Abstract
Background
Depression is a common neuropsychiatric disease that shows astrocyte pathology. Ginsenoside Rf (G-Rf) is a saponin found in Panax ginseng which has been used to treat neuropsychiatric diseases. We aimed to investigate antidepressant properties of G-Rf when introduced into the L-alpha-aminoadipic acid (L-AAA)–infused mice model which is representative of a major depressive disorder that features diminished astrocytes in the brain.
Methods
L-AAA was infused into the prefrontal cortex (PFC) of mice to induce decrease of astrocytes. Mice were orally administered G-Rf (20 mg/kg) as well as vehicle only or imipramine (20 mg/kg) as controls. Depression-like behavior of mice was evaluated using forced swimming test (FST) and tail suspension test (TST). We observed recovery of astroglial impairment and increased proliferative cells in the PFC and its accompanied change in the hippocampus by Western blot and immunohistochemistry to assess the effect of G-Rf.
Results
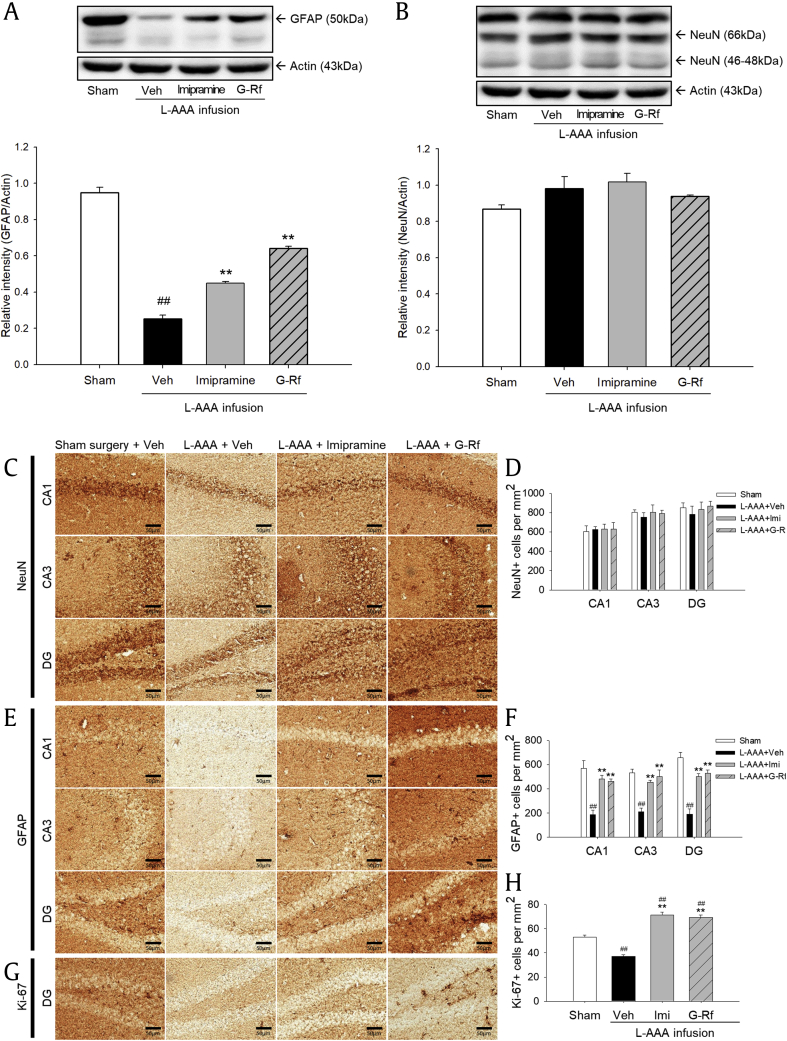
After injection of L-AAA into the PFC, mice showed increased immobility time in FST and TST and loss of astrocytes without significant neuronal change in the PFC. G-Rf–treated mice displayed significantly more decreased immobility time in FST and TST than did vehicle-treated mice, and their immobility time almost recovered to those of the sham mice and imipramine-treated mice. G-Rf upregulated glial fibrillary acidic protein (GFAP) expression and Ki-67 expression in the PFC reduced by L-AAA and also alleviated astroglial change in the hippocampus.
Conclusion
G-Rf markedly reversed depression-like behavioral changes and exhibited protective effect against the astrocyte ablation in the PFC induced by L-AAA. These protective properties suggest that G-Rf might be a therapeutic agent for major depressive disorders.
Keywords: Astrocyte, Depression, Ginsenoside Rf, Glial fibrillary acidic protein (GFAP), L-Alpha-Aminoadipic Acid (L-AAA)
1. Introduction
Depression is a neuropsychiatric syndrome that afflicts about 350 million people worldwide and produces a major, multi–billion dollar annual socioeconomic source of stress [1]. Those afflicted with depression experience sadness, apathy, anhedonia, or suicidal ideation by psychophysiological transformation. Notwithstanding the intensive research accomplished over the past decades, the therapies currently implemented to treat patients with a major depressive disorder (MDD) have been effective in less than 50% of those patients with MDD whose symptoms require chronic treatment [2].
Extensive studies on brains of patients with MDD or those of animal models of depression found that density of astrocytes diminished, whereas neurons did not show significant change or only atrophied [1], [3], [4], [5]. In the postmortem autopsy of the brains of patients with MDD, a reduced number of astrocytes were observed in the prefrontal cortex (PFC) [4], [6] and hippocampus [7]. Astrocytes take part in trophic support, neuronal differentiation, and synaptic efficacy [8]. Astrocytic abnormality has been shown to induce a decrease in glutamate uptake/cycling and an accumulation of glutamate, which is a main excitatory amino acid neurotransmitter keeping balance with gamma-aminobutyric acid (GABA), and may bring about depression [9]. These changes could be identified by a corresponding level of astrocytic markers, such as glial fibrillary acidic protein (GFAP).
L-alpha-aminoadipic acid (L-AAA) induces local glial degeneration by transitory ablation of astrocytes and an increased number of microglia around the lesion [10]. L-AAA enters cells by a sodium (Na+)-dependent transporter and induces glial cell death by blocking required cellular functions involving glutamate [11]. According to previous studies, infusing gliotoxin of L-AAA in the PFC region establishes a depression model as it presents depression-like behaviors and mimics the histological features of the brain of patients with MDD [9], [11], [12]. Compared with conventional animal models of depression in which animals were subjected to chronic stress, L-AAA–infused model has the advantage that it can equally modulate the change in relative animal subjects and thus evaluate the precise effect by intervention.
