Abstract
Adsorption of organic pollutants onto microplastics has been reported in prior studies indicating the potential of these particles to serve as vectors of pollutants. Most prior investigations, however, have been conducted in laboratories under conditions with relatively little environmental relevance. Here we report the results of in-situ experiments to investigate the adsorption of pharmaceuticals (atenolol, sulfamethoxazole, and ibuprofen) on to eight types of test materials (pellets from five types of widely-used polymers, small pieces of straws, fragments of bags, and glass beads for control). Three sample sets survived 28 days of deployment in New York City waterways. Concentrations of each analyte in water samples taken at these sites were also measured. Adsorption coefficients were calculated based on mass and surface area for each type. Mass-based coefficients showed much higher values for straw and bag samples than other types, consistent with their greater surface area to mass ratios. The surface area-based coefficients were similar among the plastic materials tested as well as the glass beads, indicating that surface area is a major determinant of the pharmaceutical adsorption, regardless of material type. Rapid biofouling, which was observed on all samples, appeared to be the predominant factor controlling the sorption capacity of the plastics. Our observations suggest that extensive biofouling and the formation of biofilms in nutrient-enriched waters can significantly impact the adsorption of pharmaceuticals onto plastics.
Keywords: microplastics, pharmaceuticals, adsorption, urban coastal waterways, vectors, biofilm
Introduction
Plastics are a family of resilient, inexpensive, inert, and ubiquitous materials on which society continues to have a constant and growing dependency; we are rarely more than one step away from something made of plastic. Of the 6.3 billion tons of plastic discarded to-date, less than 9% has been recycled (Jambeck et al, 2015). As of 2015, it was estimated that an annual average of 8.8 million metric tons (Mt) of plastic debris ends up in the ocean (Jambeck et al, 2015) where it degrades into increasingly smaller fragments. While the durability and convenience of plastic has been lauded since the 1950s, plastics do not biodegrade readily, but when subjected to photo-degradation and physical weathering, they can become friable and fracture into micro-sized particles classified as microplastics (< 5 mm) (Andrady, 2011; GESAMP, 2015).
Similar to many other urban coastal water bodies, the waterways surrounding New York City are severely contaminated with microplastics, nutrients, and pharmaceuticals (Cantwell et al., 2018; Conover et al., 2016; Lim, 2018; Magadini et al., 2017). On average NYC waterways, carry about 165 million floating plastic particles, with majority of them (>95%) being microplastics (<5 mm) (Conover et al., 2016). This is presumably due to: 1) the inability of wastewater treatment plants (WWTPs) to filter all microplastics from over one billion gallons of daily influent, as well as 2) the episodic release of raw sewage from combined sewer overflow (CSO) outlets (Carr et al., 2016; Geis et al., 2018; Mason et al., 2016; NYC-DEP, Wastewater, 2019). Similarly, high concentrations of pharmaceuticals are also discharged on a daily basis from WWTPs into NYC’s waterways. As NYC continues to grow, so also do the volumes and range of pharmaceutical contaminants present in the estuary. In an extensive study covering 72 sampling sites throughout the Hudson River Estuary, the analytes we targeted as well as 16 other pharmaceuticals were measured (Cantwell et al., 2018). While pharmaceuticals are not persistent pollutants, by sheer volume and regular discharge into urban waterways, they are pseudo-persistent (Boxall et al., 2014), at concentrations high enough to cause harm to many species of marine organisms (Cantwell et al., 2018). Pharmaceutical interactions with aquatic fauna are known to have toxic effects at metabolic and cellular levels (Aguirre-Martínez et al., 2015; Daughton, 2001; Fabbri & Franzellitti, 2016).
Given that both microplastics and pharmaceuticals are released from sewage systems into urban coastal waters on a continual basis, there is urgent need to study the potential of microplastics as non-biological concentrators and vectors of pharmaceuticals under natural environmental conditions. Studies have shown that pharmaceuticals will adsorb on to microplastics in laboratory settings (Kleinteich et al., 2018; Razanajatovo et al., 2018; Wu et al., 2016). Though these experiments can be informative in investigating adsorption kinetics, they have limitations. For instance, in laboratory experiments, test microplastics were confined to just one or several types of particles (to limit the competition of sorption sites), while in polluted urban waters, many different types of microplastic particles, comprised of different types of polymers exist, and also co-exist with non-plastic particles such as black carbon, organic particles, silt, clay, etc. Furthermore, under experimental conditions, only one chemical or a simple chemical mixture was tested, but in urban aquatic environments, thousands of natural and anthropogenic chemicals coexist. Additionally, in order to reduce the effect of bacterial biofilm formation onto the experimental microplastics, previous studies have relied on biocides to arrest bacterial growth (Kleinteich et al., 2018; Razanajatovo et al., 2018; Wu et al., 2016). In the real world, especially in nutrient-rich waterways, biofouling is inevitable within one week of microplastics residing in the water (Lobelle & Cunliffe, 2011); therefore, these laboratory experiments have relatively limited environmental relevance.
The overall purpose of this study was to investigate the sorption of pharmaceuticals onto microplastics in NYC waterways through in-situ experiments. Given the inevitable growth of biofilm on microplastic surfaces, we hypothesized that the sorption capacity of pollutants among different types of polymers is comparable because the newly formed biofilm acts as the true active layer for pollutant sorption. To test the hypothesis, five different types of commonly used plastic polymers were selected for piecing together a broader picture of the disruptive effects that microplastics can pose to the marine ecosystem. The pharmaceutical compounds that we selected for examination are: atenolol - a drug commonly used as a beta-blocker to treat high blood pressure, ibuprofen - a common, nonsteroidal anti-inflammatory drug, and sulfamethoxazole - an antibiotic (Table 1). In their study, Cantwell et al. (2018) showed that these three compounds are ubiquitous in the waterways around New York City.
