Abstract
Background
Ginsenoside compound-Mc1 (Mc1) is a member of the deglycosylated ginsenosides obtained from ginseng extract. Although several ginsenosides have a cardioprotective effect, this has not been demonstrated in ginsenoside Mc1.
Methods
We treated H9c2 cells with hydrogen peroxide (H2O2) and ginsenoside Mc1 to evaluate the antioxidant effects of Mc1. The levels of antioxidant molecules, catalase, and superoxide dismutase 2 (SOD2) were measured, and cell viability was determined using the Bcl2-associated X protein (Bax):B-cell lymphoma-extra large ratio, a cytotoxicity assay, and flow cytometry. We generated mice with high-fat diet (HFD)–induced obesity using ginsenoside Mc1 and assessed their heart tissues to evaluate the antioxidant effect and the fibrosis-reducing capability of ginsenoside Mc1.
Results
Ginsenoside Mc1 significantly increased the level of phosphorylated AMP-activated protein kinase (AMPK) in the H9c2 cells. The expression levels of catalase and SOD2 increased significantly after treatment with ginsenoside Mc1, resulting in a decrease in the production of H2O2-mediated reactive oxygen species. Treatment with ginsenoside Mc1 also significantly reduced the H2O2-mediated elevation of the Bax:Bcl2 ratio and the number of DNA-damaged cells, which was significantly attenuated by treatment with an AMPK inhibitor. Consistent with the in vitro data, ginsenoside Mc1 upregulated the levels of catalase and SOD2 and decreased the Bax:B-cell lymphoma-extra large ratio and caspase-3 activity in the heart tissues of HFD-induced obese mice, resulting in reduced collagen deposition.
Conclusion
Ginsenoside Mc1 decreases oxidative stress and increases cell viability in H9c2 cells and the heart tissue isolated from HFD-fed mice via an AMPK-dependent mechanism, suggesting its potential as a novel therapeutic agent for oxidative stress–related cardiac diseases.
Keywords: AMP-activated protein kinases, Antioxidant, Cardiomyocyte
1. Introduction
Oxidative stress in cardiomyocytes plays a critical role in cardiovascular diseases (CVDs) such as coronary artery disease, hypertensive and diabetic cardiomyopathy, and congestive heart failure [1], [2], [3], [4]. Increased levels of reactive oxygen species (ROS) activate several hypertrophy-signaling kinases and transcription factors that induce apoptosis by damaging DNA and mitochondria [5], [6]. Cardiac cell death promotes changes in the extracellular matrix composition of cardiac tissue, leading to increased cardiac fibrosis, inflammation, and eventually cardiac dysfunction [7]. No effective therapeutic strategies are currently available to reduce oxidative stress in cardiomyocytes and thereby to prevent CVD.
Ginseng has been widely used in Asia as a traditional herbal medication for thousands of years. There is growing evidence that ginseng has beneficial effects against diabetes, obesity, stroke, and CVDs [8], [9], [10], [11]. Within the published research on the whole extract of ginseng, numerous studies have focused on specific chemical compounds derived from ginseng extracts and their biological properties [12]. Ginsenosides (more than 150 have been isolated) are the main constituents of ginseng extract, for which antioxidant effects have been widely reported [13], [14], [15], [16]. With regard to cardiomyocytes, ginsenoside Re significantly inhibited both exogenous and endogenous oxidative stress in chick embryonic ventricular myocytes [14]. Ginsenoside Rb1 protects cardiomyocytes from oxidative injury by acting as a radical scavenger and attenuating mitochondrial ROS generation in a process mediated by the c-Jun N-terminal kinase pathway [17]. Ginsenosides Re and Rb1 are glycosylated ginsenosides, as are more than 80% of all the ginsenosides in wild ginseng [18]. Glycosylated ginsenosides have low absorption rates and low bioavailability. In contrast, owing to their smaller size and greater ability to permeate across cell membranes, deglycosylated ginsenosides are more pharmacologically active [19]. Diverse deglycosylated ginsenosides can be obtained by highly selective hydrolysis of the sugar moieties of ginsenosides. Ginsenoside compound-Mc1 (Mc1) is a newly identified deglycosylated ginsenoside that is converted from the major ginsenoside Rc by cloned ginsenosidase [20]. Until now, the antioxidant effects of ginsenoside Mc1 have not been studied or observed in an animal model. Furthermore, recent evidence has shown that antioxidant effects of ginsenosides occur through an AMP-activated protein kinase (AMPK)-dependent pathway in the liver and skeletal muscle [21], [22], [23]; however, that mechanism has not been investigated in cardiomyocytes or heart tissue.
Therefore, to examine the potential protective effects of ginsenoside Mc1 in cardiomyocytes, we investigated the following: (i) whether ginsenoside Mc1 induces AMPK phosphorylation; (ii) whether ginsenoside Mc1 reduces ROS production and increases cell viability by stimulating the production of antioxidants in H9c2 cardiomyocytes; and (iii) whether ginsenoside Mc1 inhibits collagen deposition and cardiac fibrosis in heart tissue obtained from C57BL/6 mice.
2. Materials and methods
2.1. Cell culture and compounds
We obtained H9c2 cells from the Korean Cell Line Bank (Seoul, Korea) and cultured them in Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, CA, USA), containing 10% (v/v) fetal bovine serum (Invitrogen), 50 U/mL penicillin, and 50 g/mL streptomycin (Invitrogen), at 37°C and at 5% CO2. The cells were subcultured in 24- or 96-well plates until they exceeded 90% confluence and were then incubated with ginsenoside Mc1 (Ambo Institute, Seoul, Korea) and other additives. Ginsenoside Mc1 was prepared at the Ambo Institute by the deglycosylation of ginsenoside Rc using beta galactosidase from Aspergillus oryzae (Sigma-Aldrich, MO, USA) in 0.1 M acetate buffer (pH 4.5) at 40°C [24]. In more detail, one gram of β-galactosidase from A. oryzae(Sigma-Aldrich) was dissolved with ginsenoside Rc (2 g) in 20 mL methanol plus 200 mL of 0.1M acetate buffer. The mixture was incubated at 40°C for 36 hours. The reaction mixture was extracted with n-butanol and then was chromatographed over silica gel to collect the ginsenoside Mc1 fraction. The ginsenoside Mc1 fraction was chromatographed over the RiChroprep RP18 column (Merck Millipore, MA, USA), and the purity of ginsenoside Mc1 was found to be 98.95% by HPLC analysis (Supplementary Fig. S1). Hydrogen peroxide (H2O2; Sigma-Aldrich) was used to induce oxidative stress. Ginsenoside Mc1, compound C (AMPK inhibitor; Sigma-Aldrich), and N-acetylcysteine (NAC; Cayman Chemical, MI, USA) were dissolved in dimethyl sulfoxide (Sigma-Aldrich). NAC, a well-known antioxidant, was used as a positive control to determine the antioxidant effect of ginsenoside Mc1 [25]. All the H9c2 cells (control group, H2O2-treated group, ginsenoside Mc1-treated group, ginsenoside Mc1 plus compound C–treated group, and NAC-treated group) received the same concentration (<0.1%) of the dissolving solution (dimethyl sulfoxide) to ensure the suitability of the H2O2-treated H9c2 cells as a negative control group.