Panax ginseng Meyer (P. ginseng) has traditionally been used to treat a variety of disorders in the nervous system including MDD. The antidepressant effect of P. ginseng extract was previously studied using a chronic mild stress model [13], chronic restraint stress model [14], menopause depression model [15], and an addiction-withdrawal model [16]. As active ingredients and metabolites of P. ginseng, Rg1 [17], [18], Rb1 [15], Rb3 [19], 20 (S)-protopanaxadiol [20], and compound K [15] were reported to ameliorate depression-like behaviors in rodents.
Among approximately thirty kinds of ginsenosides found in P. ginseng, ginsenoside Rf (G-Rf) is a steroid glycoside and classified as protopanaxatriol-type ginsenosides. As a genuine ingredient in Asian ginseng, G-Rf is a trace ginsenoside among other diverse ginsenosides, and it is one of the biologically active saponins found in P. ginseng [21]. Previous studies demonstrated that G-Rf had analgesic and antinociceptive effects and was related to inhibition of Ca2+ channels in sensory neurons and the role of adrenergic receptors [21], [22], [23]. An antiinflammatory effect of G-Rf was reported for pain [23] and atopic and contact dermatitis [24]. A series of previous studies suggest a potential effect of P. ginseng, especially G-Rf, on MDD, which is a prominent neurological disorder. However, there is barely any research on the effects of P. ginseng or G-Rf on diseases relevant to affective disorders. Therefore, in this study, we hypothesized that G-Rf might significantly improve depression-like behavior and histological changes using an L-AAA–induced mouse model of depression and focused mainly on the pathology of astrocytes, which are deeply involved in the etiology of MDD.
2. Materials and methods
2.1. Reagents
Ginsenoside Rf (PubChem CID: 441922, CAS Number: 52286-58-5) was purchased from Interpharm, Inc. (Koyang, Korea). Neuronal-specific nuclear protein (NeuN) was purchased from Millipore, Inc. (Bedford, MA, USA), and β-actin and GFAP were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Ki-67 was purchased from Abcam (Cambridge, MA, USA). Secondary anti-mouse antibody and anti-rabbit antibody were obtained from Pierce Biotechnology, Inc. (Rockford, IL, USA).
2.2. Animals
Eight-week-old male C57Bl/6 mice (Orient Bio, Inc., Korea) weighing 20–22 g were used. The mice were housed in acrylic cages (22 × 27 × 12 cm), with constantly automatically monitored and controlled temperature (22 ± 2°C) and relative humidity (60 ± 10%), with free access to water and food, under a twelve-hour light/dark cycle. All behavioral tests were performed between 9:00 and 17:00. All procedures were approved by the Kyung Hee University Medical Center Institutional Animal Care (approval number; KHMC-IACUC 16-028).
2.3. Drug administration
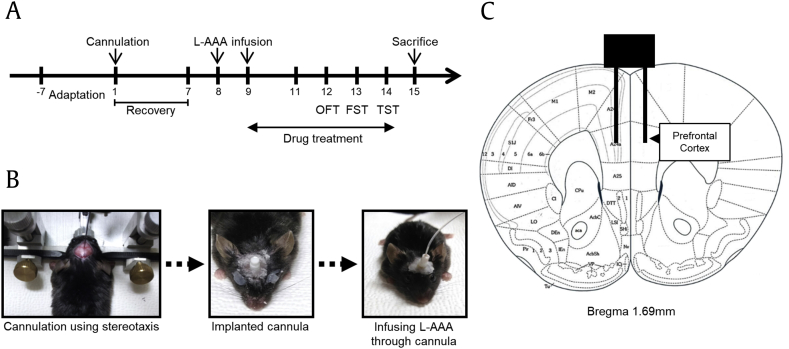
Mice were randomly assigned to four groups: (i) Sham control (Sham), distilled water per os (po) + sham surgery (n = 7); (ii) Vehicle (Veh), distilled water po + L-AAA infusion (n = 7); (iii) Imipramine (Imi), imipramine (20 mg/kg) po + L-AAA infusion (n = 7), (iv) G-Rf, G-Rf (20 mg/kg) po + L-AAA infusion (n = 7). G-Rf and imipramine were completely dissolved in distilled water and were administered to the mice orally. Oral administration continued until the mice were sacrificed. All experiments followed the time schedule shown in Fig. 1A.
Fig. 1.
(A) Timeline of the experiment. (B) The image depicts the course of cannula implantation. Guide cannulas were implanted, and L-AAA was infused through them (1.2 μl/mouse). (C) Location of L-AAA infusion. Guide cannula was aimed at the prefrontal cortex using the following coordinates: AP 1.7 mm, ML ±0.3 mm, and DV −2.5 mm from the bregma. FST, forced swimming test; L-AAA, L-alpha-aminoadipic acid; OFT, open-field test; TST, tail suspension test; AP, anterior-posterior; ML, medial-lateral; DV, dorsal-ventral.
2.4. Cannula implantation
Mice were anesthetized using intraperitoneal injections of 100 mg/kg of ketamine + 10 mg/kg of xylazine and were placed on a stereotaxic apparatus (Vernier Stereotaxic Instrument; Leica Biosystems, Nussloch, Germany). The mice were implanted with a guide cannula (RWD Life science Co., Ltd., Shenzhen, China) in the PFC region using the following coordinates: 1.7 mm anterior-posterior (AP), ±0.3 mm medial-lateral (ML), and −2.5 mm dorsal-ventral (DV) from the bregma. All animals then recuperated within seven days.
2.5. Injection of L-AAA
After one week of recovery, we infused L-AAA (100 μg/μl; Sigma) bilaterally at a rate of 0.1 μl/min for six minutes using an injection cannula and a microdrive pump (Pump 11 Elite Nanomite; Harvard Apparatus, Holliston, MA, USA). We administered it once daily for two days (Fig. 1). Mice in the vehicle, imipramine, and G-Rf groups were injected with L-AAA into the PFC through the previously implanted guide cannula. The sham mice did not undergo injection after cannula implantation.
2.6. Behavioral tests
The open-field test (OFT) was conducted to estimate locomotor activity. Each mouse was placed in the center of a transparent acrylic box arena (50 cm × 50 cm × 50 cm), and behavior was recorded for ten minutes by means of a video-recording device. The horizontal locomotor activity was defined as total ambulatory distance. Locomotor activity was analyzed using Smart 3.0 (Panlab SL, Barcelona, Spain)
We performed the forced swimming test (FST) and the tail suspension test (TST) to find out if the mice exhibited depression-like behavior. The FST was applied as described previously with slight modifications [25]. Briefly, FST was assessed using a swimming chamber consisting of an acrylic cylindrical tank (height, 46 cm; diameter, 20 cm) with 23–25°C water filled to a depth of 15 cm. Each mouse was individually placed in the cylinder for six minutes. Immobility time was measured during the last four minutes. The TST was performed as previously described [26]. Mice were suspended 50 cm above the floor by using adhesive tape, the other end of which was placed at the tip of the tail. The immobility time was calculated by video recording during the last four minutes of the six-minute-long testing time.