Table 1.
Information for selected pharmaceuticals
| Compounds and their solubilities (μg/L) | Molecular Formula | Chemical Structure |
|---|---|---|
| Atenolol (3300 mg/L) | C14H22N2O3 | 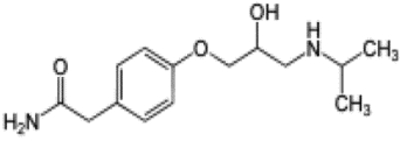 |
| Ibuprofen (21 mg/L) | C13H18O | 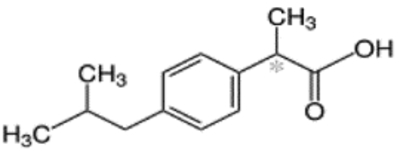 |
| Sulfamethoxazole (610 mg/L) | C10H11N3OcS | 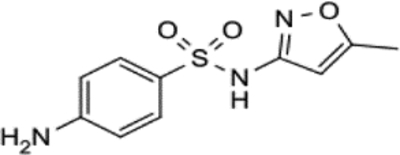 |
Methods
Site selection
Six sites were selected within the waterways surrounding NYC: three sites are in the Hudson River; at Englewood Boat Basin, the North River Wastewater Treatment Plant (NRWWTP), and Pier 25; and three sites are connected to the East River at Newtown Creek, Flushing Bay, and the Harlem River (Fig.1). The site at Newtown Creek is least subjected to tidal flux which can affect the concentration of pollutant given its higher residence time (Cantwell et al., 2018). Operating WWTPs which discharge wastewater on a daily basis, are located close to three sites: North River WWTP in Hudson, the East River, which is connected to Newtown Creek, and Flushing Bay. Additionally, numerous CSO outlets are located along the waterways of all site locations except site #1; the Englewood Boat Basin.
Figure 1:
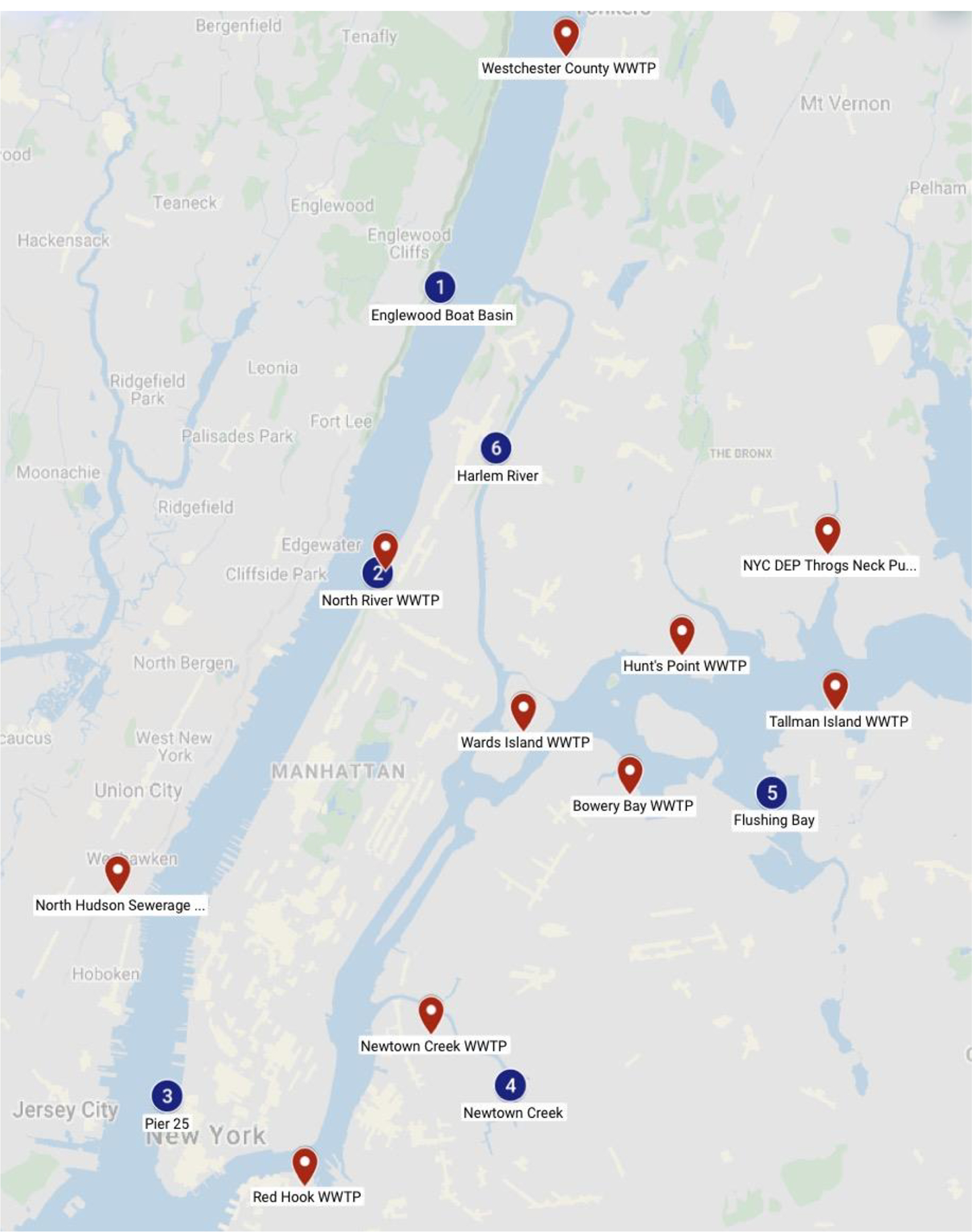
Sampling locations in blue dots: 1) Englewood Boat Basin, 2) North River Wastewater Treatment Plant, 3) Pier 25, 4) Newtown Creek, 5) Flushing Bay, and 6) Harlem River; and Wastewater Treatment Plants in red balloons. Only samples at locations 4,5,6 were successfully retrieved.