2.2. Western blotting
We extracted total protein using PRO-PREP™ solution (iNtRON, Sungman-si, Korea) and quantified it using the Bradford assay (Bio-Rad Laboratories, CA, USA). Equal amounts of proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (Amersham Biosciences, Westborough, MA, USA). The nitrocellulose membranes were incubated sequentially with blocking solution [0.05% TBST (a mixture of Tris-buffered saline and Tween 20) containing 5% nonfat dry milk or 5% bovine serum albumin], blocking solution plus primary antibodies, and blocking solution plus horseradish peroxidase–conjugated secondary antibodies. Immunoreactive bands were detected in the dark using a chemiluminescence solution (Bio-Rad Laboratories, CA, USA), after which band density was determined using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
We used the following antibodies in the experiments: anti–beta actin mouse monoclonal IgG antibody (1:5,000 dilution), anti–beta actin rabbit polyclonal IgG antibody (1:1,000 dilution), anti–catalase mouse monoclonal IgG antibody (1:1,000 dilution), anti–superoxide dismutase 2 (SOD2) mouse monoclonal IgG antibody (1:500 dilution), anti–collagen type I alpha I mouse monoclonal antibody (1:1,000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti–phosphorylated AMPK rabbit monoclonal antibody (1:1,000 dilution), anti–total AMPK rabbit monoclonal antibody (1:2,000 dilution), anti–Bcl2-associated X protein (Bax) rabbit polyclonal antibody (1:1,000 dilution), and anti–B-cell lymphoma-extra large rabbit polyclonal antibody (1:1,000 dilution; Cell Signaling Technology, Boston, MA, USA).
2.3. Detection of superoxide levels
To measure the intracellular superoxide levels, we incubated the cells with 10 μM dihydroethidium (red; Invitrogen) for 60 min at 37°C and then fixed them in 4% formaldehyde solution for 10 min. We then incubated the fixed cells with 4′,6-diamidino-2-phenylindole (blue; Sigma-Aldrich) to visualize the nuclei. The intracellular dihydroethidium was examined under a fluorescence microscope (Olympus, Japan) and quantified using ImageJ software (National Institutes of Health).
2.4. Measurement of cytotoxicity
We measured cell viability using an EZ-CYTOX kit (Daeil Lab Service, Seoul, Korea) according to the user manual. The cells were incubated with EZ-CYTOX solution for 30 min at 37°C, after which optical density was measured using a microplate reader (Bio-Rad).
2.5. Hoechst staining
We stained the cells with Hoechst (1 μM; Invitrogen) according to the user manual to determine the shape of their nuclei. DNA-damaged cells were observed and counted under a fluorescence microscope.
2.6. Flow cytometry
To measure the rate of cell apoptosis, we stained H9c2 cells with Annexin V–fluorescein isothiocyanate and propidium iodide (Cell Signaling Technology). The stained cells were washed and then investigated using a Cytomics FC500 flow cytometry analyzer (Beckman Coulter, CA, USA).
2.7. Animals
We purchased 5-week-old male C57BL/6 mice from SLC (Shizuoka, Japan) and randomly divided them into the following 3 groups: a normal diet group (C; n = 5), a high-fat diet (HFD; 45% fat, 20% protein, and 35% carbohydrate) group (H; n = 5), and a HFD plus ginsenoside Mc1 group (Mc1; n = 5). The vehicle and ginsenoside Mc1 (10 mg per kg) were administered to the mice by intraperitoneal injection at intervals of 2 days [26], [27]. After 4 months, the heart tissues were harvested. All the mice were maintained on a 12-h light/12-h dark cycle and had ad libitum access to food and water. The present study was approved by the Institutional Animal Care and Use Committee of Korea University (Seoul, Korea), and all procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (NIH publication, 8th edition, 2011).
2.8. Caspase-3 activity assay
We used equal amounts of proteins obtained from heart tissues to detect caspase-3 activity using a Caspase-3 Assay Kit (Abcam, MA, USA) according to the user manual. The optical density values were obtained using a microplate reader.
2.9. Immunohistochemistry and Sirius Red staining
We fixed heart tissues in 4% formaldehyde and embedded them in paraffin. The paraffin tissue blocks were cut to a thickness of 6 μm, after which the sections were adhered to slides, deparaffinized, and rehydrated. To visualize the accumulated level of collagens, the slides were incubated with primary collagen I antibody plus secondary fluorescein isothiocyanate antibody (Santa Cruz Biotechnology) or Sirius Red solution (Polysciences, PA, USA) following the protocols in the user manuals.
2.10. Measurement of total collagen levels
We used an EnzyFluo™ Collagen Assay Kit (BioAssay Systems, CA, USA) according to the user manual to calculate the total collagen levels in the heart tissues. The fluorescence intensities were determined by a spectrofluorometer (PerkinElmer, Bridgeville, PA, USA).
2.11. Statistical analysis
The significance of differences between groups was determined by analysis of variance. All graphs present data as the mean ± standard deviation of 3 experiments. Differences were considered to be significant at p < 0.05.
