Abstract
Background
It is well recognized that gut microbiota is involved in the biotransformation of ginsenosides by converting the polar ginsenosides to nonpolar bioactive ginsenosides. However, the roles of the gut microbiota on the pharmacokinetics of ginsenosides in humans have not yet been fully elucidated.
Methods
Red ginseng (RG) or fermented red ginseng was orally administered to 34 healthy Korean volunteers, and the serum concentrations of the ginsenosides were determined using liquid chromatography–tandem mass spectrometry. In addition, the fecal ginsenoside Rd– and compound K (CK)–forming activities were measured. Then, the correlations between the pharmacokinetic profiles of the ginsenosides and the fecal ginsenoside–metabolizing activities were investigated.
Results
For the RG group, the area under the serum concentration–time curve values of ginsenosides Rd, F2, Rg3, and CK were 8.20 ± 11.95 ng·h/mL, 4.54 ± 3.70 ng·h/mL, 36.40 ± 19.68 ng·h/mL, and 40.30 ± 29.83 ng·h/mL, respectively. For the fermented red ginseng group, the the area under curve from zero to infinity (AUC∞) values of ginsenosides Rd, F2, Rg3, and CK were 187.90 ± 95.87 ng·h/mL, 30.24 ± 41.87 ng·h/mL, 28.68 ± 14.27 ng·h/mL, and 137.01 ± 96.16 ng·h/mL, respectively. The fecal CK-forming activities of the healthy volunteers were generally proportional to their ginsenoside Rd–forming activities. The area under the serum concentration–time curve value of CK exhibited an obvious positive correlation (r = 0.566, p < 0.01) with the fecal CK-forming activity.
Conclusion
The gut microbiota may play an important role in the bioavailability of the nonpolar RG ginsenosides by affecting the biotransformation of the ginsenosides.
Keywords: Gut microbiota, Pharmacokinetics, Protopanaxadiol ginsenosides, Red ginseng
1. Introduction
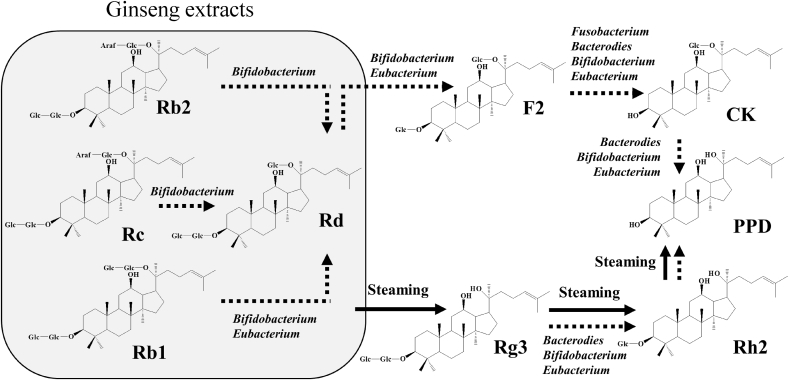
Red ginseng (RG), which is the steamed root of Panax ginseng Meyer (family Araliaceae), has been used widely as a dietary supplement and as a traditional Chinese medical treatment for cancer, diabetes, and inflammatory diseases [1], [2], [3]. RG contains ginsenosides Rb1, Rg1, Re, Rc, Rb2, and Rd, which are components of white ginseng, and Rg3, which is a characteristic constituent [4], [5]. Among these, ginsenosides Rb1, Rc, Rb2, Rd, and Rg3 are categorized as protopanaxadiol ginsenosides [6]. These protopanaxadiol ginsenosides exhibit a variety of pharmacological activities, such as antitumor, antidiabetic, antiinflammatory, and neuroprotective activities [7], [8], [9], [10], [11]. When RG is administered orally, the polar ginsenosides Rb1, Rb2, and Rc can be metabolized to nonpolar ginsenoside 20-O-β-(D-glucopyranosyl)-20(S)-protopanaxadiol, which is called compound K (CK), through ginsenoside Rd; or ginsenoside Rg3 can be metabolized to protopanaxadiol through ginsenoside Rh2 by the gut microbiota [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22]. In a previous study, when RG was orally administered to mice, ginsenosides Rd, Rg3, and CK were detected mainly in the plasma [20], [21]. In addition, CK has been detected in the blood of healthy volunteers treated with ginseng or ginsenoside Rb1 [23], [24], [25], [26], [27], [28]. These nonpolar ginsenoside metabolites of ginsenosides Rd, Rg3, and CK are known to exhibit more potent biological activities, including antitumor and antiinflammatory activities both in vitro and in vivo than their parent ginsenosides [29], [30]. Based on these findings, to increase the pharmacological activity of RG, various bioactive ginsenoside-enforcing products have been developed, such as heat-processed, microwave-treated, enzyme-treated, and fermented ginseng products [21], [31], [32], [33], [34]. Heat treatments such as steaming mainly produce ginsenoside Rg3 by removing a diglucoside at the C20 position, whereas bacterial enzymes are involved in glycosidic bond hydrolysis to eliminate the terminal monosaccharide (Fig. 1).
Fig. 1.
Transformation pathways of protopanaxadiol-type ginsenosides by steaming and gut microbiota. CK, compound K; PPD, protopanaxadiol.
As mentioned previously, the gut microbiota may play an important role in exerting the pharmacological activity of ginseng by converting the polar ginsenosides to nonpolar bioactive ginsenosides. There are many articles reporting the pharmacokinetics of ginsenosides after the administration of ginseng or ginsenosides [20], [21], [23], [35], [36], [37], [38], [39], [40], [41]. However, information about the pharmacokinetic parameters of various ginsenoside metabolites in humans after intake of RG is still limited. Moreover, the roles of the gut microbiota on the pharmacokinetics of ginsenosides in humans have not yet been fully elucidated, although it is well recognized that ginsenosides can be metabolized by the enzymes of gut microbes.
In this study, we investigated the pharmacokinetic properties of the protopanaxadiol ginsenosides, including Rd, Rg3, F2, and CK, in humans after the intake of conventional RG and fermented red ginseng (FRG). Then, we analyzed the relationships between the pharmacokinetic profiles of the ginsenoside metabolites and ginsenoside-metabolizing activity of human fecal microbiota.
2. Materials and methods
2.1. Materials
Ginsenoside standards for Rg3, Rd, F2, and CK (purity ≥98%) were obtained from ChemFaces (Wuhan, China). RG and FRG were donated as a paste form from Korea Yakult Research Institute (Yongin, Korea) [20]. Digitoxin as an internal standard (IS) and formic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA). Acetonitrile and methanol (HPLC grade) were purchased from J.T. Baker (Phillipsburg, NJ, USA). Distilled water was prepared using a Milli-Q purification system (Millipore, Billerica, MA, USA). The Oasis HLB 96-well solid-phase extraction (SPE) cartridge was purchased from Waters (Milford, MA, USA).