In situ deployment of plastic particles:
Virgin pre-production plastic pellets, ranging in diameter from 2.3 mm to 5 mm, of Polyethylene Terephthalate (PET), High Density Polyethylene (HDPE), Polyvinyl Chloride (PVC), Low Density Polyethylene (LDPE), and Polypropylene (PP) were purchased from the Society of Plastic Engineers. Plastic shopping bags (LDPE) and plastic straws (PP) were obtained and cut into pieces ≤ 35 mm in size. Glass beads, ~ 3 mm in diameter, were used as a control (Fig. 2). The surface morphology of these particles is shown in the supplementary material (Fig. S1). Prior to deployment, all materials were cleaned with 100% methanol and dried at room temperature. After the cleaning process, pellets and glass beads were segregated and placed into stainless-steel mesh tea infusers, of 38 mm in diameter, with an approximate mesh size of 1mm. Fragments of plastic bags and cut segments of straws were placed in 100 mm diameter tea infusers given their large volume. The volume of experiment materials was approximately 1/3 of the volume of tea infusers used. After filling with one particle type, each infuser was tightly sealed with stainless steel wire and tagged (stainless steels tags) with the type of particle inside. The eight infusers, one for each particle type, were then placed into a galvanized steel mesh basket, labeled, and wired shut. A total of six baskets were deployed; one at each of the six sites previously described. Each basket was submerged at a level below the lowest spring tide, and secured to moorings or to a buoy at each site. Date, time, GPS coordinates, and surface water temperature and salinity were recorded at the time of each deployment. The surface water temperatures of the sites during the deployments in July ranged from 18.5 to 22.7 °C and salinity ranged from 15 to 22 PSU.
Figure 2:
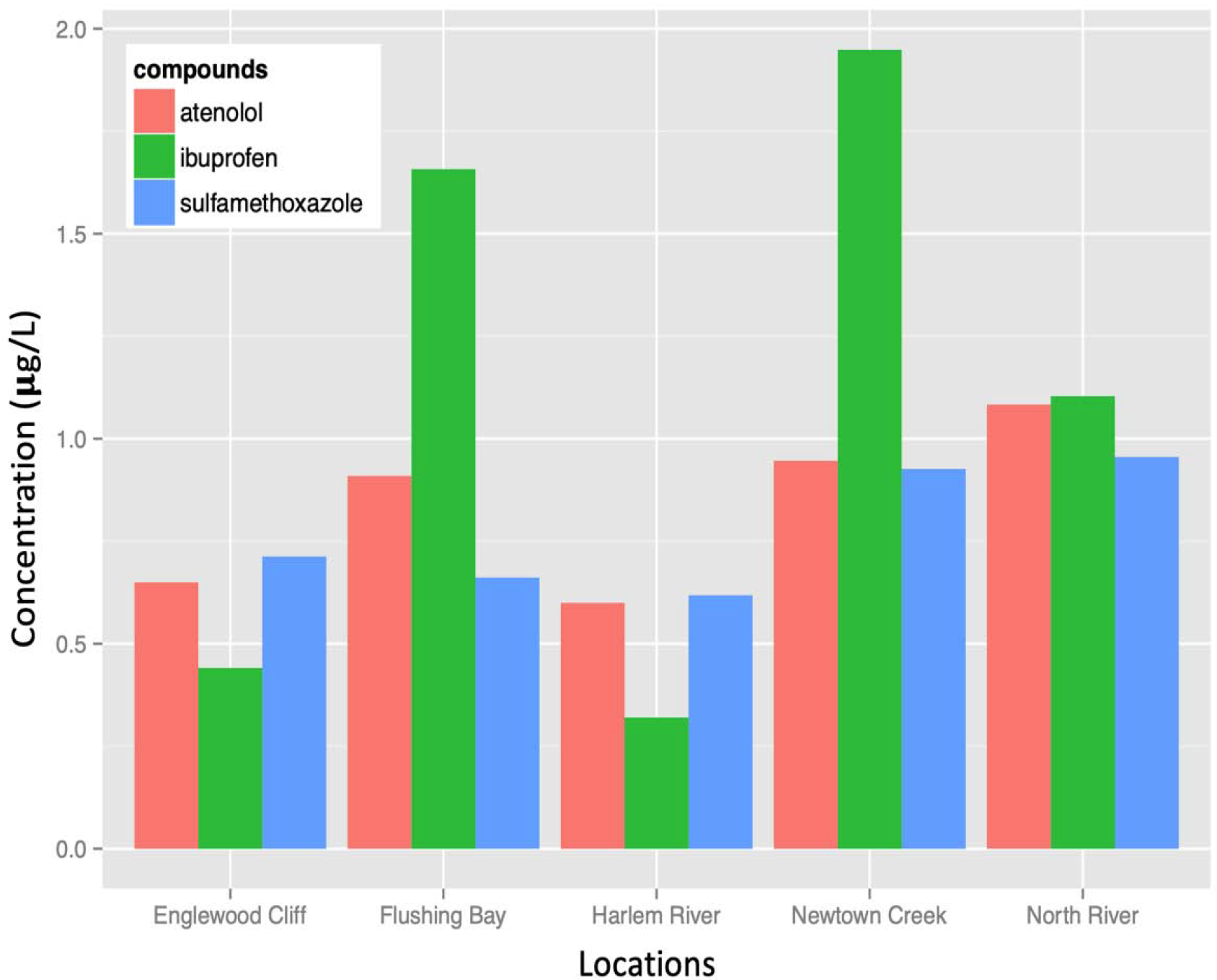
Concentrations of atenolol (μg/L), sulfamethoxazole and ibuprofen in surface water samples from Englewood Boat Basin, The North River Wastewater Treatment Plant, Newtown Creek, Flushing Bay, and the Harlem River.