3. Results
3.1. Ginsenoside Mc1 reduced H2O2-induced oxidative stress via an AMPK-dependent mechanism in H9c2 cells
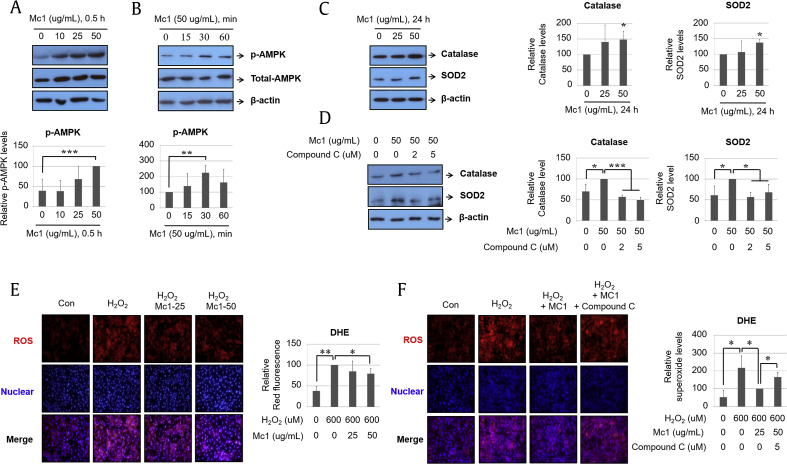
Western blotting revealed that AMPK phosphorylation was increased by ginsenoside Mc1 treatment in a dose-dependent manner (Fig. 1A) and peaked after 30 min of treatment (Fig. 1B). The expression levels of the antioxidants catalase and SOD2 increased after treatment with ginsenoside Mc1 (Fig. 1C), and those increases were blocked by the specific AMPK inhibitor compound C (Fig. 1D). Furthermore, H2O2-mediated ROS production decreased after treatment with ginsenoside Mc1 (Fig. 1E) and was restored by compound C treatment (Fig. 1F). These results suggest that ginsenoside Mc1 attenuates H2O2-mediated oxidative stress in H9c2 cells through an AMPK-dependent mechanism. As a positive control, the treatment of H9c2 cells with NAC significantly reduced H2O2-mediated ROS production by an amount that did not differ significantly from that resulting from ginsenoside Mc1 treatment (Supplementary Fig. S2).
Fig. 1.
Ginsenoside Mc1 inhibited hydrogen peroxide (H2O2)–mediated reactive oxygen species (ROS) production by increasing the expression of antioxidant proteins in H9c2 cells. (A) Cells were stimulated with various doses (0, 10, 25, or 50 μg/mL) of ginsenoside Mc1 for 30 min. (B) H9c2 cells were incubated with ginsenoside Mc1 (50 μg/mL) for the indicated times (0, 15, 30, or 60 min). The extent of AMPK phosphorylation was determined by Western blotting. (C) Cells were incubated with several doses (0, 25, or 50 μg/mL) of ginsenoside Mc1 for 24 h. (D) H9c2 cells were stimulated with ginsenoside Mc1 (50 μg/mL) or ginsenoside Mc1 plus compound C (2 or 5 μM) for 24 h. Catalase and SOD2 levels were determined by Western blotting. (E) Cells were pretreated with ginsenoside Mc1 (25 or 50 μg/mL) for 24 h and then stimulated with H2O2 (600 μM) for 2 h. (F) H9c2 cells were preincubated with ginsenoside Mc1 (50 μg/mL) or ginsenoside Mc1 plus compound C (2 or 5 μM) for 24 h and then stimulated with H2O2 (600 μM) for 2 h. The stimulated cells were stained with dihydroethidium (DHE) solution to assess the prevalence of intracellular ROS. The mean ± standard deviation was obtained from 3 separate experiments [*, p < 0.05; **, p < 0.005; ***, p < 0.0005; analysis of variance (ANOVA)]. AMPK, AMP-activated protein kinase; SOD2, superoxide dismutase 2.
3.2. Ginsenoside Mc1 reduced H2O2-mediated cell death via an AMPK-dependent mechanism in H9c2 cells
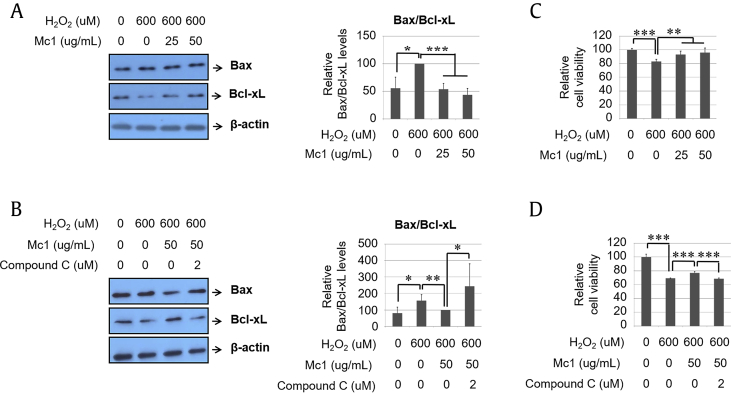
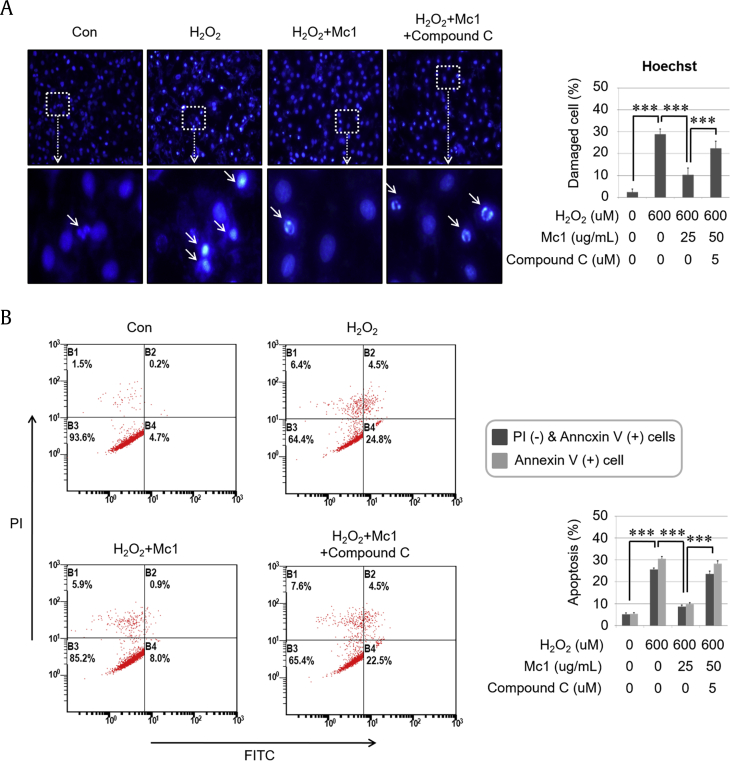
To verify the cytoprotective effects of ginsenoside Mc1 during H2O2 treatment, we measured the Bax:Bcl2 ratio and cell viability. The H2O2-mediated elevation of the Bax:Bcl2 ratio decreased significantly after treatment with ginsenoside Mc1 (Fig. 2A), and that protective effect was attenuated by compound C treatment (Fig. 2B). The H2O2-mediated decrease in cell viability was also prevented by ginsenoside Mc1 treatment (Fig. 2C), which was again reversed by treatment with compound C (Fig. 2D). The degree of recovery of cell viability after ginsenoside Mc1 treatment was almost the same as that after NAC treatment (Supplementary Fig. S3). Furthermore, the number of DNA-damaged cells after H2O2 treatment decreased after ginsenoside Mc1 treatment, and that effect was also canceled by compound C (Fig. 3A). H2O2-mediated annexin V–positive/propidium iodide–negative (early apoptotic cells) and annexin V–positive (total dead cells) populations were reduced after treatment with ginsenoside Mc1, which was reversed by treatment with compound C (Fig. 3B), suggesting that the antiapoptotic effect of ginsenoside Mc1 against H2O2-mediated oxidative stress depends on AMPK signaling.