2.7. Tissue collection
After the behavioral tests were completed, all mice were anesthetized and sacrificed. Some mice were designated for rapid removal of the hippocampi from the brains. The hippocampi were dissected and stored at −80°C for Western blotting analysis. The remaining mice in each group were anesthetized using diethyl ether and were perfused with phosphate-buffered saline, followed by 4% paraformaldehyde solution. Collected brains were subsequently submerged in 4% paraformaldehyde for 24 hours at 4°C and transferred to a 20% sucrose solution. The brains were stored at −80°C for immunohistochemical analysis.
2.8. Histochemical and immunohistochemical analysis
All frozen brains were cut into 10-μm-thick sections using a cryostat microtome (Leica CM1850; Leica Microsystems, Wetzlar, Germany). Damaged cells in the PFC were identified by hematoxylin and eosin (H&E) staining to confirm tissue injury by cannula. Sections from the PFC and hippocampus were also incubated with primary antibodies GFAP (1:200), NeuN (1:200), and Ki-67 (1:500) overnight to verify changes of astrocytes, neurons, and proliferative cells. The sections were incubated with the secondary antibody for one hour at room temperature. After incubation with avidin-conjugated peroxidase complex (ABC kit; Vector Laboratories, CA, USA), signals were detected using 3, 3′-diaminobenzidine tetrahydrochloride (Dako, CA, USA). Microscopic analysis was performed using Olympus BX51 microscope (Olympus, Tokyo, Japan). Immunopositive cells were quantified using the method followed by David et al [27] using ImageJ v.1.44 software (NIH, Bethesda, MD, USA). The number of cells was counted in three 250 μm × 250 μm contours per region. Cell counts were obtained by averaging the total number of cells per section of that brain area.
2.9. Western blot
The hippocampus tissue was homogenized in 1 × RIPA buffer (Pierce Biotechnology, IL, US). The homogenate was centrifuged at 12,000 × g for 15 minutes at 4°C, and the supernatant was collected. Protein was quantified using the BCA protein assay kit (Pierce Biotechnology). The protein extract (40 μg per lane) was loaded onto 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel followed by transferring onto polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). The membranes were incubated with the primary antibody against NeuN (1:1000) and GFAP (1:1000) overnight at 4°C. The membranes were then incubated with goat anti–mouse IgG secondary antibody (1:5000) for one hour at room temperature. Immunoreactive bands were detected using an enhanced chemiluminescent kit (Pierce Biotechnology). The blots were imaged using Davinch-Chemi (Celltagen, Seoul, Korea) and were quantified using ImageJ v.1.44 software (NIH).
2.10. Statistical analysis
Data were assessed using a one-way analysis of variance followed by Tukey post hoc test comparisons for the behavioral and biochemical analysis studies. All results were expressed as means ± standard error of mean, p values < 0.05 were considered statistically significant, and all calculations were made using SPSS version 22.0 (Chicago, IL, USA).
3. Results
3.1. Effects of G-Rf on depression-like behavior of L-AAA–infused depressive mice
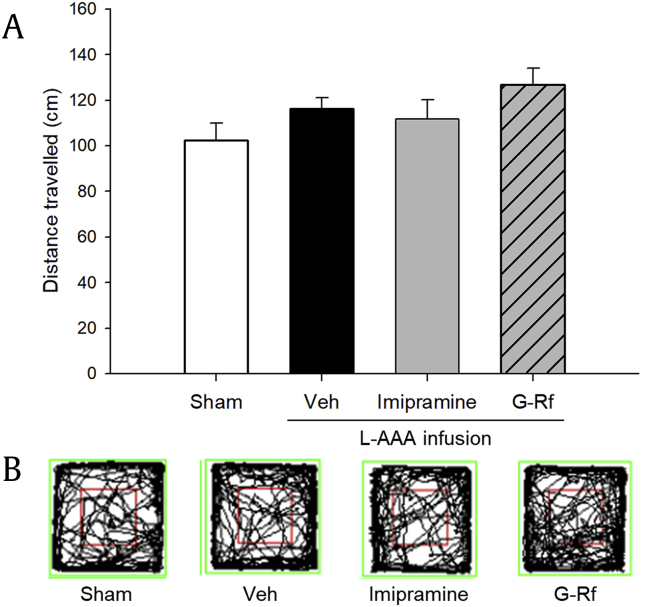
We first assessed whether L-AAA affected locomotor ability of each group. In the OFT, there was no statistically significant difference in total ambulatory distance between groups (p > 0.05, Fig. 2). Accordingly, sensorimotor deficits associated with intracerebral infusion were not detected during behavioral assessment.
Fig. 2.
(A) Effect of ginsenoside Rf (G-Rf) on the open-field test in mice that were injected with L-AAA in the prefrontal cortex. Locomotion was assessed by total distance traveled by mice in whole grids, and no significant difference was observed between groups. The results are expressed as the mean ± SEM. Representative motion tracks for each group are presented in (B). L-AAA, L-alpha-aminoadipic acid; SEM, standard error of mean.
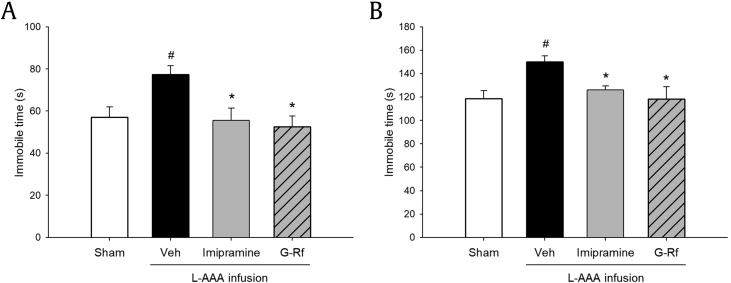
The FST and TST were performed to explore the antidepressant effects of G-Rf on the behavior of L-AAA–infused mice. In the FST, vehicle-treated mice showed significantly increased immobility time than did the sham group (p < 0.05), whereas the G-Rf–treated and imipramine-treated mice displayed decreased immobility time similar to that of the sham mice (p < 0.05 relatively, Fig. 3A). Similarly, in the TST, the immobility time was significantly increased in the vehicle-treated mice as compared with sham mice (p < 0.05), and G-Rf and imipramine treatment decreased immobility time (p < 0.05 relatively, Fig. 3B). The obtained results indicate that G-Rf is able to recover depression-like behavior which is induced by glial loss in the PFC of mice.