We returned to these sites after 28 days. Upon retrieval, the baskets deployed in the Hudson River at sites #1–3 were missing from their moorings, presumably lost to the strong tidal action of the Hudson River. The baskets at Newtown Creek, Flushing Bay, and the Harlem River which are sheltered compared to the other sites, were retrieved successfully. On the day the baskets were retrieved, surface water samples were collected from Englewood Boat Basin, NRWWTP, Newtown Creek, Harlem River, and Flushing Bay. Water samples were collected in pre-combusted 2.5 L amber glass bottles.
Measuring pharmaceuticals in surface water samples
Upon our return to the laboratory, each 2.5 L water sample was processed within 24–48 hours of collection. Each 2.5L sample was spiked with 200 mL of saturated mercuric chloride solution (~74 g/L) to arrest microbial activity and 20 mL of 5% EDTA to reduce adsorption of organics to the wall of glass bottles. An aliquot of 475 mL (the volume of the glass bottles used to hold the sample) of each water sample was filtered through a pre-combusted Whatman 47mm GF/F filter. Pharmaceuticals were extracted from the filtered water using solid phase extraction columns (Waters Oasis HLB, 500mg, 6 mL, P/N: 186003365) (SI for details). The eluent was then analyzed for atenolol, sulfamethoxazole and ibuprofen using Sciex 6500+ liquid chromatography tandem mass spectroscopy (LC/MS/MS) for both positive and negative ionization modes. Field blank samples, lab blank samples, and isotopically-labeled compounds were used as QA/QC measures.
Treatment of pellets after retrieval
After removal from individual stainless-steel infusers, the eight test materials (one from each of the three sites) were separately washed with 100 mL of filtered surface water from each site to dislodge loose biofilm and environmental particles. Each sample was immersed in the water for two minutes and then gently stirred with a glass rod for three minutes. Plastics and glass beads were then removed from the surface water and then transferred into separate vials containing 2 mL of a 1:1 methanol acetonitrile solution for 3 hours for extractions of the analytes from glass beads and the plastic micro-pellets. Pieces of plastic straws and shreds of plastic bags were treated with 22 mL of this mixture due to their larger volume using an auto-mix (Gilson, Middleton, WI) for 3 hours. A 1mL portion of each extract was then centrifuged (Eppendorf, Germany) at 12,300 rotations per minute (RPM) for 20 minutes after which the supernatant was filtered through a filter vial. After filtration, 10 uL of the extracts were injected for analyzing atenolol, sulfamethoxazole and ibuprofen using LC/MS/MS. Extracts from the fragments of plastic bags and plastic straws were filtered through 47 mm GF/F filters (Whatman, United Kingdom), concentrated to a volume of approximately 0.3 ml, and then 10 uL was injected for LC/MS/MS analysis (See SI for details).
Calculation of sorption coefficients:
The net transfer of pharmaceuticals, from water (liquid phase) sorbed to plastics (solid) is calculated by the formula:
where Kpw [Lwater/kgplastic] represents the sorption coefficient and the concentrations of the chemical in solid phases (based on mass of plastic pellets, glass beads, straw, or plastics bags) to water are denoted by CP [μg/kgplastics] and CW [μg/Lwater] (Endo and Koelmans, 2019)
We also calculated the coefficients based on surface area of testing materials. The sorption coefficient based on surface area is equal to the concentration of sorbed pharmaceutical per millimeter squared of surface area divided by the concentration of pharmaceuticals present in the water sample from the corresponding site (i.e., the ratio of CP [μg/mm2] and CW [μg/Lwater]). Surface area was measured based on geometry of pellets, glass beads, straws, and plastic bags.
Results
Water samples
All three analytes were detected in the surface water samples at the five sites and their average concentration are very close, with sulfamethoxazole being the lowest at 0.77 μg/L, atenolol 0.84 μg/L, and ibuprofen the highest at 1.09 μg/L. Among five sites, sulfamethoxazole and atenolol had less spatial variation, while ibuprofen showed the largest spatial variation: 0.32 μg/L in the Harlem River and > 1.6 μg/L in Newtown Creek and Flushing Bay (Fig. 2). For all three compounds, the Englewood Cliff and Harlem River sites had lower concentrations than the other three sites nearer to wastewater treatment plant discharge sites (North River WWTP site, Newton Creek, and Flushing Bay).