Fig. 2.
Ginsenoside Mc1 exhibited a protective effect after the treatment of H9c2 cells with hydrogen peroxide (H2O2). (A and B) Cells were preincubated with ginsenoside Mc1 (25 or 50 μg/mL) or ginsenoside Mc1 plus compound C (2 μM) for 24 h and then stimulated with H2O2 (600 μM) for 2 h. Bax and Bcl2 levels were determined by Western blotting. (C and D) H9c2 cells were pretreated with ginsenoside Mc1 or ginsenoside Mc1 plus compound C for 24 h and then stimulated with H2O2 for 4 h. Cell viability was measured using an EZ-CYTOX kit. The mean ± standard deviation was obtained from 3 separate experiments [*, p < 0.05; **, p < 0.005; ***, p < 0.0005; analysis of variance (ANOVA)]. Bax, Bcl2-associated X protein.
Fig. 3.
Ginsenoside Mc1 inhibited hydrogen peroxide (H2O2)–induced apoptotic events in H9c2 cells. (A) Cells were preincubated with ginsenoside Mc1 (50 μg/mL) or ginsenoside Mc1 plus compound C (2 μM) for 24 h and then stimulated with H2O2 (600 μM) for 2 h. The shape of the nucleus was determined by Hoechst staining. White arrows indicate DNA-damaged cells. (B) H9c2 cells were pretreated with ginsenoside Mc1 or ginsenoside Mc1 plus compound C for 24 h and then stimulated with H2O2 for 4 h. The rate of apoptosis was determined using annexin V/propidium iodide (PI) double staining and flow cytometry. The mean ± standard deviation was obtained from 3 separate experiments [***, p < 0.0005; analysis of variance (ANOVA)]. FITC, fluorescein isothiocyanate.
3.3. Ginsenoside Mc1 inhibited HFD-mediated cell death events in the heart tissues of C57BL/6 mice
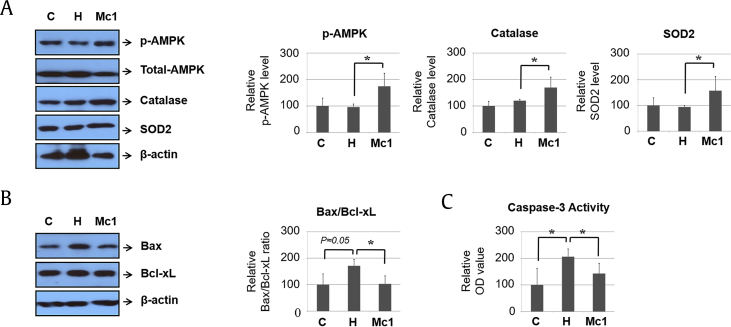
To determine the function of ginsenoside Mc1 in vivo, we administered ginsenoside Mc1 to mice by intraperitoneal injection. Ginsenoside Mc1 treatment did not significantly affect calorie intake, but it did tend to reduce body weight, although not to a statistically significant extent (Supplementary Fig. S4). Treatment with ginsenoside Mc1 was related to increased AMPK phosphorylation, which led to the upregulation of catalase and SOD2 (Fig. 4A). Furthermore, HFD-mediated increases in the Bax:B-cell lymphoma-extra large ratio and caspase-3 activity were significantly reduced by ginsenoside Mc1 (Fig. 4B and C). These data imply that HFD-mediated cell death was prevented by ginsenoside Mc1 in the heart tissues of the C57BL/6 mice.
Fig. 4.
Ginsenoside Mc1 reduced high-fat diet–induced, cell death–related events in heart tissues from C57BL/6 mice. (A) Western blotting was performed to determine the levels of phosphorylated AMPK, catalase, and SOD2. (B) The ratio of Bax to Bcl2 was determined by Western blotting. (C) Caspase-3 activity was measured using a caspase-3 activity assay kit. All graphs were obtained using 5 mice per group. Error bars represent mean ± standard deviation [*, p < 0.05; analysis of variance (ANOVA)]. AMPK, AMP-activated protein kinase; Bax, Bcl2-associated X protein; OD, optical density; SOD2, superoxide dismutase 2.