Fig. 3.
Effect of ginsenoside Rf (G-Rf) on depression-like behavior in (A) forced swimming test (FST) and (B) tail suspension test (TST). Immobility time in FST and that in TST were assessed to observe depression-like behavior. Administration of G-Rf (20 mg/kg) reversed the immobility time increased by L-AAA infusion as much as imipramine (20 mg/kg) did in both tests. The results are expressed as the mean ± SEM. #p < 0.05, significantly different from sham control group (Sham); *p < 0.05 and **p < 0.01, significantly different from vehicle-treated group (Veh). FST, forced swimming test; L-AAA, L-alpha-aminoadipic acid; SEM, standard error of mean; TST, tail suspension test.
3.2. Effect of G-Rf administration on the number of GFAP-positive cells in the PFC of mice under L-AAA infusion
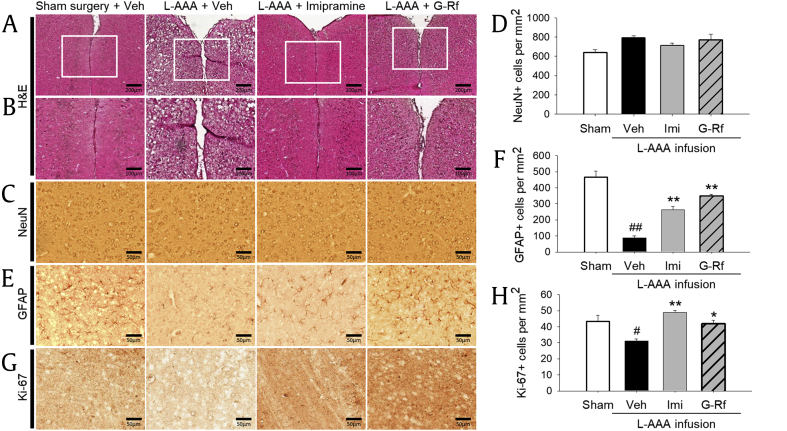
Fig. 4 presents representative sections from the PFC. Histological analysis of sections stained with H&E showed the proper localization of the injection sites (Fig. 4A, original magnification × 100; Fig. 4B, original magnification × 200). In all groups, H&E-stained sections revealed no marked traces of lesions or necrosis caused by the cannula implantation because stained neurons were found near the scar.
Fig. 4.
Localization of injection site and effect of ginsenoside Rf (G-Rf) on expression of neuron-specific nuclear protein (NeuN), glial fibrillary acidic protein (GFAP), and Ki-67 in the prefrontal cortex. (A, B) Hematoxylin and eosin (H&E) staining showed the proper location of the injection sites. In every group, stained tissue adjacent to the implanted site did not reveal a prominent extent of necrosis compared with the sham control group. (C, D) Effect of G-Rf on NeuN expression in the prefrontal cortex. NeuN expression did not significantly differ between groups after L-AAA treatment. (E, F) Effect of G-Rf on GFAP expression in the prefrontal cortex. After L-AAA treatment, GFAP extremely diminished, but oral administration of G-Rf (20 mg/kg) reversed astrocyte degeneration, making the GFAP expression of G-Rf group similar to that of the sham control group. (G, H) Effect of G-Rf on Ki-67 expression in the prefrontal cortex. After L-AAA injection, proliferative cells diminished, but G-Rf protected loss of proliferative cells. Representative results from H&E staining and immunohistochemistry and quantitative analysis are shown. All values are expressed as the mean ± SEM. #p < 0.05 significantly different from sham control group (Sham); *p < 0.05 and **p < 0.01, significantly different from vehicle-treated group (Veh). Calibration bars for (A), 200 μm; for (B), 100 μm; and for (C), (E), and (G), 50 μm. L-AAA, L-alpha-aminoadipic acid; SEM, standard error of mean.
A reduced number of astrocytes have been detected in brains from patients with depression rather than neuronal change. To determine the effect of G-Rf on astrogliosis, prefrontal cortical sections of each group were immunostained with antibodies to GFAP and NeuN. To determine the extent of astrocytic or neuronal activation, we quantified immunoreactive cells (Fig. 4C, E, original magnification × 400). There was no apparent difference after consecutive injection of L-AAA, as demonstrated by the similar NeuN expression level among groups (p > 0.05, Fig. 4C and D). As opposed to the NeuN, GFAP-positive cells were almost removed in vehicle-treated mice implying severe astrogliosis, when compared with the sham control mice (p < 0.01). Accordingly, L-AAA injection affected solely astrocytes without degrading neurons, which is similar to the brain state of patients with MDD. Oral treatment of G-Rf and imipramine improved density of GFAP-stained astrocytes decreased by L-AAA (p < 0.01 respectively), and G-Rf especially reversed it more than imipramine, confirming antidepressant effects of G-Rf. These data suggest that repeated administration of G-Rf may block astrocyte impairment which characterizes depressive disorder.
3.3. Effect of G-Rf administration on proliferative cells in the PFC of mice
To evaluate loss of proliferative cells by L-AAA and the effect of G-Rf on their recovery, we explored whether proliferation-related marker Ki-67 altered in the PFC. When treated with L-AAA, the number of Ki-67–positive cells significantly decreased (p < 0.05 vs. sham surgery group). In contrast, G-Rf treatment and imipramine treatment increased the number of Ki-67–positive cells in the glial lesion (p < 0.05 and p < 0.01, respectively, vs. vehicle-treated group, Fig. 4G and H). These results provide evidence that proliferative effect of G-Rf may be related to its protective effect on astrocyte impairment.
3.4. Effect of G-Rf administration on the NeuN, GFAP, and Ki-67 level in the hippocampi of mice
Dysfunction in specific brain region can lead to subsequent change in other regions [28], [29]. The PFC, hippocampus, and amygdala are considered to be involved in pathology of depression [30]. The recent study demonstrated that L-AAA injection into the prelimbic cortex resulted in anhedonia and apathy-related behaviors, while injection into CA3 of the hippocampus resulted in learning deficit [27]. If G-Rf acted on astrocyte dysfunction of both regions, G-Rf could manage a broad range of depression-related symptoms. We examined the effects of G-Rf on the NeuN and GFAP expression levels in the mice hippocampi by Western blotting. The results demonstrated no change in the NeuN expression level, which was found to be consistent in all the mice hippocampi examined (p > 0.05, Fig. 5A). It therefore appears that L-AAA injection did not influence the neurons in the hippocampus. On the other hand, GFAP expression level was observed to significantly decrease in the vehicle-treated mice hippocampi (p < 0.01). However, G-Rf treatment yielded significantly increased hippocampal GFAP expression level compared with that of the sham (p < 0.01), and it was higher than that of mice under imipramine treatment (Fig. 5B).