Sorption coefficients based on mass
Figures 3a–3c shows the box plot of sorption coefficients in the unit of L/Kg of three compounds based on mass of materials. With respect to atenolol, plastic bags, which were very thin (~ 0.04 mm in thickness), had the largest coefficient (~ 0.2 L/Kg), about 4 to 5 times higher than straws but 2 orders of magnitude higher than other test materials. Interestingly the five plastics (PETE, HDPE, PVC, LDPE, and PP) showed very similar coefficients (~0.005 L/Kg), regardless of their difference in polymer type. Surprisingly glass beads, which were less hydrophobic compared to plastics, also had similar range of coefficients to plastics pellets. Ibuprofen and sulfamethoxazole showed a consistent pattern in coefficients among eight test materials as that of atenolol (Figures 3a – 3c). The mean of the coefficient of atenolol for all five plastics pellets is about 0.034 L/Kg, higher than ibuprofen and sulfamethoxazole (~0.025 L/Kg).
Figure 3:
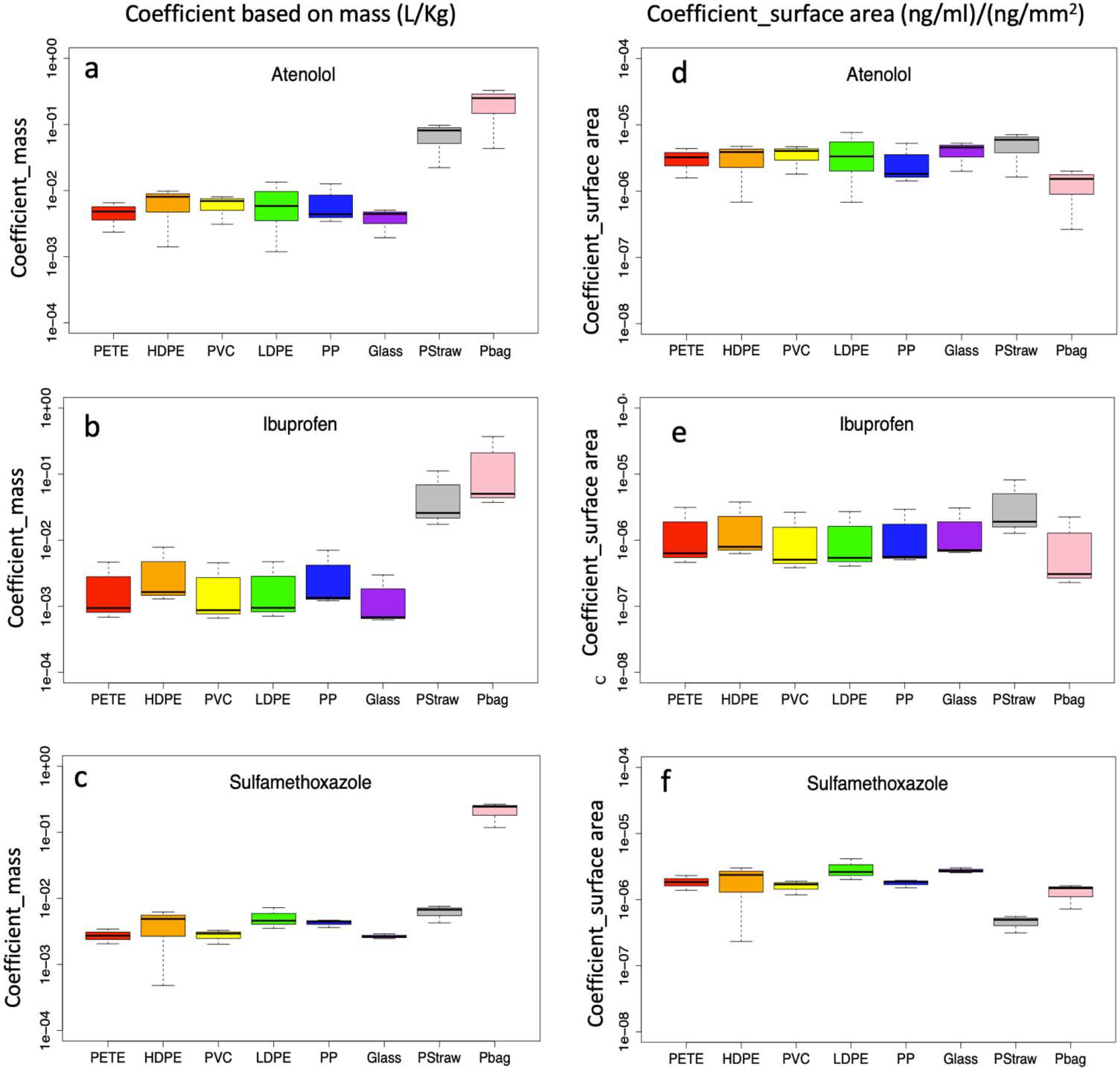
Sorption coefficients based on mass and surface area for atenolol, ibuprofen, and sulfamethoxazole over three sites (Newtown Creek, Flushing Bay, and Harlem River). Test materials includes: polyethylene terephthalate(PET), high density polyethylene(HDPE), polyvinyl chloride(PVC), low density polyethylene (LDPE), polypropylene(PP), glass beads(glass), plastic straws(PStraw), and plastic bags (Pbag).