3.4. Ginsenoside Mc1 reduced HFD-mediated fibrotic events in the heart tissues of C57BL/6 mice
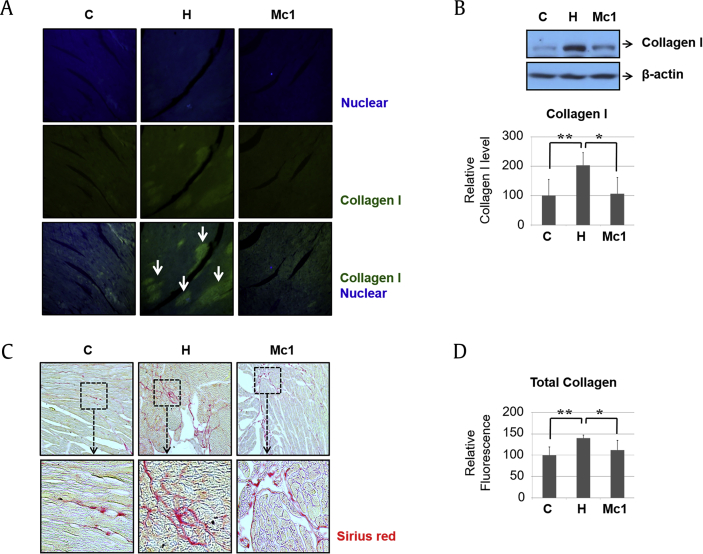
To determine the function of ginsenoside Mc1 in HFD-induced cardiac fibrotic events, we measured the rate of collagen accumulation in the heart tissues. Immunohistochemistry and Western blotting revealed that an HFD increased the abundance of collagen I in the heart, and that increase was attenuated after ginsenoside Mc1 administration (Fig. 5A and B). We also measured the total collagen level using Sirius Red staining and a collagen assay kit. The elevated accumulation of collagen induced by an HFD was reduced by treatment with ginsenoside Mc1 (Fig. 5C and D), suggesting that HFD-mediated fibrotic events in the heart tissues of C57BL/6 mice were attenuated by ginsenoside Mc1 administration.
Fig. 5.
Ginsenoside Mc1 reduced high-fat diet–mediated fibrotic events in heart tissues from C57BL/6 mice. (A and B) The abundance of collagen I was determined by immunohistochemistry and Western blotting. White arrows indicate collagen I–positive areas. (C) Fibrotic areas were identified by staining with Sirius Red. (D) The total amount of accumulated collagen was determined using a collagen assay kit. All graphs were obtained using 5 mice per group. Error bars represent mean ± standard deviation [*, p < 0.05; **, p < 0.005; analysis of variance (ANOVA)].
4. Discussion and conclusions
In the present study, we found that ginsenoside Mc1 reduced oxidative stress and protected H2O2-treated cardiomyocytes from apoptosis via an AMPK-dependent mechanism. The effect was also confirmed in heart tissues from HFD-induced obese mice, which showed increased levels of antioxidant molecules, a decreased tendency toward apoptosis, and significantly reduced cardiac fibrosis compared with the mice that did not receive ginsenoside Mc1.
Under normal physiological conditions, cells maintain a redox state in which the production and removal of ROS (through catalase, SODs, and glutathione) are balanced. However, increased ROS production caused by ischemia, hyperglycemia, or dyslipidemia induces oxidative stress and leads to cell damage. The antioxidant effects of ginsenosides have been widely reported [13], [14], [15], [16]. Gao et al. [28] showed that Rg1 prevents cisplatin-induced hepatotoxicity in mice, mainly by increasing the production of antioxidant proteins through the nuclear factor erythroid 2-related factor 2 (nrf2) signaling pathway. Treatment with ginsenoside Rg1 also prevented the cognitive impairment induced by isoflurane anesthesia via antioxidant, antiinflammatory, and antiapoptotic effects [29]. Excessive oxidative stress plays a pivotal role in the onset and development of CVDs as well as neurodegenerative and hepatic disorders, suggesting that ginsenosides could have beneficial effects on CVDs. Xie et al. [14] reported that ginsenoside Re is endowed with significant antioxidant properties and can effectively protect cardiomyocytes from acute oxidant injury. More recently, Li et al. [16] demonstrated that ginsenoside Rg1 exerts a strong protective effect on cardiomyocytes, primarily through its potent antioxidant properties, which activate the NF-E2–related factor 2/heme oxygenase-1 axis and inhibit the c-Jun N-terminal kinase pathway. Although several major ginsenosides exert cardioprotective effects by functioning as antioxidants, the present study is the first to show that treatment with the minor ginsenoside Mc1 effectively reduces oxidative stress and protects cardiomyocytes from apoptosis. The deglycosylated minor ginsenoside Mc1 is more pharmacologically active than the major ginsenosides because it is smaller and crosses cell membranes more readily. Diverse deglycated ginsenosides can be obtained by the highly selective hydrolysis of the sugar moieties of ginsenosides [30]. Ginsenoside Mc1 is a protopanaxadiol derivative with 2 different sugar moieties at C-3 (Glu) and C-20 (α-l-arabinofuranosyl (1→6) Glu) [30]. Our results are distinct in that they constitute the first in vitro and preliminary in vivo data to demonstrate the protective effect of ginsenoside Mc1 against cardiac apoptosis induced by oxidative stress.
In the present study, all the antioxidant and antiapoptotic effects of ginsenoside Mc1 depended on AMPK phosphorylation. In cardiomyocytes, consistent evidence has indicated that an activated AMPK signal pathway enhances glucose uptake and inhibits apoptosis and ischemic injury [31], [32]. AMPK is a key enzyme that regulates cellular energy by activating catabolic pathways to generate ATP efficiently and inhibit anabolic pathways [33]. The enzyme is also involved in antioxidant and antiinflammatory pathways, and a growing number of studies have shown that it maintains cardiac cell homeostasis to prevent apoptosis [34], [35], [36]. Recent studies on ginsenosides have also shown that their antioxidant effects occur through AMPK-dependent pathways in the liver and skeletal muscles [21], [22], [23]. Ginsenoside Rb2 activates AMPK, and suppression of hepatic gluconeogenesis by Rb2 is AMPK dependent [37]. Moreover, ginsenoside Rg1 stimulates glucose uptake in insulin-resistant C2C12 muscle cells, which positively increases glucose transporter type 4 expression through the AMPK pathway [23]. Huang et al. [22] reported that ginsenoside Rb2 promotes hepatic autophagy via AMPK/Sirt1-dependent pathways and consequently alleviates hepatic lipid accumulation. However, the antioxidant effect of ginsenosides through the AMPK pathway had not been evaluated in cardiomyocytes before the present study. In the present study, we verified that ginsenoside Mc1 increased the levels of antioxidant molecules and inhibited ROS production via an AMPK-dependent mechanism in the heart tissues of mice, as well as in H9c2 cells. These findings confirm that ginsenoside Mc1 has cardiobeneficial effects through AMPK signaling and has potential for therapeutic use in the future.