Fig. 5.
Effect of ginsenoside Rf (G-Rf) on expression of neuron-specific nuclear protein (NeuN) and glial fibrillary acidic protein (GFAP) in the hippocampus of the mice treated with L-AAA in the prefrontal cortex as determined by Western blot. (A) Effect of G-Rf (20 mg/kg) on relative expression of NeuN in the hippocampus. NeuN expression did not significantly differ between groups after L-AAA treatment. (B) Effect of G-Rf on relative expression of GFAP in the hippocampus. After L-AAA treatment, GFAP diminished, but oral administration of G-Rf ameliorated astrocyte degeneration. (C, D) Effect of G-Rf on NeuN expression in the hippocampus. NeuN expression did not significantly differ between groups after L-AAA treatment in CA1, CA3, or dentate gyrus (DG). (E, F) Effect of G-Rf on GFAP expression in the hippocampus. While GFAP expression significantly decreased after L-AAA injection, oral administration of G-Rf (20 mg/kg) increased the number of GFAP-positive cells in CA1, CA3, and DG. (G, H) Effect of G-Rf on Ki-67 expression in DG of the hippocampus. L-AAA injection altered the number of proliferative cells, and G-Rf rescued diminished proliferative cells. The quantitative results are expressed as the mean ± SEM. #p < 0.05 and ##p < 0.01, significantly different from the sham control group (Sham); *p < 0.05 and **p < 0.01, significantly different from the vehicle-treated group (Veh). Representative results from Western blot and immunohistochemistry are also presented. Calibration bars 50 μm. L-AAA, L-alpha-aminoadipic acid; SEM, standard error of mean.
We further identified the change of neuron, astrocytes, and proliferative cells in the hippocampus using NeuN-, GFAP-, and Ki-67–stained sections. We used sections for CA1, CA3, and dentate gyrus (DG) to examine the overall changes in the hippocampus regarding neurons and astrocytes and sections of DG to observe proliferative cells. The number of NeuN-positive cells showed no significant changes in CA1, CA3, and DG (p > 0.05, Fig. 5C and D). As confirmed by the results of the Western blot, the L-AAA injection significantly affected the number of GFAP-positive cells in CA1, CA3, and DG (p < 0.01). G-Rf treatment significantly recovered the number of GFAP-positive cells which was decreased by L-AAA injection in each region (p < 0.01). The number of GFAP-positive cells was significantly increased in hippocampal CA1, CA3, and DG regions by imipramine treatment (p < 0.01, Fig. 5E and F). We also investigated whether G-Rf treatment altered the number of proliferative cells which was reduced by L-AAA infusion in the hippocampus. Ki-67–positive cells decreased in the DG of the mice injected with L-AAA into the PFC (p < 0.01). In G-Rf–treated mice, we observed a significant increase in the number of Ki-67–positive cells as in imipramine-treated mice (p < 0.01, Fig. 5G and H). In addition, G-Rf–treated mice and imipramine-treated mice showed an increased number of Ki-67 compared with the sham group. These results suggest that G-Rf can have a protective role over other brain regions affected by depressive disorder including the hippocampus.
4. Discussion
To suggest G-Rf as possibly being a useful antidepressant, we evaluated its efficacy on neurobehavioral change and histopathological deficit induced by astrocyte impairment in the PFC. We also investigated its protective effect on proliferative cells. Conventional animal models for depression, such as chronic mild stress model and learned helplessness model, have disadvantages in having low reliability or in that not all animals respond to intervention [31]. Because of homogeneous modulation across patients and similarity with postmortem brain tissues of patients with MDD, we focused on astrocyte dysfunction in depression and adopted the selective astrocyte toxin, L-AAA, in the PFC. Behavioral and neurological alteration derived by astrocyte ablation is repeatable by fine surgery. Although the definite cause or mechanism of MDD has not been discovered, a reduced number of astrocytes were observed in the PFC and hippocampi of patients with MDD in postmortem studies [3], [4], [5], [6], [7]. It was also suggested that the astrocyte approach can be a key to the cognitive deficit found in depression [32]. The focus on glial pathology may provide better candidates for explaining the disease [32]. Accordingly, this study was intended to investigate whether administration of G-Rf ameliorated depression-like behavior and astroglial pathology of depression.
Antidepressant effects of G-Rf were observed in behavioral tests. Although injection of L-AAA did not show locomotor deficit in the OFT, astrocyte-ablated mice showed longer immobility time in the FST and TST, which indicates a depressive state. Mice treated with G-Rf had significantly as much less depression-like behaviors as those treated with imipramine. The results of the behavioral studies indicate that G-Rf treatment improved depression, especially as induced by astrocyte pathology.
We also verified the protective effect of G-Rf on the brain by using immunohistochemical and immunoblotting analyses. H&E staining of all groups confirmed the proper localization of the injection sites. Intra-PFC injection of gliotoxin diminished GFAP level, which indicates decreased astrocytes and resembles the typical feature of patients with MDD. The relative expression of NeuN was not decreased in the PFC in immunohistochemistry, which indicates that we established a mouse model that resembles traits of depression, as intended [32], [33]. A reduced number of astrocytes was observed in the PFC of patients with MDD, and it was more prominent in patients with familial MDD [4], [6]. In a recent meta-analysis of neurometabolites in the PFC by proton magnetic resonance spectroscopy, the absolute values of glutamatergic metabolites in patients with MDD were significantly decreased compared with those in healthy controls without reduction in the respective level of glutamate, implying engagement of astrocytes [34]. In the previous study, loss of astrocytes in the PFC by L-AAA resulted in anhedonia, anxiety, and helplessness in rats as exhibited in a chronic unpredictable stress model, whereas lesions in the PFC induced by neurotoxins did not provoke behavioral change [11]. Some studies imply that modulation of astrocytes is crucial in exploring the pathology of depression. Therefore, an astrocyte-ablated model is effective in implementing features of depression in animals and in assessing antidepressant effects on astrocytic change in MDD.