Sorption coefficients based on surface area
Figures 3d –3f shows the results of the surface area-based coefficients, which were very different than those based on mass of materials (Figures 3a – 3c). Based on surface area, plastic bags had relatively low coefficients (~20% less) for all three compounds compared to the plastic pellets. The five types of plastic pellets and glass beads exhibited similar coefficients to each other by this metric.
Discussion
Concentration of pharmaceuticals in surface water samples
Atenolol, sulfamethoxazole and ibuprofen were detected in all water samples from the five sites tested. High concentrations of ibuprofen were found in Newtown Creek and Flushing Bay where nearby wastewater treatment plants are in operation (Fig 1). Levels of atenolol and sulfamethoxazole are at the high end of the range reported in Cantwell et al., (2018), a study which monitored the spatial distribution of pharmaceuticals in Hudson River Estuary.
The high pharmaceutical concentrations measured in the surface water samples collected around NYC are not surprising given the high volume of treated wastewater that is discharged into the river on a daily basis as well as the known limitations of wastewater management systems to effectively capture and decompose pharmaceuticals (Boxall et al., 2014). NYC WWTPs release about 1.3 billion gallons of treated sewage water daily (NYC-DEP, Wastewater, 2019), thus, it would be expected to hold higher levels of these chemical contaminants in NYC waterways. Atenolol is a drug that is not fully metabolized in humans so the parent compound is ubiquitous in wastewater effluent and easily detected in the surface waters of NYC (Cantwell et al., 2018). Low exposure of atenolol is shown to have mildly chronic effects on larval growth of the fat head minnow, Pimephales promelas (Winter et al., 2008). For sulfamethoxazole, the concentrations measured (0.5 to 1 μg/L) were in the range that can impact behavior of a soil nematode, Caenorhabditis elegans such as body bending frequency and reversal movement (Yu et al., 2011). Ibuprofen is a pharmaceutical with the lowest solubility of the three analytes. The level of ibuprofen reported here at ~1.09 μg/L, is well within range of levels reported to be highly toxic to larval development of sea urchin, Parencendrotus lividus specimens that were exposed to extremely low concentrations of ibuprofen (0.001 μg/L to 15 μg/L) in Washington’s Puget Sound (Aguirre-Martínez et al., 2015).
Sorption of pharmaceuticals based on mass and solubility
The target pharmaceuticals were found adsorbed on to all of the test materials. The majority of plastic pellets had similar sorption coefficients indicating that plastic composition, and molecular structures may not play a significant role in the sorption of pharmaceuticals to microplastics in NYC waterways. The mass-based coefficients of the three compounds in this study are in the range of 0.1 to 0.001 L/Kg, 3 to 6 orders of magnitude lower than coefficients of different pharmaceutical compounds reported in Wu et al. (2016). Two possible reasons can lead to the discrepancy; the particle size in Wu et al. study is 0.25 mm, which is about 10 times smaller than in this study (Wu et al., 2016), thus two orders of magnitude higher in surface area; another possible reason is lower solubility of compounds studied in Wu et al. than this work. The compound with highest Kd in Wu et al. was 4-methylbenzylidene camphor, which is poorly soluble in water (~1.3 mg/L of solubility) (European Commission, 2006), in contrast with the solubilities of 13,300 mg/L (at 25 °C) for atenolol, 680 mg/L (at 37 °C) for sulfamethoxazole, and 21 mg/L (at 25°C) for ibuprofen. In addition to particle size and solubility, there are some other reasons that can lead to the coefficient discrepancy between ours and Wu et al. (2016). Carbamazepine has a solubility of 17 mg/L (Pubchem, 2019), comparable to ibuprofen. However, the coefficient of carbamazepine is about four orders of magnitude higher than that of ibuprofen, thus there is about two orders of magnitude difference that cannot be explained.
Sorption based on surface area
Our data indicates that surface area can have a greater influence on pharmaceutical sorption than plastic composition as our findings show that these pharmaceuticals will sorb to all ranges of polymers in this specific marine environment. There were higher sorption coefficients based on surface area for all three compounds for plastic bags, mainly due to high surface area to mass ratio of plastic bags. Amorphous regions on the surface, glassy or rubbery structures of the polymer, weathering, and the age of polymers each play important roles in sorption capacities of plastics (Teuten et al., 2009); however, the pellets we used were each a homogeneous polymeric sphere of virgin material, of similar size and age, without exposure to weathering or other environmental degradation.
Influence of biofilm on sorption capacity
We suspect that the fast growth of biofilm onto the test materials plays an important role in the sorption of pharmaceuticals on particles in aquatic ecosystems. In our study, biofilm was observed on the second day after deployment. The basket initially deployed at the site Englewood Boat Basin broke loose from its buoy and traveled about 10 miles south along the Hudson River. Within 40 hours it was taken from the water and deployed back at the initial site. A thick layer of biofilm was observed at the time of retrieval.
The glass beads had similar sorption coefficients to the plastic pellets, which may be also due to the biofilm as observed. We are limited in our understanding of whether biofilm contributes or diminishes sorption capacity. Wunder et al. (2011) measured the sorption coefficient and found that the bacterial biofilm, which, in general, is negatively charged, does not necessarily increase sorption capacity and was shown to have little effect on the sorption of sulfamethoxazole to biofilm (Wunder et al., 2011). In another study, biofilm has been shown to facilitate sorption of metals to plastics coated in biofilm (Richard et al., 2019). Examining the influence biofilm on sorption of pharmaceuticals to microplastics as part of our future studies, will help shed light on its potential contribution to sorption dynamics.