2.2. Participants
Physically healthy men and women, aged between 20 and 45 years, were recruited from H Plus Yangji Hospital (Seoul, Korea). Participants were recruited if antibacterial, antidiarrhea, and laxative medications were not given within 1 month before the study. The study protocol and consent forms were approved by the Institutional Review Boards of H Plus Yangji Hospital, and all participants provided written informed consent to participate in the study. All study procedures were conducted in compliance with the principles of the Declaration of Helsinki and Korean Good Clinical Practice guidelines (IRB HYJ-18-011).
2.3. Study design
RG or FRG (3 g) was orally administered to volunteers once a day for 3 days to acclimatize to RG or FRG. The volunteers were admitted to the hospital 2 days after the final administration and were randomly assigned to receive RG or FRG. The RG group consisted of 13 men and 4 women, and the FRG group consisted of 13 men and 5 women. All volunteers were given standardized meals, and no food intake was permitted after 20:00 pm. The next day, the volunteers orally received 3 g of either RG or FRG with 47 mL of tap water. Any food or water intake was not allowed during the first 4 h after administration of ginseng extracts. Venous blood samples (10 mL) were collected before the administration and at 1, 3, 6, 9, 12, and 24 h after the administration of RG or FRG in dry nonheparinized centrifuge tubes. Serum was collected by centrifugation at 10,000 × g for 5 min.
2.4. HPLC analysis of ginsenosides
The RG and FRG samples were determined by an Agilent 1200 series HPLC system with a UV detector (Agilent Technologies, Palo Alto, CA, USA). The chromatographic separation of ginsenosides was achieved using a Discovery C18 column (4.6 × 250 mm, 5 μm; Sigma-Aldrich, MO, USA). The mobile phase consisted of water (solvent A) and acetonitrile (solvent B). The mobile phase flow rate was 1 mL/min. The solvent composition was initially set at 15% B solvent, with gradient elution as 0–5 min, 15%; 5–17 min, 20%; 17–57 min, 39%; 57–70 min, 48%; 70–80 min, 70%; 80–82 min, 90%; 82–94 min, 15%; and 94–115 min, 15% of solvent B. The injection volume was 10 μL, and the UV detection wavelength was set at 203 nm.
2.5. Preparation of calibration standards and quality control samples
Stock solutions of Rd, Rg3, F2, and CK were prepared to obtain a concentration of 1 mg/mL in methanol. Working standard solutions were prepared by diluting the stock solution with methanol to final concentration ranges: 0.2–30 ng/mL for Rg3, F2, and CK and 0.5–30 ng/mL for Rd. The IS solution was digitoxin dissolved in methanol at a concentration of 200 ng/mL. These working standard solutions (10 μL) were spiked to blank human serum (1 mL) to yield calibration standards of range from 0.2 ng/mL to 30 ng/mL for Rg3, F2, and CK and from 0.5 ng/mL to 30 ng/mL for Rd. Quality control (QC) samples were prepared at a final concentration of 0.2 ng/mL [low-level QC (LQ)], 3 ng/mL [(medium-level QC (MQ)], and 10 ng/mL [high-level QC (HQ)] for Rg3, F2, and CK and 0.5 ng/mL (LQ), 3 ng/mL (MQ), and 10 ng/mL (HQ) for Rd.
2.6. Sample preparation
Human serum samples (1 mL) were mixed with 100 μL of IS solution and prepared for loading in the SPE cartridge. Activating the SPE cartridge was done by washing it with 1 mL of methanol. The cartridges were equilibrated and washed twice with 1 mL distilled water and eluted with 1 mL methanol. The eluate was dried under a flow of nitrogen at 35°C. The residue was dissolved in 100 μL of methanol and centrifuged for LC–MS/MS analysis.
2.7. LC–MS/MS instrumentation
All samples were separated from the matrix components using a Thermo Hypersil Gold column (2.1 × 50 mm, 3 μm; Thermo Fisher Scientific, Bellefonte, PA, USA). The mobile phase consisted of 0.1% formic acid in distilled water (solvent A) and 0.1% formic acid in acetonitrile (solvent B). A gradient program was used for HPLC separation, with a flow rate of 0.3 mL/min. The initial composition of the mobile phase was 10% solvent B, which was changed to 40% solvent B over 2.0 min and 90% solvent B over 5.0 min and followed by 11 min reequilibration to the initial condition over 0.1 min. The ginsenosides Rg3, Rd, F2, and CK were measured using an Agilent 6460 triple quadrupole tandem mass spectrometer in a multiple reaction monitoring mode using positive/negative electrospray ionization source. The precursor–product ion pairs monitored were 829.5→783.4 for Rg3 and F2, 969.5→789.4 for Rd, and 667.4→621.3 for CK. Nitrogen gas was used as a nebulizer at a pressure of 35 psi, as a carrier gas at 10 L/min (at 300°C), and as a sheath gas at 11 L/min (at 350°C).
2.8. Linearity, accuracy, and precision
Calibration curves were generated by plotting the peak area ratio of the analyte to the IS versus the concentration of the analyte, using least-square linear regression. The intraday and interday accuracy and precision of the assay were evaluated by repeated analyses of samples at LQ, MQ, and HQ for three consecutive days.
2.9. Pharmacokinetic analysis
The Phoenix WinNonlin Enterprise program (version 5.3; Pharsight Inc., St. Louis, MO, USA) was used with a noncompartmental statistical model to determine the following pharmacokinetic parameters: Cmax (maximum serum concentration), Tmax (the time taken to reach maximum), AUCt (the area under curve from zero to the last time point), AUC∞ (the area under curve from zero to infinity), and t1/2 (elimination half-life).
2.10. Assay of fecal ginsenoside Rd– and CK–forming activity
The human fecal specimens (approximately 0.2 g) were collected in plastic cups after 1–12 h, suspended with 1.8 mL cold saline, and centrifuged at 500 × g for 5 min [20], [21]. The supernatant were further centrifuged at 10,000 × g for 30 min, and the resulting precipitate was suspended in 5 mL of phosphate buffered saline (PBS), sonicated, and centrifuged at 10,000 × g for 30 min. The resulting supernatant was used as a crude fecal enzyme solution. For the fecal metabolizing activities, the reaction mixture (2 mL) containing 0.2 mL of the crude enzyme solution and 0.2 mL of 0.1 mg/mL RG extract was incubated at 37°C for 12 h, and the reaction was stopped by the addition of 2 mL of MeOH. The reaction mixture is centrifuged at 3000 × g for 10 min, and the levels of ginsenosides Rd, F2, and CK in the resulting supernatant are analyzed by HPLC. Ginsenoside Rd–forming activity was indicated as the amount required to catalyze the formation of 1.0 nmole of ginsenosides Rd, F2, and CK from the RG extract per hour under the standard assay conditions. CK-forming activity was indicated as the amount required to catalyze the formation of 1.0 nmol of ginsenoside CK from the RG extract per hour under the standard assay conditions. Specific activity was defined as nmol/h per g of feces.