Cardiac fibrosis is seen in nearly all forms of heart disease, including acute coronary artery disease, atrial fibrillation, and hypertensive or diabetic cardiomyopathy [38], [39], [40], [41]. Compared with other organs, the heart has limited regeneration capacity after various injuries, and after damage, necrotic cardiomyocytes are replaced with fibrotic tissue [40]. Pathologically, increased cardiac fibrosis leads to ventricular hypertrophy, pathologic chamber dilatation, and ultimately heart failure [42], [43]. Previous studies have shown that excessive amounts of ROS cause cellular dysfunction and protein and lipid peroxidation, which eventually lead to cardiac cell damage and death [1], [44]. Therefore, reducing the production of ROS is critical for inhibiting cardiac cell death and fibrosis and could help to prevent and treat heart failure. In the present study, we demonstrated that ginsenoside Mc1 reduced the deposition of type I collagen and increased antioxidant levels in the heart tissues of mice with HFD-induced obesity. By Sirius Red staining and using a collagen assay kit for fibrosis, we also observed decreased fibrosis in ginsenoside Mc1-treated heart tissue from mice fed on an HFD. These results confirm the antifibrotic effect of ginsenoside Mc1 in cardiomyocytes. Our experiments indicate that the antifibrotic effect of ginsenoside Mc1 is similar to that of ginsenoside Rg1, which has recently been reported to reduce cardiac fibrosis and hypertrophy of the left ventricle [45]. The antifibrotic effect of ginsenoside Rg1 is attributed to Akt activation and p38 MAPK inhibition, in contrast to the effects of ginsenoside Mc1, which occur through the AMPK pathway. Although the types of ginsenoside and the pathways involved in attenuating fibrosis differ, the collective results suggest that ginsenosides have a protective effect on cardiac fibrosis through various pathways. Our preliminary in vivo experiment used only a single dosage of ginsenoside Mc1y and did not examine cardiac systolic or diastolic functions. Therefore, further in vivo studies are needed to examine the biological and physiological activities of ginsenoside Mc1 in more depth using various dosages and animal echocardiography.
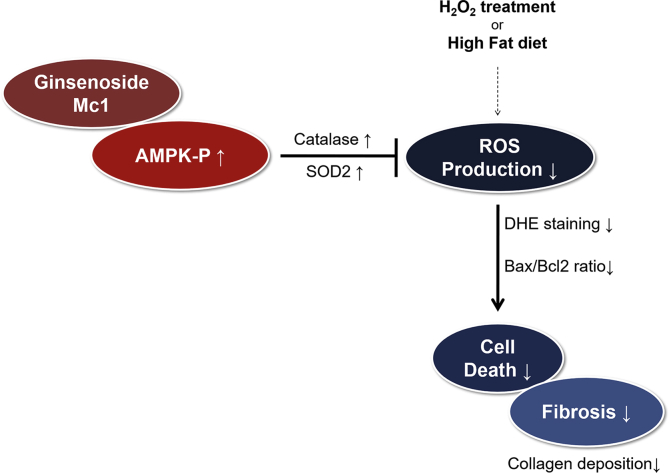
In conclusion, we have demonstrated for the first time that ginsenoside Mc1 helps prevent cardiac fibrosis by reducing ROS production and increasing cell viability in mice with HFD-induced obesity (Fig. 6). Ginsenoside Mc1 inhibited ROS production by enhancing the level of catalase and SOD2 in H2O2-treated cardiomyocytes. These effects were attenuated by treatment with compound C, which is known to specifically inhibit AMPK [46]. These findings indicate that ginsenoside Mc1 could be a novel therapeutic target for the treatment of cardiac fibrosis and the prevention of various cardiac diseases associated with oxidative stress.
Fig. 6.
Schematic diagram of ginsenoside Mc1 function. Treatment with ginsenoside Mc1 increased the expression of antioxidant proteins and inhibited high-fat diet–mediated cell death and fibrosis-related events in the heart by regulating AMPK phosphorylation. AMPK, AMP-activated protein kinase; Bax, Bcl2-associated X protein; DHE, dihydroethidium; ROS, reactive oxygen species; SOD2, superoxide dismutase 2.
Author contributions
S.-h.H., H.-J.H., J.A.K., Y.B.L., E.R., and H.J.Y. participated in the design of the study. H.J.H., J.W.K., S.-h.H., and E.R. performed the experiments. S.-h.H. and H.J.H. drafted the manuscript, and K.M.C., S.H.B., and H.J.Y. revised the manuscript. All authors read and approved the final manuscript.