Pharmaceutical solubility
Unlike the predictable relationship between Kd and the octanol/water partition coefficient (Kow), for hydrophobic organics such as PAHs and PCBs (Bergmann et al., 2015; Koelmans et al., 2013), Kow is not sufficient for predicting Kd for pharmaceuticals. For example, among three chemicals, ibuprofen has the highest log Kow (3.97), but it has the lowest Kd. This finding is consistent with the study of Wunder et al. (2011), which concluded that hydrophobic interactions (predicted by Kow) do not determine sorption of relatively hydrophilic antibiotics. In Wunder’s study, Kd of sulfamethoxazole on a bacteria biofilm was reported to be (4000 ± 1000 L/kg), which is about 7 orders of magnitude higher than our results (3.5 ×10−3 L/kg). Since we did not measure the weight of biofilm on the surface of our test materials, the two results cannot be compared directly.
Deployment period
We used a deployment period of 28 days in this study for several reasons; Rochman et al., found that equilibrium time decreases with Kow; for compounds with Kow > 5 such as high molecular weight PAHs and PCBs, the equilibrium time was typically more than 6 months (Rochman et al., 2013); for compounds with Kow < 5 (e.g., fluorene, phenanthrene, and anthracene), sorption did not fit the exponential sorption curve, indicating much less time needed to reach equilibrium (probably in days to a few months). Another issue considered is that the bioavailability of pharmaceutical compounds with low Kow is high, leading to relatively short half-lives (Bu et al., 2016). Rochman even observed the decreasing trend in concentration over time for compounds with low Kow (Rochman et al., 2013).
Other factors:
We are aware that many other factors can affect the interaction between the investigated pharmaceuticals and MPs, such as the types and concentrations of organic components, particulate species (e.g., soot, iron oxides, clay), dissolved species, which we acknowledge is a limitation of this study. However, in our field experiment settings, it was impossible to measure pharmaceuticals that are associated with these components since the isolation steps will change the partitioning coefficients. For example, in order to completely remove organics from particulate species, we need to use acid to remove organics first, which will affect pharmaceuticals adsorbed on to particle surface.
Limitations and future plans for addressing shortcomings of present study
We acknowledge other limitations of this study: 1) three of our sample sets were lost due to tidal action or human interference, 2) our results were based on deployments of samples over a period of 28 days; 3) replicates were not conducted due to limited funds, 4) it does not account for changes in adsorption due to changes in water temperatures, salinity and other soluble inorganic and organic constituents, and 5) it lacks information on the sorption kinetics of pharmaceuticals to biofilms. These limitations will be considered in our future studies. A repeat of this study would involve acquiring saltwater resistant stainless-steel baskets in order to complete wider sampling in the lower regions of the Hudson River. Replicates will need to be executed for more robust data on sorption coefficients. Further study on the factors which may influence the kinetics of sorption capacity are microplastic shape and surface texture, relative age and amount of weathering of microplastic particles, as well as pore size of the infuser’s steel mesh which restricted movement of the water and sorption capacity of pharmaceuticals in relation to the presence or non-presence of organic biofilm. Despite the limitations our study, the data collected clearly demonstrate that the coefficients are not determined by plastic types. Given this observation, it is reasonable to infer that the difference in replicates would have been minimal and therefore would not affect the general conclusion of this study.
Current and future implications
This study has strong implications; all of the connecting waterways of New York are of vital ecological and economic importance, yet the creeks, channels and rivers surrounding the city have historically been subjected to pollutants and poor water quality management. Both microplastics and pharmaceuticals are known to be ubiquitous pollutants in the waterways surrounding NYC (Lim, 2018; Palmer et al., 2008). The ability of WWTPs to effectively sort and filter out these pollutants prior to being released into the environment is highly limited. Newtown Creek in particular is subjected to high volumes of raw sewage primarily from four of the twenty-two CSO outlets along the creek. The Newtown Creek CSOs discharge an estimated 1.2 billion gallons of untreated run-off annually (NCA, 2017). This high level of treated and untreated wastewater impacts the water quality of NYC’s estuary and the organisms that inhabit it.
To the best of our knowledge, our study is the first of its kind to examine the affinity between microplastics and pharmaceuticals in-situ within the waterways of NYC. The data supports the claim that pharmaceutical contaminants are sorbing to the microplastic particles present in these waterways, therefore leading to the premise that the microplastics will act as vectors for the transport of pharmaceuticals and other organic pollutants into the ecological food web of this marine environment. In future, investigating the average concentrations of microplastics ingested by benthic filter feeders in future studies would allow us to better define the ecological impact of pharmaceuticals and microplastics.
Supplementary Material
Highlights.