2.11. Statistics
All pharmacokinetic (PK) data were summarized as mean ± standard deviation. A one-way analysis of variance followed by the Student t-test was conducted to compare the means of different groups using SPSS (version 24; IBM Corporation, Armonk, NY, USA). Spearman's rank correlation analysis was conducted using SPSS to determine the correlation coefficient between the area under the serum concentration–time curve (AUC) of ginsenosides and fecal bacterial ginsenoside-forming activity. A p-value less than 0.05 was considered to be statistically significant.
3. Results and discussion
3.1. Contents of ginsenosides in RG and FRG extracts
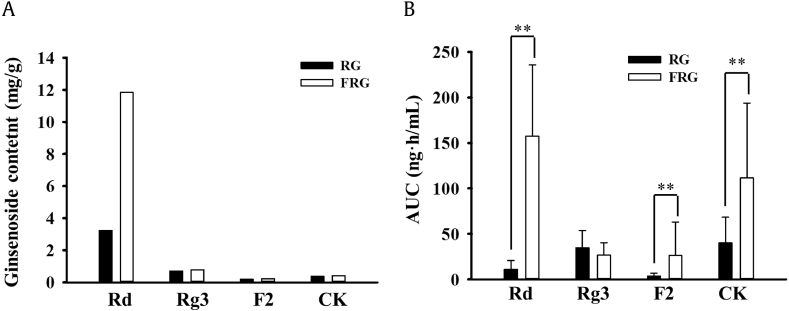
Ginsenosides of RG and FRG were quantified using HPLC. The contents of ginsenosides Rb1, Rd, Rg3, F2, and CK were 16.06 mg/g, 3.24 mg/g, 0.71 mg/g, 0.20 mg/g, and <0.40 mg/g, respectively, in RG; and 11.46 mg/g, 11.84 mg/g, 0.79 mg/g, 0.23 mg/g, and 0.42 mg/g, respectively, in FRG.
3.2. Bioanalytical method for ginsenosides
The serum concentrations of ginsenosides Rd, F2, Rg3, and CK were determined using LC-MS/MS analysis. A quantitative method for ginsenosides in the human serum sample was developed and validated. The calibration curve equations were y = 0.0527x – 0.0063 for Rg3; y = 0.0131x + 0.0009 for F2; y = 0.0152x + 0.0050 for CK; and y = 0.0076x – 0.0023 for Rd. The correlation coefficients of the calibration curves were more than 0.99. The intraday and interday precisions were found to be <12.6%, <13.3%, <11.8%, and <12.6%, and the accuracies were 86.1–114.6%, 87.9–110.2%, 89.8–106.0%, and 93.5–107.7% for Rg3, F2, CK, and Rd, respectively.
3.3. Pharmacokinetic profiles of ginsenosides Rd, F2, Rg3, and CK in the healthy volunteers
Ginseng contains hydrophilic protopanaxadiol ginsenosides Rb1, Rb2, and Rc as principal bioactive constituents. When ginseng is administered orally, portions of these hydrophilic protopanaxadiol ginsenosides are metabolized to ginsenosides Rd, F2, and CK by the gut microbiota. A variety of gut bacteria that belong to Bifidobacterium, Eubacterium, Fusobacterium, and Bacterodies are known to be involved in the biotransformation of ginsenosides (Fig. 1) [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22]. When fresh ginseng is steamed to prepare RG, portions of ginsenosides Rb1, Rb2, and Rc are transformed to ginsenoside Rg3 (Fig. 1) [17], [20], [21], [22], [34]. The FRG was prepared by probiotic fermentation with Bifidobacterium lactis HY8002 and Bifidobacterium adolescentis HY8502 [20]. Thus, microbial metabolism facilitated the biotransformation of the polar ginsenosides to ginsenoside Rd and consequently enriched the ginsenoside Rd contents when compared with RG.
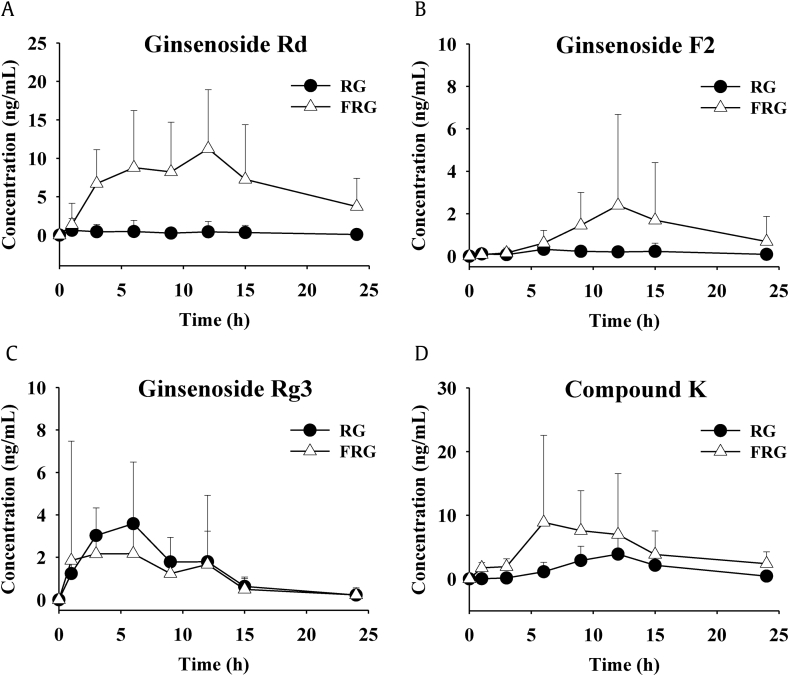
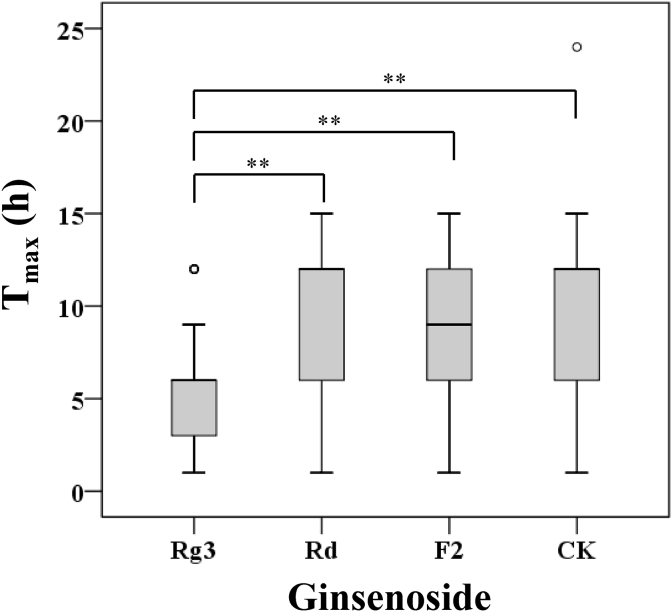
The mean serum concentration–time curves of ginsenosides Rd, F2, Rg3, and CK are presented in Fig. 2, and the pharmacokinetic parameters are summarized in Table 1. The Cmax values of Rd, F2, Rg3, and CK were 1.77 ± 2.09 ng/mL, 0.68 ± 0.32 ng/mL, 4.32 ± 2.81 ng/mL, and 4.57 ± 3.16 ng/mL, respectively, for the RG group. The AUC∞ values of Rd, F2, Rg3, and CK were 8.20 ± 11.95 ng·h/mL, 4.54 ± 3.70 ng·h/mL, 36.40 ± 19.68 ng·h/mL, and 40.30 ± 29.83 ng·h/mL, respectively, for the RG group. For the FRG group, the Cmax values of Rd, F2, Rg3, and