Conflicts of interest
The authors have declared that they have no conflicts of interest.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2018R1D1A1B07047587) and by a Korea University grant (K1710551).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jgr.2019.08.006.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Tsutsui H., Kinugawa S., Matsushima S. Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol. 2011;301:H2181–H2190. doi: 10.1152/ajpheart.00554.2011. [DOI] [PubMed] [Google Scholar]
- 2.Symons J.D., McMillin S.L., Riehle C., Tanner J., Palionyte M., Hillas E., Jones D., Cooksey R.C., Birnbaum M.J., McClain D.A. Contribution of insulin and Akt1 signaling to endothelial nitric oxide synthase in the regulation of endothelial function and blood pressure. Circ Res. 2009;104:1085–1094. doi: 10.1161/CIRCRESAHA.108.189316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wende A.R., Symons J.D., Abel E.D. Mechanisms of lipotoxicity in the cardiovascular system. Curr Hypertens Rep. 2012;14:517–531. doi: 10.1007/s11906-012-0307-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wold L.E., Ceylan-Isik A.F., Ren J. Oxidative stress and stress signaling: menace of diabetic cardiomyopathy. Acta Pharmacol Sin. 2005;26:908–917. doi: 10.1111/j.1745-7254.2005.00146.x. [DOI] [PubMed] [Google Scholar]
- 5.Sabri A., Hughie H.H., Lucchesi P.A. Regulation of hypertrophic and apoptotic signaling pathways by reactive oxygen species in cardiac myocytes. Antioxid Redox Signal. 2003;5:731–740. doi: 10.1089/152308603770380034. [DOI] [PubMed] [Google Scholar]
- 6.Cesselli D., Jakoniuk I., Barlucchi L., Beltrami A.P., Hintze T.H., Nadal-Ginard B., Kajstura J., Leri A., Anversa P. Oxidative stress-mediated cardiac cell death is a major determinant of ventricular dysfunction and failure in dog dilated cardiomyopathy. Circ Res. 2001;89:279–286. doi: 10.1161/hh1501.094115. [DOI] [PubMed] [Google Scholar]
- 7.Segura A.M., Frazier O.H., Buja L.M. Fibrosis and heart failure. Heart Fail Rev. 2014;19:173–185. doi: 10.1007/s10741-012-9365-4. [DOI] [PubMed] [Google Scholar]
- 8.Gui Q.F., Xu Z.R., Xu K.Y., Yang Y.M. The efficacy of ginseng-related therapies in type 2 diabetes mellitus: an updated systematic review and meta-analysis. Medicine (Baltimore) 2016;95 doi: 10.1097/MD.0000000000002584. e2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y., Yu L., Cai W., Fan S., Feng L., Ji G., Huang C. Protopanaxatriol, a novel PPARγ antagonist from Panax ginseng, alleviates steatosis in mice. Sci Rep. 2014;4:7375. doi: 10.1038/srep07375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim J.-H. Cardiovascular diseases and Panax ginseng: a review on molecular mechanisms and medical applications. J Ginseng Res. 2012;36:16–26. doi: 10.5142/jgr.2012.36.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rastogi V., Santiago-Moreno J., Doré S. Ginseng: a promising neuroprotective strategy in stroke. Front Cell Neurosci. 2015;8:457. doi: 10.3389/fncel.2014.00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christensen L.P. Ginsenosides chemistry, biosynthesis, analysis, and potential health effects. Adv Food Nutr Res. 2009;55:1–99. doi: 10.1016/S1043-4526(08)00401-4. [DOI] [PubMed] [Google Scholar]
- 13.Zhou W., Chai H., Lin P.H., Lumsden A.B., Yao Q., Chen C. Ginsenoside Rb1 blocks homocysteine-induced endothelial dysfunction in porcine coronary arteries. J Vasc Surg. 2005;41:861–868. doi: 10.1016/j.jvs.2005.01.054. [DOI] [PubMed] [Google Scholar]
- 14.Xie J.T., Shao Z.H., Vanden Hoek T.L., Chang W.T., Li J., Mehendale S., Wang C.Z., Hsu C.W., Becker L.B., Yin J.J. Antioxidant effects of ginsenoside Re in cardiomyocytes. Eur J Pharmacol. 2006;532:201–207. doi: 10.1016/j.ejphar.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 15.He F., Guo R., Wu S.L., Sun M., Li M. Protective effects of ginsenoside Rb1 on human umbilical vein endothelial cells in vitro. J Cardiovasc Pharmacol. 2007;50:314–320. doi: 10.1097/FJC.0b013e3180cab12e. [DOI] [PubMed] [Google Scholar]
- 16.Li Q., Xiang Y., Chen Y., Tang Y., Zhang Y. Ginsenoside Rg1 protects cardiomyocytes against hypoxia/reoxygenation injury via activation of Nrf2/HO-1 signaling and inhibition of JNK. Cell Physiol Biochem. 2017;44:21–37. doi: 10.1159/000484578. [DOI] [PubMed] [Google Scholar]
- 17.Li J., Shao Z.H., Xie J.T., Wang C.Z., Ramachandran S., Yin J.J., Aung H., Li C.Q., Qin G., Vanden Hoek T. The effects of ginsenoside Rb1 on JNK in oxidative injury in cardiomyocytes. Arch Pharm Res. 2012;35:1259–1267. doi: 10.1007/s12272-012-0717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noh K.H., Oh D.K. Production of the rare ginsenosides compound K, compound Y, and compound Mc by a thermostable beta-glycosidase from Sulfolobus acidocaldarius. Biol Pharm Bull. 2009;32:1830–1835. doi: 10.1248/bpb.32.1830. [DOI] [PubMed] [Google Scholar]
- 19.Xu Q.F., Fang X.L., Chen D.F. Pharmacokinetics and bioavailability of ginsenoside Rb1 and Rg1 from Panax notoginseng in rats. J Ethnopharmacol. 2003;84:187–192. doi: 10.1016/s0378-8741(02)00317-3. [DOI] [PubMed] [Google Scholar]
- 20.Liu C.Y., Zhou R.X., Sun C.K., Jin Y.H., Yu H.S., Zhang T.Y., Xu L.Q., Jin F.X. Preparation of minor ginsenosides C-Mc, C-Y, F2, and C-K from American ginseng PPD-ginsenoside using special ginsenosidase type-I from Aspergillus niger g.848. J Ginseng Res. 2015;39:221–229. doi: 10.1016/j.jgr.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen L., Xiong Y., Wang D.Q., Howles P., Basford J.E., Wang J., Xiong Y.Q., Hui D.Y., Woods S.C., Liu M. Ginsenoside Rb1 reduces fatty liver by activating AMP-activated protein kinase in obese rats. J Lipid Res. 2013;54:1430–1438. doi: 10.1194/jlr.M035907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang Q., Wang T., Yang L., Wang H.Y. Ginsenoside Rb2 alleviates hepatic lipid accumulation by restoring autophagy via induction of Sirt1 and activation of AMPK. Int J Mol Sci. 2017;18:E1063. doi: 10.3390/ijms18051063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee H.M., Lee O.H., Kim K.J., Lee B.Y. Ginsenoside Rg1 promotes glucose uptake through activated AMPK pathway in insulin-resistant muscle cells. Phytother Res. 2012;26:1017–1022. doi: 10.1002/ptr.3686. [DOI] [PubMed] [Google Scholar]