In-situ microplastic sorption experiments were conducted in NYC waterways
Higher level of pharmaceutics on locations close to WWTP discharge sites
Similar sorption coefficients of pharmaceutics among different plastics and glass
Rapid biofouling appeared to be the predominant factor controlling the sorption
High coefficients for plastic straws and bags due to large surface area
Acknowledgements
We would like to thank Kali McKee for her time and assistance with our analysis in the lab, to Keyence Corporation of America for microscopic imaging, to the Riverkeeper organization for the dedication of time and resources for our study, and to Robert Newton for encouragement and support. Undergraduate students were supported by Lamont Summer Intern Program. Additional support was provided by the Hudson River Foundation and the US NIEHS (ES009089) and NIH shared instrument grant (1S10OD020058-01A1)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References:
- Aguirre-Martínez GV, Owuor MA, Garrido-Pérez C, Salamanca MJ, Del Valls TA, & Martín-Díaz ML (2015). Are standard tests sensitive enough to evaluate effects of human pharmaceuticals in aquatic biota? Facing changes in research approaches when performing risk assessment of drugs. Chemosphere, 120, 75–85. 10.1016/j.chemosphere.2014.05.087 [DOI] [PubMed] [Google Scholar]
- Boxall ABA, Keller VDJ, Straub JO, Monteiro SC, Fussell R, & Williams RJ (2014). Exploiting monitoring data in environmental exposure modelling and risk assessment of pharmaceuticals. Environment International, 73, 176–185. 10.1016/j.envint.2014.07.018 [DOI] [PubMed] [Google Scholar]
- Cantwell MG, Katz DR, Sullivan JC, Shapley D, Lipscomb J, Epstein J, Juhl AR, Knudson C, & O’Mullan GD (2018). Spatial patterns of pharmaceuticals and wastewater tracers in the Hudson River Estuary. Water Research, 137, 335–343. 10.1016/j.watres.2017.12.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr SA, Liu J, & Tesoro AG (2016). Transport and fate of microplastic particles in wastewater treatment plants. Water Research, 91, 174–182. 10.1016/j.watres.2016.01.002 [DOI] [PubMed] [Google Scholar]
- Conover D, Keith Cooper, Marcus Eriksen, Sherri Mason, James Nickels, & Beth Ravit. (2016). NY-NJ HARBOR ESTUARY PLASTIC COLLECTION REPORT. 19. [Google Scholar]
- Daughton CG (2001). Pharmaceuticals and Personal Care Products in the Environment: Overarching Issues and Overview. In Pharmaceuticals and Care Products in the Environment (Vol. 791, pp. 2–38). American Chemical Society. 10.1021/bk-2001-0791.ch001 [DOI] [Google Scholar]
- Fabbri E, & Franzellitti S (2016). Human pharmaceuticals in the marine environment: Focus on exposure and biological effects in animal species. Environmental Toxicology and Chemistry, 35(4), 799–812. 10.1002/etc.3131 [DOI] [PubMed] [Google Scholar]
- Geis E, LeNoble J, & Noël M (2018). Retention of microplastics in a major secondary wastewater treatment plant in Vancouver, Canada. ; Marine Pollution Bulletin, 133(553), 56. [DOI] [PubMed] [Google Scholar]
- Kleinteich J, Seidensticker S, Marggrander N, & Zarfl C (2018). Microplastics Reduce Short-Term Effects of Environmental Contaminants. Part II: Polyethylene Particles Decrease the Effect of Polycyclic Aromatic Hydrocarbons on Microorganisms. International Journal of Environmental Research and Public Health, 15(2), 287 10.3390/ijerph15020287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S (2018). Distribution of Microplastics in the Estuarine Waters around the New York Metropolitan Area. Barnard College, Dept of Environmental Science. [Google Scholar]
- Lobelle D, & Cunliffe M (2011). Early microbial biofilm formation on marine plastic debris. Marine Pollution Bulletin, 62(1), 197–200. 10.1016/j.marpolbul.2010.10.013 [DOI] [PubMed] [Google Scholar]
- Magadini DL, Louw RS, Perez-perez Y, Sarker RT, Torres T, & Goes JI (2017). Microplastics in Faunal Tissue of Marine Organisms. https://www.riverkeeper.org/wp-content/uploads/2018/04/Microplastics-2-2017-Poster.pdf [Google Scholar]
- Mason SA, Garneau D, Sutton R, Chu Y, Ehmann K, Barnes J, Fink P, Papazissimos D, & Rogers DL (2016). Microplastic pollution is widely detected in US municipal wastewater treatment plant effluent. Environmental Pollution, 218, 1045–1054. 10.1016/j.envpol.2016.08.056 [DOI] [PubMed] [Google Scholar]
- NYC Wastewater Treatment System—DEP. (2019). NYC Environmental Protection. https://www1.nyc.gov/site/dep/water/wastewater-treatment-system.page
- Palmer PM, Wilson LR, O’Keefe P, Sheridan R, King T, & Chen C-Y (2008). Sources of pharmaceutical pollution in the New York City Watershed. Science of The Total Environment, 394(1), 90–102. 10.1016/j.scitotenv.2008.01.011 [DOI] [PubMed] [Google Scholar]
- Razanajatovo RM, Ding J, Zhang S, Jiang H, & Zou H (2018). Sorption and desorption of selected pharmaceuticals by polyethylene microplastics. Marine Pollution Bulletin, 136, 516–523. 10.1016/j.marpolbul.2018.09.048 [DOI] [PubMed] [Google Scholar]
- Winter MJ, Lillicrap AD, Caunter JE, Schaffner C, Alder AC, Ramil M, Ternes TA, Giltrow E, Sumpter JP, & Hutchinson TH (2008). Defining the chronic impacts of atenolol on embryo-larval development and reproduction in the fathead minnow (Pimephales promelas). Aquatic Toxicology, 86(3), 361–369. 10.1016/j.aquatox.2007.11.017 [DOI] [PubMed] [Google Scholar]
- Wu C, Zhang K, Huang X, & Liu J (2016). Sorption of pharmaceuticals and personal care products to polyethylene debris. Environmental Science and Pollution Research, 23(9), 8819–8826. 10.1007/s11356-016-6121-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