CK were 16.69 ± 6.95 ng/mL, 2.79 ± 4.29 ng/mL, 4.83 ± 5.92 ng/mL, and 13.93 ± 15.17 ng/mL, respectively. The AUC∞ values of Rd, F2, Rg3, and CK for the FRG group were 187.90 ± 95.87 ng·h/mL, 30.24 ± 41.87 ng·h/mL, 28.68 ± 14.27 ng·h/mL, and 137.01 ± 96.16 ng·h/mL, respectively. The extract contents and AUC values of each ginsenoside are presented in Fig. 3. The range of Tmax values of each ginsenoside for all the participants is depicted in Fig. 4. The mean Tmax value of Rg3 was 5.69 ± 3.38 h, and the mean Tmax values of Rd, F2, and CK were 9.41 ± 4.42 h, 9.32 ± 3.38 h, and 10.35 ± 4.06 h, respectively. Statistically significant differences in the pharmacokinetic parameters between the men and women were not observed in either group.
Fig. 2.
Mean serum concentration-time profiles of ginsenosides in health volunteers after oral administration of RG and FRG. (A) Ginsenoside Rd, (B) ginsenoside F2, (C) ginsenoside Rg3, and (D) compound K. FRG, fermented red ginseng; RG, red ginseng.
Table 1.
Pharmacokinetic parameters of ginsenosides Rd, F2, Rg3, and CK in healthy volunteers orally treated with RG or FRG
| Ginsenoside | Parameter | RG group (n = 17) |
FRG group (n = 18) |
||||
|---|---|---|---|---|---|---|---|
| Total (n = 17) | Male (n = 13) | Female (n = 4) | Total (n = 18) | Male (n = 13) | Female (n = 5) | ||
| Rd | Cmax (ng/mL) | 1.77 ± 2.09 | 1.53 ± 2.18 | 2.55 ± 1.81 | 16.69 ± 6.95 | 17.52 ± 6.58 | 14.53 ± 8.18 |
| AUCt (ng·h/mL) | 7.85 ± 11.24 | 6.93 ± 11.39 | 10.83 ± 11.78 | 163.82 ± 77.56 | 167.83 ± 75.03 | 153.38 ± 92.12 | |
| AUC∞(ng·h/mL) | 8.20 ± 11.95 | 6.93 ± 11.39 | 12.30 ± 14.60 | 187.90 ± 95.87 | 190.75 ± 101.98 | 180.48 ± 88.12 | |
| Tmax (h) | 15.12 ± 9.35 | 6.67 ± 4.55 | 8.33 ± 7.02 | 10.50 ± 3.88 | 9.92 ± 3.33 | 12.00 ± 5.20 | |
| F2 | Cmax (ng/mL) | 0.68 ± 0.32 | 0.57 ± 0.22 | 1.03 ± 0.39 | 2.79 ± 4.29 | 3.09 ± 5.03 | 2.01 ± 1.14 |
| AUCt (ng·h/mL) | 4.33 ± 3.25 | 2.98 ± 2.01 | 8.70 ± 2.61 | 27.17 ± 37.25 | 29.17 ± 43.24 | 21.98 ± 15.51 | |
| AUC∞(ng·h/mL) | 4.54 ± 3.70 | 3.08 ± 2.24 | 9.28 ± 3.71 | 30.24 ± 41.87 | 32.98 ± 48.59 | 23.13 ± 16.68 | |
| Tmax (h) | 8.76 ± 5.52 | 6.85 ± 3.18 | 12.75 ± 4.50 | 10.83 ± 3.28 | 10.15 ± 3.58 | 10.80 ± 2.68 | |
| Rg3 | Cmax (ng/mL) | 4.32 ± 2.81 | 4.43 ± 3.02 | 3.97 ± 2.33 | 4.83 ± 5.92 | 5.83 ± 6.74 | 2.25 ± 0.96 |
| AUCt (ng·h/mL) | 35.63 ± 18.69 | 35.27 ± 20.73 | 36.80 ± 11.86 | 27.34 ± 14.11 | 31.04 ± 14.97 | 17.71 ± 3.72 | |
| AUC∞(ng·h/mL) | 36.40 ± 19.68 | 36.14 ± 21.88 | 37.23 ± 12.22 | 28.68 ± 14.27 | 32.24 ± 14.97 | 19.41 ± 6.72 | |
| Tmax (h) | 6.00 ± 3.52 | 5.54 ± 3.84 | 7.50 ± 1.73 | 5.39 ± 3.42 | 5.85 ± 3.85 | 4.20 ± 1.64 | |
| CK | Cmax (ng/mL) | 4.57 ± 3.16 | 3.80 ± 2.98 | 7.09 ± 2.58 | 13.93 ± 15.17 | 9.99 ± 10.94 | 24.18 ± 20.91 |
| AUCt (ng·h/mL) | 39.04 ± 28.85 | 30.49 ± 25.60 | 66.83 ± 21.72 | 111.54 ± 84.70 | 94.80 ± 76.77 | 155.04 ± 97.63 | |
| AUC∞(ng·h/mL) | 40.30 ± 29.83 | 31.31 ± 26.33 | 69.52 ± 21.98 | 137.01 ± 96.16 | 128.10 ± 97.96 | 160.20 ± 97.89 | |
| Tmax (h) | 10.69 ± 2.89 | 10.50 ± 3.00 | 11.25 ± 2.87 | 10.06 ± 5.03 | 10.92 ± 5.66 | 7.80 ± 1.64 | |
AUC∞, the area under curve from zero to infinity; AUCt, the area under curve from zero to the last time point; CK, compound K; Cmax, maximum serum concentration; FRG, fermented red ginseng; RG, red ginseng; Tmax, the time taken to reach maximum
Fig. 3.