The decreased level of GFAP was reversed by G-Rf and imipramine in both Western blot and immunohistochemical analysis. It was consistent with the results from the FST and TST. Considering the human neuroimaging study that found the levels of glutamate to be correlated with severity of symptoms [34], a drug that can modulate astrocytes can influence mood disorders. Furthermore, we detected a significant reduction in GFAP expression in the hippocampus after L-AAA injection. Even though glial toxin was locally infused, its effect could generate global change over the brain. There is a previous study that injecting carbachol in lateral hypothalamus induced change in the ventral tegmental area, hippocampus, and PFC [29]. In previous studies using L-AAA, the author mentioned that a marker of GABA neuron changed in the hippocampus six days after injection into the PFC [28]. Even though this finding showed compensatory effect, it implies that remote structure can change after histological change. One previous study reported that the number of BrdU-positive cells did not change two days after L-AAA injection [35]. However, because GFAP expression in the lesion did not change until 3 days after injection, it is expected that prolonged observation is required to verify the change of other structures [12], [28]. The PFC is closely related to the hippocampus, and its direct and indirect interaction routes have been suggested [36], [37]. As abnormal interaction between the PFC and the hippocampus is connected to deficits in emotional regulation, the symptoms of neuropsychiatric disorders such as MDD, schizophrenia, or specific phobias [38], which means that a drug that ameliorates deterioration of the PFC and hippocampus and their interaction, can be a promising candidate. In this study, administration of G-Rf reversed both direct and indirect alterations by glial toxins in the PFC and hippocampus just as imipramine did. Thus, G-Rf and P. ginseng may provide an effective strategy for treatment of MDD. Further immunohistochemical study and investigation on its mechanism should be carried out.
Previous studies have found that P. ginseng affects neurological disorders, and its neuroprotective effect was revealed [39]. White ginseng saponin has regulated neuroinflammation of glial cells in vitro [40]. A recent study reported that oral administration of P. ginseng extract ameliorated depressive behavior in mice under chronic restraint stress, and its effect was related to inhibition of neuroinflammation and oxidative stress in the amygdala [14]. Even though G-Rf has rarely been studied in terms of neurological diseases, including affective disorders, G-Rf can work on the nervous system. In this study, we focused on the action of astrocytes and cell proliferation, but there are other clues about its mode of action that remain unclear. G-Rf showed an inhibitory effect on interleukin-1β, interleukin-6, and nuclear factor-k beta [23], [41] and its antiinflammatory effects could influence depressive symptoms, in that allostatic load is reported to contribute to its onset and interrupt its recovery. G-Rf has been found to regulate the nervous system and modulate neurotransmission in the brain [21], [42], [43]. G-Rf inhibited N-type and other Ca2+ channels in sensory neurons as much as opioids did [42]. In previous studies using adrenal chromaffin cells, G-Rf hampered catecholamine secretion evoked by acetylcholine better than did saponins from other plants [44], [45], inhibited Ca2+ currents, and had the most remarkable regulatory effect on the change in cell membrane capacitance among various ginsenosides [46]. G-Rf also inhibited inward currents in oocytes expressing nicotinic acetylcholine receptor subtypes [47]. These support the additional role of G-Rf in treating depression by its potential antistress effect [43]. G-Rf regulated GABAA receptors in a rat brain [48] and activated G protein–coupled inwardly rectifying K+ channels, which result in postsynaptic hyperpolarization, mainly in the olfactory bulb, hippocampus, DG, and cortex [49]. Even though the safety of G-Rf has not been tested, most ginsenosides and ginseng have proved to be safe, so G-Rf can be expected to be an effective and safe agent [50]. Considering these results, G-Rf works on the central nervous system and may be a potent candidate for MDD medication.
Our study was designed to document the potential antidepressant properties of G-Rf and its underlying mechanism. One limitation of this study is that we concentrated on the astrocyte-related pathology of depressive disorder. Even though astrocytes are deeply associated with the pathophysiology of depression, an approach from various perspectives would be beneficial to explain the efficacy of G-Rf. Moreover, the underlying mechanisms of G-Rf that regulate astrocytes, such as the glutamatergic system, gap junction, neurotrophic factors, and relation with other cells such as neurons, should be elucidated. Despite no statistical significance, the mice that administered G-Rf showed increased activity in OFT compared with other groups, so this factor can be considered in interpreting the antidepressant effect of G-Rf.
In conclusion, our study shows that orally administered G-Rf ameliorated depression-like behavior, astrocytic degeneration, and loss of proliferative cells in the PFC and alterations of the hippocampus of mice treated with a glial toxin, L-AAA. Because investigation of medications for depressive disorder is still a problem to be solved, G-Rf, the genuine component in P. ginseng, can be proposed as a potential antidepressant agent because these findings were verified in the animal model that resembles features in the brains of patients with MDD. A further understanding of the mechanisms underlying the effect of G-Rf is required.
Conflicts of interest
There are no conflicts of interest to declare.
