126.Yoshihara K., Hirose Y. The sesquiterpenes of ginseng. Bull Chem Soc Jpn. 1975;48:2078. [Google Scholar]
- 127.Sohn H.J., Heo J.N., Nho K.B., Kim M.W. A comparison of the composition of the major headspace volatiles between the Korean ginseng and the Chinese ginseng. J Ginseng Res. 1997;21:196–200. [Google Scholar]
- 128.Park M.H., Sohn H.J., Jeon B.S., Kim N.M., Park C.K., Kim A.K., Kim K.C. Studies on flavor components and organoleptic properties in roasted red Ginseng Marc. J Ginseng Res. 1999;23:211–216. [Google Scholar]
- 129.Chung B.S. Studies on the oil soluble constituents of Korean ginseng -Part 1. On the composition of ginseng sterols. Korean J Pharmacog. 1974;5:173–177. [Google Scholar]
- 130.Shon M.S., Kim J.S., Song J.H., Jang H.D., Kim G.N. Anti-oxidant activity of oil extracted from Korean Red ginseng and its moisturizing function. Kor J Aesthet Cosmetol. 2013;11:489–494. [Google Scholar]
- 131.Bak M.J., Jun M.R., Jeong W.S. Antioxidant and hepatoprotective effects of the Red ginseng essential oil in H2O2-treated HepG2 cells and CCl4-treated mice. Int J Mol Sci. 2012;13:2314–2330. doi: 10.3390/ijms13022314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bak M.J., Truong V.L., Ko S.Y., Nguyen X.N., Jun M., Hong S.G., Lee J.W., Jeong W.S. Induction of Nrf2/ARE-mediated cytoprotective genes by red ginseng oil through ASK1-MKK4/7-JNK and p38 MAPK signaling pathways in HepG2 cells. J Ginseng Res. 2016;40:423–430. doi: 10.1016/j.jgr.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bak M.J., Hong S.G., Lee J.W., Jeong W.S. Red ginseng marc oil inhibits iNOS and COX-2 via NFκB and p38 pathways in LPS-stimulated RAW 264.7 macrophages. Molecules. 2012;17:13769–13786. doi: 10.3390/molecules171213769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lee S., Youn K., Jeong W.S., Ho C.T., Jun M. Protective effects of Red ginseng oil against Aβ25-35-induced neuronal apoptosis and inflammation in PC12 cells. Int J Mol Sci. 2017;18(10):2218. doi: 10.3390/ijms18102218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Reyes A.W.B., Hop H.T., Arayan L.T., Huy T.X.N., Park S.J., Kim K.D., Min W., Lee H.J., Rhee M.H., Kwak Y.S. The host immune enhancing agent Korean Red ginseng oil successfully attenuates Brucella abortus infection in a murine model. J Ethnopharmacol. 2017;198:5–14. doi: 10.1016/j.jep.2016.12.026. [DOI] [PubMed] [Google Scholar]
- 136.Truong V.L., Bak M.J., Lee C., Jun M., Jeong W.S. Hair regenerative mechanisms of Red ginseng oil and its major components in the testosterone-induced delay of anagen entry in C57BL/6 mice. Molecules. 2017;8(9):E1505. doi: 10.3390/molecules22091505. 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lee M.S., Lee J.H., Kwon T.O., Namkoong S.B. Increment of germanium contents in Angelica keiskei Koidz. and Panax Ginseng G.A. Meyer by in vitro propagation. Korean J Medical Crop Sci. 1995;3:251–258. [Google Scholar]
- 138.Han B.H., Huh B.H., Lee I.R. Lignan components from Panax ginseng C. A. Meyer. J Ginseng Res. 1990;14:217–220. [Google Scholar]