Contents of ginsenosides in extracts and their AUCs in PK studies. (A) Contents of ginsenosides in RG and FRG extracts; (B) AUCs of ginsenosides in the RG- and FRG-treated groups. AUC, area under the serum concentration–time curve; FRG, fermented red ginseng; RG, red ginseng.
Fig. 4.
Box plot of Tmax for ginsenosides Rg3, Rd, F2, and CK in all participants. **P < 0.01. The circle represents the outlier data points. CK, compound K; Tmax, the time taken to reach maximum.
After the RG or FRG intake, the mean AUC values of ginsenosides Rd, F2, and CK in the FRG group were 187.90 ± 95.87 ng·h/mL, 30.24 ± 41.87 ng·h/mL, and 137.01 ± 96.16 ng·h/mL, respectively, and those in the RG group were 8.20 ± 11.95 ng·h/mL, 4.54 ± 3.70 ng·h/mL, and 40.30 ± 29.83 ng·h/mL, respectively, which were significantly higher than those in the RG group, even when considering the differences in the ginsenoside contents of each extract. The ginsenoside Rd amount in the FRG group (11.84 mg/g) was approximately 3.5 times higher than that in the RG group (3.24 mg/g), but the mean AUC value of ginsenoside Rd in the FRG group was more than 20 times higher than that in the RG group. In addition, the contents of ginsenosides F2 and CK were very low and comparable in RG and FRG extracts. However, the AUC values of ginsenosides F2 and CK in the FRG group were more than six and three times higher, respectively, than those in the RG group. The greater ginsenoside Rd absorption in the FRG group suggests the additional formation of ginsenoside Rd, possibly from other polar ginsenosides after the FRG intake. The FRG that was administered may have contained some of the probiotics that were used for the fermentation. The probiotics may not have been alive, but they could have acted as prebiotics. Thus, probiotics may activate the metabolic activity of the gut microbiota to promote the formation of ginsenoside Rd. In turn, this increase in the ginsenoside Rd formation may have enhanced the biotransformation into ginsenosides F2 and CK. These sequential reactions may have resulted in the increase in the nonpolar ginsenosides in the FRG group.
There was no difference in the AUC values of ginsenoside Rg3 between the two groups. As shown in Fig. 1, ginsenoside Rg3 is known to be generated by heat processing, such as steaming, rather than microbial biotransformation. The data from the FRG group revealed that ginsenoside Rg3 was not likely generated from ginsenoside Rd or the other polar ginsenosides via the gut microbiota enzymes. The Tmax profiles of Rg3 also support this. When ginseng is taken orally, the polar ginsenosides are metabolized to hydrophobic ginsenosides by the gut microbiota, and then, they are absorbed into the blood. Consequently, it should take longer for the hydrophobic ginsenosides to be absorbed. According to Fig. 4, the Tmax values of Rd, F2, and CK reached mostly no sooner than 6 h after the dosing. This indicates that Rd, F2, and CK were generated by gut metabolism, and then, they were absorbed, rather than being absorbed directly from the ginseng extracts that were taken. However, the Tmax values of Rg3 were somewhat earlier (approximately 3 to 6 h) than those of the other three ginsenosides. This shows that Rg3 was mainly absorbed directly from the ginseng extracts that were taken. The portion of Rg3 that was absorbed after the biotransformation of the polar ginsenosides into Rg3 by the gut microbiota metabolism was assumed to be minimal. Actually, studies have mainly reported that polar ginsenosides including ginsenoside Rb1 are transformed into CK by the intestinal microflora [17], [34], [42], [43], whereas the metabolism into ginsenoside Rg3 by gut microbiota was not demonstrated. According to Kong et al, the deglycosylation of ginsenosides by gut microbiota mainly occurs at the, C3 position whereas the deglycosylation under acidic conditions takes place at the C20 position. These observations may partially support our deduction mentioned previously [44].
3.4. Fecal ginsenoside Rd– and CK–forming activities
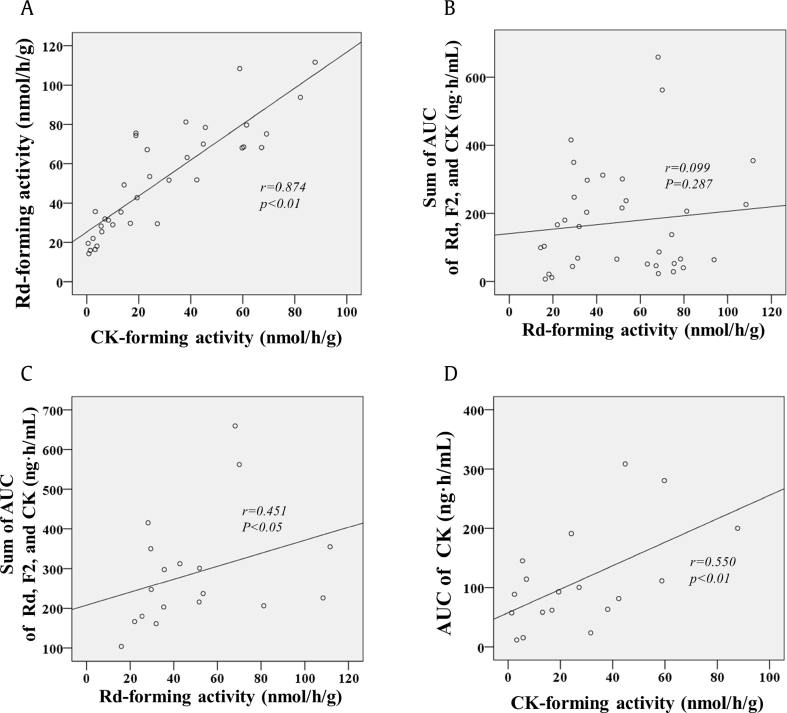
To understand the relationships between the serum concentration levels of the ginsenosides and the ginsenoside-metabolizing activities of the gut microbiota, we measured the fecal ginsenoside Rd– and CK–forming activities of the study participants (Table 2). The ginsenoside Rd–forming activity was 51.65 ± 27.24 nmol/g/h in all the participants; it was 46.91 ± 26.15 nmol/g/h in the men and 66.15 ± 24.9 nmol/g/h in the women. The CK-forming activity was 29.02 ± 25.70 nmol/g/h in all the participants; it was 26.51 ± 27.22 nmol/g/h in the men and 36.25 ± 17.90 nmol/g/h in the women. Significant differences were not seen in the fecal ginsenoside Rd– and CK–forming activities between the men and women. The fecal CK-forming activities of all the study participants were generally proportional to the Rd-forming activities (Fig. 5A).