- 24.Ko S.R., Suzuki Y., Suzuki K., Choi K.J., Cho B.G. Marked production of ginsenosides Rd, F2, Rg3, and compound K by enzymatic method. Chem Pharm Bull (Tokyo) 2007;55:1522–1527. doi: 10.1248/cpb.55.1522. [DOI] [PubMed] [Google Scholar]
- 25.Rushworth G.F., Megson I.L. Existing and potential therapeutic uses for N-acetylcysteine: the need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol Ther. 2014;141:150–159. doi: 10.1016/j.pharmthera.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 26.Guo M., Xiao J., Sheng X., Zhang X., Tie Y., Wang L., Zhao L., Ji X. Ginsenoside Rg3 mitigates atherosclerosis progression in diabetic apoE-/- mice by skewing macrophages to the M2 phenotype. Front Pharmacol. 2018;9:464. doi: 10.3389/fphar.2018.00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Q.W., Yu X.F., Xu H.L., Zhao X.Z., Sui D.Y. Ginsenoside Re improves isoproterenol-induced myocardial fibrosis and heart failure in rats. Evid Based Complement Alternat Med. 2019;2019:3714508. doi: 10.1155/2019/3714508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao Y., Chu S., Shao Q., Zhang M., Xia C., Wang Y., Li Y., Lou Y., Huang H., Chen N. Antioxidant activities of ginsenoside Rg1 against cisplatin-induced hepatic injury through Nrf2 signaling pathway in mice. Free Radic Res. 2017;51:1–13. doi: 10.1080/10715762.2016.1234710. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y., Zhang Z., Wang H., Cai N., Zhou S., Zhao Y., Chen X., Zheng S., Si Q., Zhang W. Neuroprotective effect of ginsenoside Rg1 prevents cognitive impairment induced by isoflurane anesthesia in aged rats via antioxidant, anti-inflammatory and anti-apoptotic effects mediated by the PI3K/AKT/GSK-3beta pathway. Mol Med Rep. 2016;14:2778–2784. doi: 10.3892/mmr.2016.5556. [DOI] [PubMed] [Google Scholar]
- 30.Shin K.C., Oh D.K. Classification of glycosidases that hydrolyze the specific positions and types of sugar moieties in ginsenosides. Crit Rev Biotechnol. 2016;36:1036–1049. doi: 10.3109/07388551.2015.1083942. [DOI] [PubMed] [Google Scholar]
- 31.Russell R.R., 3rd, Li J., Coven D.L., Pypaert M., Zechner C., Palmeri M., Giordano F.J., Mu J., Birnbaum M.J., Young L.H. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest. 2004;114:495–503. doi: 10.1172/JCI19297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang J., Tong C., Yan X., Yeung E., Gandavadi S., Hare A.A., Du X., Chen Y., Xiong H., Ma C. Limiting cardiac ischemic injury by pharmacological augmentation of macrophage migration inhibitory factor-AMP-activated protein kinase signal transduction. Circulation. 2013;128:225–236. doi: 10.1161/CIRCULATIONAHA.112.000862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carling D. The AMP-activated protein kinase cascade--a unifying system for energy control. Trends Biochem Sci. 2004;29:18–24. doi: 10.1016/j.tibs.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 34.Ruderman N.B., Carling D., Prentki M., Cacicedo J.M. AMPK, insulin resistance, and the metabolic syndrome. J Clin Invest. 2013;123:2764–2772. doi: 10.1172/JCI67227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hardie D.G., Ross F.A., Hawley S.A. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagendran J., Waller T.J., Dyck J.R. AMPK signalling and the control of substrate use in the heart. Mol Cell Endocrinol. 2013;366:180–193. doi: 10.1016/j.mce.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 37.Lee K.T., Jung T.W., Lee H.J., Kim S.G., Shin Y.S., Whang W.K. The antidiabetic effect of ginsenoside Rb2 via activation of AMPK. Arch Pharm Res. 2011;34:1201–1208. doi: 10.1007/s12272-011-0719-6. [DOI] [PubMed] [Google Scholar]
- 38.Leask A. Potential therapeutic targets for cardiac fibrosis: TGFbeta, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circ Res. 2010;106:1675–1680. doi: 10.1161/CIRCRESAHA.110.217737. [DOI] [PubMed] [Google Scholar]
- 39.Dzeshka M.S., Lip G.Y., Snezhitskiy V., Shantsila E. Cardiac fibrosis in patients with atrial fibrillation: mechanisms and clinical implications. J Am Coll Cardiol. 2015;66:943–959. doi: 10.1016/j.jacc.2015.06.1313. [DOI] [PubMed] [Google Scholar]
- 40.Travers J.G., Kamal F.A., Robbins J., Yutzey K.E., Blaxall B.C. Cardiac fibrosis: the fibroblast awakens. Circ Res. 2016;118:1021–1040. doi: 10.1161/CIRCRESAHA.115.306565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Russo I., Frangogiannis N.G. Diabetes-associated cardiac fibrosis: cellular effectors, molecular mechanisms and therapeutic opportunities. J Mol Cell Cardiol. 2016;90:84–93. doi: 10.1016/j.yjmcc.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cleutjens J.P., Verluyten M.J., Smiths J.F., Daemen M.J. Collagen remodeling after myocardial infarction in the rat heart. Am J Pathol. 1995;147:325–338. [PMC free article] [PubMed] [Google Scholar]
- 43.Khan R., Sheppard R. Fibrosis in heart disease: understanding the role of transforming growth factor-beta in cardiomyopathy, valvular disease and arrhythmia. Immunology. 2006;118:10–24. doi: 10.1111/j.1365-2567.2006.02336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsushima S., Kuroda J., Ago T., Zhai P., Park J.Y., Xie L.H., Tian B., Sadoshima J. Increased oxidative stress in the nucleus caused by Nox4 mediates oxidation of HDAC4 and cardiac hypertrophy. Circ Res. 2013;112:651–663. doi: 10.1161/CIRCRESAHA.112.279760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y.J., Zhang X.L., Li M.H., Iqbal J., Bourantas C.V., Li J.J., Su X.Y., Muramatsu T., Tian N.L., Chen S.L. The ginsenoside Rg1 prevents transverse aortic constriction-induced left ventricular hypertrophy and cardiac dysfunction by inhibiting fibrosis and enhancing angiogenesis. J Cardiovasc Pharmacol. 2013;62:50–57. doi: 10.1097/FJC.0b013e31828f8d45. [DOI] [PubMed] [Google Scholar]
- 46.Zhou G., Myers R., Li Y., Chen Y., Shen X., Fenyk-Melody J., Wu M., Ventre J., Doebber T., Fujii N. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.