Acknowledgments
This work was supported by a grant from Kyung Hee University in 2016. (KHU- 20161702)
References
- 1.Rial D., Lemos C., Pinheiro H., Duarte J.M., Goncalves F.Q., Real J.I., Prediger R.D., Goncalves N., Gomes C.A., Canas P.M. Depression as a glial-based synaptic dysfunction. Front Cell Neurosci. 2015;9:521. doi: 10.3389/fncel.2015.00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee S., Jeong J., Kwak Y., Park S.K. Depression research: where are we now? Mol Brain. 2010;3:8. doi: 10.1186/1756-6606-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rajkowska G., Stockmeier C A. Astrocyte pathology in major depressive disorder: insights from human postmortem brain tissue. Curr Drug Targets. 2013;14:1225–1236. doi: 10.2174/13894501113149990156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miguel-Hidalgo J.J., Baucom C., Dilley G., Overholser J.C., Meltzer H.Y., Stockmeier C.A., Rajkowska G. Glial fibrillary acidic protein immunoreactivity in the prefrontal cortex distinguishes younger from older adults in major depressive disorder. Biol Psychiatry. 2000;48:861–873. doi: 10.1016/s0006-3223(00)00999-9. [DOI] [PubMed] [Google Scholar]
- 5.Wang Q., Jie W., Liu J.H., Yang J.M., Gao T.M. An astroglial basis of major depressive disorder? An overview. Glia. 2017;65:1227–1250. doi: 10.1002/glia.23143. [DOI] [PubMed] [Google Scholar]
- 6.Öngür D., Drevets W.C., Price J.L. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proceed Natl Acad Sci. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cobb J.A., O’Neill K., Milner J., Mahajan G.J., Lawrence T.J., May W.L., Miguel-Hidalgo J., Rajkowska G., Stockmeier C.A. Density of GFAP-immunoreactive astrocytes is decreased in left hippocampi in major depressive disorder. Neuroscience. 2016;316:209–220. doi: 10.1016/j.neuroscience.2015.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Volterra A., Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci. 2005;6:626. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- 9.Domin H., Szewczyk B., Pochwat B., Wozniak M., Smialowska M. Antidepressant-like activity of the neuropeptide Y Y5 receptor antagonist Lu AA33810: behavioral, molecular, and immunohistochemical evidence. Psychopharmacology (Berl) 2017;234:631–645. doi: 10.1007/s00213-016-4495-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takada M., Hattori T. Fine structural changes in the rat brain after local injections of gliotoxin, alpha-aminoadipic acid. Histol Histopathol. 1986;1:271–275. [PubMed] [Google Scholar]
- 11.Banasr M., Duman R.S. Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors. Biol Psychiatry. 2008;64:863–870. doi: 10.1016/j.biopsych.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee Y., Son H., Kim G., Kim S., Lee D.H., Roh G.S., Kang S.S., Cho G.J., Choi W.S., Kim H.J. Glutamine deficiency in the prefrontal cortex increases depressive-like behaviours in male mice. J Psychiatr Neurosci JPN. 2013;38:183–191. doi: 10.1503/jpn.120024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dang H., Chen Y., Liu X., Wang Q., Wang L., Jia W., Wang Y. Antidepressant effects of ginseng total saponins in the forced swimming test and chronic mild stress models of depression. Prog Neuropsychopharmacol Biol Psychiatr. 2009;33:1417–1424. doi: 10.1016/j.pnpbp.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 14.Choi J.H., Lee M.J., Jang M., Kim H.-J., Lee S., Lee S.W. Panax ginseng exerts antidepressant-like effects by suppressing neuroinflammatory response and upregulating nuclear factor erythroid 2 related factor 2 signaling in the amygdala. J Ginseng Res. 2018 Jan;42(1):107–115. doi: 10.1016/j.jgr.2017.04.012. Epub 2017 Apr 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamada N., Araki H., Yoshimura H. Identification of antidepressant-like ingredients in ginseng root (Panax ginseng C.A. Meyer) using a menopausal depressive-like state in female mice: participation of 5-HT2A receptors. Psychopharmacology (Berl) 2011;216:589–599. doi: 10.1007/s00213-011-2252-1. [DOI] [PubMed] [Google Scholar]
- 16.Lee B., Kim H., Shim I., Lee H., Hahm D.-H. Wild ginseng attenuates anxiety-and depression-like behaviors during morphine withdrawal. J Microbiol Biotechnol. 2011;21:1088–1096. doi: 10.4014/jmb.1106.06027. [DOI] [PubMed] [Google Scholar]
- 17.Liu Z., Qi Y., Cheng Z., Zhu X., Fan C., Yu S. The effects of ginsenoside Rg1 on chronic stress induced depression-like behaviors, BDNF expression and the phosphorylation of PKA and CREB in rats. Neuroscience. 2016;322:358–369. doi: 10.1016/j.neuroscience.2016.02.050. [DOI] [PubMed] [Google Scholar]
- 18.Zheng X., Liang Y., Kang A., Ma S.-J., Xing L., Zhou Y.-Y., Dai C., Xie H., Xie L., Wang G.-J. Peripheral immunomodulation with ginsenoside Rg1 ameliorates neuroinflammation-induced behavioral deficits in rats. Neuroscience. 2014;256:210–222. doi: 10.1016/j.neuroscience.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 19.Cui J., Jiang L., Xiang H. Ginsenoside Rb3 exerts antidepressant-like effects in several animal models. J Psychopharmacol. 2012;26:697–713. doi: 10.1177/0269881111415735. [DOI] [PubMed] [Google Scholar]
- 20.Xu C., Teng J., Chen W., Ge Q., Yang Z., Yu C., Yang Z., Jia W. 20 (S)-protopanaxadiol, an active ginseng metabolite, exhibits strong antidepressant-like effects in animal tests. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1402–1411. doi: 10.1016/j.pnpbp.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 21.Mogil J.S., Shin Y.-H., McCleskey E.W., Kim S.-C., Nah S.-Y. Ginsenoside Rf, a trace component of ginseng root, produces antinociception in mice. Brain Res. 1998;792:218–228. doi: 10.1016/s0006-8993(98)00133-4. [DOI] [PubMed] [Google Scholar]
- 22.Nemmani K.V., Ramarao P. Ginsenoside Rf potentiates U-50,488 H-induced analgesia and inhibits tolerance to its analgesia in mice. Life Sci. 2003;72:759–768. doi: 10.1016/s0024-3205(02)02333-0. [DOI] [PubMed] [Google Scholar]
- 23.Kim M.K., Kang H., Baek C.W., Jung Y.H., Woo Y.C., Choi G.J. Antinociceptive and anti-inflammatory effects of ginsenoside Rf in a rat model of incisional pain. Journal of Ginseng Res. 2018 Apr;42(2):183–191. doi: 10.1016/j.jgr.2017.02.005. Epub 2017 Mar 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bae E.-A., Han M.J., Shin Y.-W., Kim D.-H. Inhibitory effects of Korean red ginseng and its genuine constituents ginsenosides Rg3, Rf, and Rh2 in mouse passive cutaneous anaphylaxis reaction and contact dermatitis models. Biol Pharm Bull. 2006;29:1862–1867. doi: 10.1248/bpb.29.1862. [DOI] [PubMed] [Google Scholar]