Table 2.
The fecal ginsenoside–metabolizing activities of healthy volunteers
| Group | Gender | Ginsenoside-metabolizing activity (nmol/g/h) |
|
|---|---|---|---|
| Rd-forming activity | CK-forming activity | ||
| RG | Male | 51.48 ± 28.36 | 30.25 ± 29.75 |
| Female | 63.18 ± 21.62 | 33.30 ± 23.90 | |
| Total | 54.23 26.78 | 30.97 ± 27.80 | |
| FRG | Male | 42.33 ± 25.14 | 22.77 ± 26.27 |
| Female | 68.53 ± 29.60 | 38.61 ± 14.02 | |
| Total | 49.61 ± 54.23 | 27.17 ± 24.22 | |
| All participants | 51.65 ± 27.24 | 29.02 ± 25.70 | |
CK, compound K; FRG, fermented red ginseng; RG, red ginseng
Fig. 5.
Correlations between the fecal ginsenoside metabolizing activities and the AUCs of the related ginsenosides. (A) Correlation between fecal CK-forming activities and fecal Rd-forming activities in whole subjects; (B) Correlation between the fecal Rd-forming activities and the sum of AUCs of ginsenosides Rd, F2, and CK in whole subjects; (C) Correlation between the fecal Rd-forming activities and the sum of AUCs of ginsenosides Rd, F2, and CK in the FRG-treated group; (D) Correlation between CK-forming activity and the AUC of ginsenoside CK in the FRG-treated group. AUC, area under the serum concentration–time curve; CK, compound K; FRG, fermented red ginseng.
Subsequently, the relationship between the fecal ginsenoside Rd–forming activities and plasma exposure of ginsenoside Rd was investigated. As ginsenoside Rd can be further metabolized to ginsenosides F2 and CK, the plasma exposure was evaluated as the sum of the AUC values of ginsenosides Rd, F2, and CK. The fecal ginsenoside Rd–forming activities did not show a correlation with the sum of the AUC values of ginsenosides Rd, F2, and CK in the whole data set containing both RG and FRG groups (Fig. 5B). However, when data set was confined to the FRG group, the correlation between the fecal Rd-forming activity and the AUC values of ginsenosides Rd, F2, and CK was statistically significant (r = 0.451, p < 0.05) (Fig. 5C). This may be because FRG is higher in ginsenoside Rd content, and the correlation regarding ginsenoside Rd can be shown more clearly in the FRG-treated group. In addition, the AUC value of CK showed an obvious positive correlation (r = 0.550, p < 0.01) with the fecal CK-forming activity in the FRG group (Fig. 5D).
For the ginsenosides, metabolic activities of the gut microbiota vary widely among individuals [27]. Our data on the fecal ginsenoside–metabolizing activities also support such interindividual differences; the fecal Rd- and CK-forming activities showed considerable variations among the individuals (Fig. 5). Meanwhile, the fecal Rd-forming activity and the fecal CK-forming activity showed a good correlation (Fig. 5A), which indicates that the gut microbiota involved in the formation of Rd may also have acted on the formation of CK. No significant correlations were observed between the fecal metabolizing activities and the pharmacokinetic profiles of the nonpolar ginsenosides in all participants. However, when the analysis data were confined to the FRG group, a significant positive correlation was found. Furthermore, the correlation between the fecal CK-forming activity and the AUC value of CK was more obvious. This may be because biotransformation occurred more actively in the FRG group and the AUC values of Rd, F2, and CK were high enough to measure the correlations as discussed previously. These findings suggest that the pharmacokinetics of ginsenosides Rd, F2, and CK may be affected by the profiles of the gut microbiota.
Several articles reporting the pharmacokinetics of RG in humans have been published [23], [24], [25], [26], [27], [28]. Most of these articles focused on the pharmacokinetic profiles of ginsenoside CK. According to these articles, fermented ginseng, in general, showed higher serum ginsenoside CK levels than the traditional RG [20], [23], [24]. Nevertheless, these findings have some limitations in explaining how the gut microbiota affects the pharmacokinetics of the ginsenosides, including ginsenoside CK. However, the present study demonstrated the role of the gut microbiota on the pharmacokinetics of the ginsenosides by analyzing the pharmacokinetic profiles of a series of the relevant ginsenoside metabolites and characterizing the relationships between the metabolizing activities of the gut microbiota and the ginsenoside serum levels.
4. Conclusion
In this study, we investigated the pharmacokinetic profiles of protopanaxadiol ginsenosides in humans after the administration of RG extracts (RG and FRG), and then, we analyzed their correlations with the fecal ginsenoside–metabolizing activities. The enzymes of the gut bacteria seemed to exert their metabolic activity mainly on the biotransformation into ginsenoside CK via ginsenoside Rd rather than ginsenoside Rg3. The fecal ginsenoside–metabolizing activities showed positive correlations with the serum levels of the transformed ginsenosides including ginsenoside CK. These findings suggest that the profile and composition of the gut microbiota may affect the bioavailability and, consequently, the pharmacological effects of ginsenosides.
Conflicts of interest
The authors declare that they have no conflict of interest.
Acknowledgments
This work was supported by the National Research Foundation of Korea (2017R1A2B4001814) and (2017R1A5A2014768).
Contributor Information
Hye Hyun Yoo, Email: yoohh@hanyang.ac.kr.
Dong-Hyun Kim, Email: dhkim@khu.ac.kr.
References
- 1.Christensen L.P. Ginsenosides chemistry, biosynthesis, analysis, and potential health effects. Adv Food Nutr Res. 2009;55:1–99. doi: 10.1016/S1043-4526(08)00401-4. [DOI] [PubMed] [Google Scholar]
- 2.Kim K.H., Lee D., Lee H.L., Kim C.E., Jung K., Kang K.S. Beneficial effects of Panax ginseng for the treatment and prevention of neurodegenerative diseases: past findings and future directions. J Ginseng Res. 2018;42:239–247. doi: 10.1016/j.jgr.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li C.P., Li R.C. An introductory note to ginseng. Am J Chin Med. 1973;1:249–261. doi: 10.1142/s0192415x73000279. [DOI] [PubMed] [Google Scholar]
- 4.Kasai R., Besso H., Tanaka O., Saruwatari Y., Fuwa T. Saponins of red ginseng. Chem Pharm Bull. 1983;31:2120–2125. [Google Scholar]
- 5.Lee S.M., Bae B.S., Park H.W., Ahn N.G., Cho B.G., Cho Y.L., Kwak Y.S. Characterization of Korean red ginseng (Panax ginseng Meyer): history, preparation method, and chemical composition. J Ginseng Res. 2015;39:384–391. doi: 10.1016/j.jgr.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Attele A.S., Wu J.A., Yuan C.S. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 7.Chang Y.S., Seo E.K., Gyllenhaal C., Block K.I. Panax ginseng: a role in cancer therapy? Integr Cancer Ther. 2003;2:13–33. doi: 10.1177/1534735403251167. [DOI] [PubMed] [Google Scholar]
- 8.Helms S. Cancer prevention and therapeutics: Panax ginseng. Altern Med Rev. 2004;9:259–274. [PubMed] [Google Scholar]
- 9.Joh E.H., Lee I.A., Jung I.H., Kim D.H. Ginsenoside Rb1 and its metabolite compound K inhibit IRAK-1 activation--the key step of inflammation. Biochem Pharmacol. 2011;82:278–286. doi: 10.1016/j.bcp.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Kim D.H., Chung J.H., Yoon J.S., Ha Y.M., Bae S., Lee E.K., Jung K.J., Kim M.S., Kim Y.J., Kim M.K. Ginsenoside Rd inhibits the expressions of iNOS and COX-2 by suppressing NF-kappaB in LPS-stimulated RAW264.7 cells and mouse liver. J Ginseng Res. 2013;37:54–63. doi: 10.5142/jgr.2013.37.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yokozawa T., Kobayashi T., Oura H., Kawashima Y. Studies on the mechanism of the hypoglycemic activity of ginsenoside-Rb2 in streptozotocin-diabetic rats. Chem Pharm Bull. 1985;33:869–872. doi: 10.1248/cpb.33.869. [DOI] [PubMed] [Google Scholar]
- 12.Bae E.A., Park S.Y., Kim D.H. Constitutive beta-glucosidases hydrolyzing ginsenoside Rb1 and Rb2 from human intestinal bacteria. Biol Pharm Bull. 2000;23:1481–1485. doi: 10.1248/bpb.23.1481. [DOI] [PubMed] [Google Scholar]
- 13.Bae E.A., Han M.J., Choo M.K., Park S.Y., Kim D.H. Metabolism of 20(S)- and 20(R)-ginsenoside Rg3 by human intestinal bacteria and its relation to in vitro biological activities. Biol Pharm Bull. 2002;25:58–63. doi: 10.1248/bpb.25.58. [DOI] [PubMed] [Google Scholar]
- 14.Bae E.A., Choo M.K., Park E.K., Park S.Y., Shin H.Y., Kim D.H. Metabolism of ginsenoside R(c) by human intestinal bacteria and its related antiallergic activity. Biol Pharm Bull. 2002;25:743–747. doi: 10.1248/bpb.25.743. [DOI] [PubMed] [Google Scholar]
- 15.Choi J.R., Hong S.W., Kim Y., Jang S.E., Kim N.J., Han M.J., Kim D.H. Metabolic activities of ginseng and its constituents, ginsenoside rb1 and rg1, by human intestinal microflora. J Ginseng Res. 2011;35:301–307. doi: 10.5142/jgr.2011.35.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jung I.H., Lee J.H., Hyun Y.J., Kim D.H. Metabolism of ginsenoside Rb1 by human intestinal microflora and cloning of its metabolizing beta-D-glucosidase from Bifidobacterium longum H-1. Biol Pharm Bull. 2012;35:573–581. doi: 10.1248/bpb.35.573. [DOI] [PubMed] [Google Scholar]
- 17.Kim D.H. Metabolism of ginsenosides to bioactive compounds by intestinal microflora and its industrial application. J Ginseng Res. 2009;33:165–176. [Google Scholar]
- 18.Kim U., Park M.H., Kim D.H., Yoo H.H. Metabolite profiling of ginsenoside Re in rat urine and faeces after oral administration. Food Chem. 2013;136:1364–1369. doi: 10.1016/j.foodchem.2012.09.050. [DOI] [PubMed] [Google Scholar]
- 19.Kim K.A., Jung I.H., Park S.H., Ahn Y.T., Huh C.S., Kim D.H. Comparative analysis of the gut microbiota in people with different levels of ginsenoside Rb1 degradation to compound K. PLoS One. 2013;8:e62409. doi: 10.1371/journal.pone.0062409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim J.K., Kim J.Y., Jang S.E., Choi M.S., Jang H.M., Yoo H.H., Kim D.H. Fermented red ginseng alleviates cyclophosphamide-induced immunosuppression and 2,4,6-trinitrobenzenesulfonic acid-induced colitis in mice by regulating macrophage activation and T cell differentiation. Am J Chin Med. 2018;46:1879–1897. doi: 10.1142/S0192415X18500945. [DOI] [PubMed] [Google Scholar]
- 21.Kim D.H. Gut microbiota-mediated pharmacokinetics of ginseng saponins. J Ginseng Res. 2018;42:255–263. doi: 10.1016/j.jgr.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H.Y., Hua H.Y., Liu X.Y., Liu J.H., Yu B.Y. In vitro biotransformation of red ginseng extract by human intestinal microflora: metabolites identification and metabolic profile elucidation using LC-Q-TOF/MS. J Pharm Biomed Anal. 2014;98:296–306. doi: 10.1016/j.jpba.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Choi I.D., Ryu J.H., Lee D.E., Lee M.H., Shim J.J., Ahn Y.T., Sim J.H., Huh C.S., Shim W.S., Yim S.V. Enhanced absorption study of ginsenoside compound K (20-O-beta-(D-Glucopyranosyl)-20(S)-protopanaxadiol) after oral administration of fermented red ginseng extract (HYFRG) in healthy Korean volunteers and rats. Evid Based Complement Alternat Med. 2016;2016:3908142. doi: 10.1155/2016/3908142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin H., Seo J.H., Uhm Y.K., Jung C.Y., Lee S.K., Yim S.V. Pharmacokinetic comparison of ginsenoside metabolite IH-901 from fermented and non-fermented ginseng in healthy Korean volunteers. J Ethnopharmacol. 2012;139:664–667. doi: 10.1016/j.jep.2011.11.052. [DOI] [PubMed] [Google Scholar]