- 25.Huang P., Tunis J., Parry C., Tallarida R., Liu-Chen L.Y. Synergistic antidepressant-like effects between a kappa opioid antagonist (LY2444296) and a delta opioid agonist (ADL5859) in the mouse forced swim test. Eur J Pharmacol. 2016;781:53–59. doi: 10.1016/j.ejphar.2016.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mineur Y.S., Belzung C., Crusio W.E. Effects of unpredictable chronic mild stress on anxiety and depression-like behavior in mice. Behav Brain Res. 2006;175:43–50. doi: 10.1016/j.bbr.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 27.David J., Gormley S., McIntosh A., Kebede V., Thuery G., Varidaki A., Coffey E., Harkin A. L-alpha-amino adipic acid provokes depression-like behaviour and a stress related increase in dendritic spine density in the pre-limbic cortex and hippocampus in rodents. Behav Brain Res. 2019;362:90–102. doi: 10.1016/j.bbr.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 28.Domin H., Szewczyk B., Wozniak M., Wawrzak-Wlecial A., Smialowska M. Antidepressant-like effect of the mGluR5 antagonist MTEP in an astroglial degeneration model of depression. Behav Brain Res. 2014;273:23–33. doi: 10.1016/j.bbr.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 29.Haghparast A., Taslimi Z., Ramin M., Azizi P., Khodagholi F., Hassanpour-Ezatti M. Changes in phosphorylation of CREB, ERK, and c-fos induction in rat ventral tegmental area, hippocampus and prefrontal cortex after conditioned place preference induced by chemical stimulation of lateral hypothalamus. Behav Brain Res. 2011;220:112–118. doi: 10.1016/j.bbr.2011.01.045. [DOI] [PubMed] [Google Scholar]
- 30.Shirayama Y., Takahashi M., Osone F., Hara A., Okubo T. Myo-inositol, glutamate, and glutamine in the prefrontal cortex, Hippocampus, and amygdala in major depression. Biol Psychiatr Cogn Neurosci Neuroimaging. 2017;2:196–204. doi: 10.1016/j.bpsc.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Slattery D.A., Cryan J.F. The ups and downs of modelling mood disorders in rodents. ILAR J. 2014;55:297–309. doi: 10.1093/ilar/ilu026. [DOI] [PubMed] [Google Scholar]
- 32.Goodwin G.M. Neuropsychological and neuroimaging evidence for the involvement of the frontal lobes in depression: 20 years on. J Psychopharmacol. 2016;30:1090–1094. doi: 10.1177/0269881116661074. [DOI] [PubMed] [Google Scholar]
- 33.Czéh B., Fuchs E., Wiborg O., Simon M. Animal models of major depression and their clinical implications. Prog Neuropsychopharmacol Biol Psychiatry. 2016;64:293–310. doi: 10.1016/j.pnpbp.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 34.Arnone D., Mumuni A.N., Jauhar S., Condon B., Cavanagh J. Indirect evidence of selective glial involvement in glutamate-based mechanisms of mood regulation in depression: meta-analysis of absolute prefrontal neuro-metabolic concentrations. Eur Neuropsychopharmacol. 2015;25:1109–1117. doi: 10.1016/j.euroneuro.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 35.Etievant A., Oosterhof C., Betry C., Abrial E., Novo-Perez M., Rovera R., Scarna H., Devader C., Mazella J., Wegener G. Astroglial control of the antidepressant-like effects of prefrontal cortex deep brain stimulation. EBioMedicine. 2015;2:898–908. doi: 10.1016/j.ebiom.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vertes R.P. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience. 2006;142:1–20. doi: 10.1016/j.neuroscience.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 37.Sigurdsson T., Duvarci S. Hippocampal-prefrontal interactions in cognition, behavior and psychiatric disease. Front Syst Neurosci. 2016;9 doi: 10.3389/fnsys.2015.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jin J., Maren S. Prefrontal-hippocampal interactions in memory and emotion. Front Syst Neurosci. 2015;9 doi: 10.3389/fnsys.2015.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim H. Neuroprotective herbs for stroke therapy in traditional eastern medicine. Neurol Res. 2005;27:287–301. doi: 10.1179/016164105X25234. [DOI] [PubMed] [Google Scholar]
- 40.Sung J.-H., Choi D.-H., Kim D.-H., Chun B.-G., Choi S.-H. White ginseng saponin upregulated the production of TNF-alpha, IL-1beta, and NO in primary cultures of mixed glial cells. J Ginseng Res. 2004;28:120–126. [Google Scholar]
- 41.Xing L., Jiang M., Dong L., Gao J., Hou Y., Bai G., Luo G. Cardioprotective effects of the YiQiFuMai injection and isolated compounds on attenuating chronic heart failure via NF-κB inactivation and cytokine suppression. J Ethnopharmacol. 2013;148:239–245. doi: 10.1016/j.jep.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 42.Nah S.-Y., Park H.-J., McCleskey E.W. A trace component of ginseng that inhibits Ca2+ channels through a pertussis toxin-sensitive G protein. Proc Natl Acad Sci. 1995;92:8739–8743. doi: 10.1073/pnas.92.19.8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nah S.-Y. Ginseng ginsenoside pharmacology in the nervous system: involvement in the regulation of ion channels and receptors. Front Physiol. 2014;5 doi: 10.3389/fphys.2014.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tachikawa E., Kudo K., Kashimoto T., Takahashi E. Ginseng saponins reduce acetylcholine-evoked Na+ influx and catecholamine secretion in bovine adrenal chromaffin cells. J Pharmacol Exp Ther. 1995;273:629–636. [PubMed] [Google Scholar]
- 45.Kudo K., Tachikawa E., Kashimoto T., Takahashi E. Properties of ginseng saponin inhibition of catecholamine secretion in bovine adrenal chromaffin cells. Eur J Pharmacol. 1998;341:139–144. doi: 10.1016/s0014-2999(97)01350-2. [DOI] [PubMed] [Google Scholar]
- 46.Kim H.S., Lee J.H., Goo Y.S., Nah S.Y. Effects of ginsenosides on Ca2+ channels and membrane capacitance in rat adrenal chromaffin cells. Brain Res Bull. 1998;46:245–251. doi: 10.1016/s0361-9230(98)00014-8. [DOI] [PubMed] [Google Scholar]
- 47.Choi S., Jung S.-Y., Lee J.-H., Sala F., Criado M., Mulet J., Valor L.M., Sala S., Engel A.G., Nah S.-Y. Effects of ginsenosides, active components of ginseng, on nicotinic acetylcholine receptors expressed in Xenopus oocytes. Eur J Pharmacol. 2002;442:37–45. doi: 10.1016/s0014-2999(02)01508-x. [DOI] [PubMed] [Google Scholar]
- 48.Kimura T., Saunders P.A., Kim H.S., Rheu H.M., Oh K.W., Ho I.K. Interactions of ginsenosides with ligand-bindings of GABA(A) and GABA(B) receptors. Gen Pharmacol. 1994;25:193–199. doi: 10.1016/0306-3623(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 49.Choi S., Jung S.Y., Ko Y.S., Koh S.R., Rhim H., Nah S.Y. Functional expression of a novel ginsenoside Rf binding protein from rat brain mRNA in Xenopus laevis oocytes. Mol Pharmacol. 2002;61:928–935. doi: 10.1124/mol.61.4.928. [DOI] [PubMed] [Google Scholar]
- 50.Nah S.Y., Kim D.H., Rhim H. Ginsenosides: are any of them candidates for drugs acting on the central nervous system? CNS Drug Rev. 2007;13:381–404. doi: 10.1111/j.1527-3458.2007.00023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]