- 25.Kim H.K. Pharmacokinetics of ginsenoside Rb1 and its metabolite compound K after oral administration of Korean Red Ginseng extract. J Ginseng Res. 2013;37:451–456. doi: 10.5142/jgr.2013.37.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim J.S., Kim Y., Han S.H., Jeon J.Y., Hwang M., Im Y.J., Kim J.H., Lee S.Y., Chae S.W., Kim M.G. Development and validation of an LC-MS/MS method for determination of compound K in human plasma and clinical application. J Ginseng Res. 2013;37:135–141. doi: 10.5142/jgr.2013.37.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee J., Lee E., Kim D., Lee J., Yoo J., Koh B. Studies on absorption, distribution and metabolism of ginseng in humans after oral administration. J Ethnopharmacol. 2009;122:143–148. doi: 10.1016/j.jep.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 28.Tawab M.A., Bahr U., Karas M., Wurglics M., Schubert-Zsilavecz M. Degradation of ginsenosides in humans after oral administration. Drug Metab Dispos. 2003;31:1065–1071. doi: 10.1124/dmd.31.8.1065. [DOI] [PubMed] [Google Scholar]
- 29.Saba E., Lee Y.Y., Kim M., Kim S.H., Hong S.B., Rhee M.H. A comparative study on immune-stimulatory and antioxidant activities of various types of ginseng extracts in murine and rodent models. Journal of Ginseng Research. 2018;42:577–584. doi: 10.1016/j.jgr.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang X.D., Yang Y.Y., Ouyang D.S., Yang G.P. A review of biotransformation and pharmacology of ginsenoside compound K. Fitoterapia. 2015;100:208–220. doi: 10.1016/j.fitote.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 31.Bae H.W., Kim J.H., Kim S., Kim M., Lee N., Hong S., Seong G.J., Kim C.Y. Effect of Korean Red Ginseng supplementation on dry eye syndrome in glaucoma patients - a randomized, double-blind, placebo-controlled study. J Ginseng Res. 2015;39:7–13. doi: 10.1016/j.jgr.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shin B.K., Park H.Y., Han J. Enzymatic biotransformation of red ginseng and the compositional change of ginsenosides. J Korean Soc Appl Bi. 2010;53:553–558. [Google Scholar]
- 33.Trinh H.T., Han S.J., Kim S.W., Lee Y.C., Kim D.H. Bifidus fermentation increases hypolipidemic and hypoglycemic effects of red ginseng. J Microbiol Biotechnol. 2007;17:1127–1133. [PubMed] [Google Scholar]
- 34.Wang C.Z., Li B.H., Wen X.D., Zhang Z.Y., Yu C.H., Calway T.D., He T.C., Du W., Yuan C.S. Paraptosis and NF-kappa B activation are associated with protopanaxadiol-induced cancer chemoprevention. Bmc Complem Altern M. 2013;13 doi: 10.1186/1472-6882-13-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akao T., Kanaoka M., Kobashi K. Appearance of compound K, a major metabolite of ginsenoside Rb1 by intestinal bacteria, in rat plasma after oral administration--measurement of compound K by enzyme immunoassay. Biol Pharm Bull. 1998;21:245–249. doi: 10.1248/bpb.21.245. [DOI] [PubMed] [Google Scholar]
- 36.Chen L., Zhou L., Huang J., Wang Y., Yang G., Tan Z., Wang Y., Zhou G., Liao J., Ouyang D. Single- and multiple-dose trials to determine the pharmacokinetics, safety, tolerability, and sex effect of oral ginsenoside compound K in healthy Chinese volunteers. Front Pharmacol. 2017;8:965. doi: 10.3389/fphar.2017.00965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen L., Zhou L., Wang Y., Yang G., Huang J., Tan Z., Wang Y., Zhou G., Liao J., Ouyang D. Food and sex-related impacts on the pharmacokinetics of a single-dose of ginsenoside compound K in healthy subjects. Front Pharmacol. 2017;8:636. doi: 10.3389/fphar.2017.00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen J., Li M., Chen L., Wang Y., Li S., Zhang Y., Zhang L., Song M., Liu C., Hua M. Effects of processing method on the pharmacokinetics and tissue distribution of orally administered ginseng. J Ginseng Res. 2018;42:27–34. doi: 10.1016/j.jgr.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim K.A., Yoo H.H., Gu W., Yu D.H., Jin M.J., Choi H.L., Yuan K., Guerin-Deremaux L., Kim D.H. Effect of a soluble prebiotic fiber, NUTRIOSE, on the absorption of ginsenoside Rd in rats orally administered ginseng. J Ginseng Res. 2014;38:203–207. doi: 10.1016/j.jgr.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim K.A., Yoo H.H., Gu W., Yu D.H., Jin M.J., Choi H.L., Yuan K., Guerin-Deremaux L., Kim D.H. A prebiotic fiber increases the formation and subsequent absorption of compound K following oral administration of ginseng in rats. J Ginseng Res. 2015;39:183–187. doi: 10.1016/j.jgr.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mancuso C., Santangelo R. Panax ginseng and Panax quinquefolius: from pharmacology to toxicology. Food Chem Toxicol. 2017;107:362–372. doi: 10.1016/j.fct.2017.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Santangelo R., Silvestrini A., Mancuso C. Ginsenosides, catechins, quercetin and gut microbiota: current evidence of challenging interactions. Food Chem Toxicol. 2019;123:42–49. doi: 10.1016/j.fct.2018.10.042. [DOI] [PubMed] [Google Scholar]
- 43.Zhang R., Huang X.M., Yan H.J., Liu X.Y., Zhou Q., Luo Z.Y., Tan X.N., Zhang B.L. Highly selective production of compound K from ginsenoside Rd by hydrolyzing glucose at C-3 glycoside using beta-glucosidase of Bifidobacterium breve ATCC 15700. J Microbiol Biotechnol. 2019;29:410–418. doi: 10.4014/jmb.1808.08059. [DOI] [PubMed] [Google Scholar]
- 44.Kong H., Wang M., Venema K., Maathuis A., van der Heijden R., van der Greef J., Xu G., Hankemeier T. Bioconversion of red ginseng saponins in the gastro-intestinal tract in vitro model studied by high-performance liquid chromatography-high resolution Fourier transform ion cyclotron resonance mass spectrometry. J Chromatogr A. 2009;1216:2195–2203. doi: 10.1016/j.chroma.2008.11.030. [DOI] [PubMed] [Google Scholar]