Abstract
Lumbar spinal stenosis (LSS) is defined as a degenerative disorder showing a narrowing of the spinal canal. The diagnosis is straightforward in cases with typical neurogenic claudication symptoms and unequivocal imaging findings. However, not all patients present with typical symptoms, and there is obviously no correlation between the severity of stenosis and clinical complaint. The radiologic diagnosis of LSS is widely discussed in the literature. The best diagnostic test for the diagnosis of LSS is magnetic resonance imaging (MRI). However, canal diameter measurements have not gained much consensus from radiologists, whereas qualitative measures, such as cerebrospinal fluid space obliteration, have achieved greater consensus. Instability can best be defined by standing lateral radiograms and flexion-extension radiograms. For cases showing typical neurogenic claudication symptoms and unequivocal imaging findings, the diagnosis is straightforward. However, not all patients present with typical symptoms, and there is obviously no correlation between the severity of stenosis (computed tomography and MRI) and clinical complaint. In fact, recent MRI studies have shown that mild-to-moderate stenosis can also be found in asymptomatic individuals. Routine electrophysiological tests such as lower extremity electromyography, nerve conduction studies, F-wave, and H-reflex are not helpful in the diagnosis and outcome prediction of LSS. The electrophysiological recordings are complementary to the neurologic examination and can provide confirmatory information in less obvious clinical complaints. However, in the absence of reliable evidence, imaging studies should be considered as a first-line diagnostic test in the diagnosis of degenerative LSS.
Key words: Canal diameter, Central stenosis, Electrophysiological recordings, Foraminal stenosis, Intraoperative neurophysiological monitoring, Lumbar spinal stenosis, Motor evoked potentials, Natural course, Somatosensory evoked potentials
Abbreviations and Acronyms: CT, Computed tomography; DSEP, Dermatomal somatosensory evoked potential; EMG, Electromyography; IONM, Intraoperative neurophysiological monitoring; LS, Likert scale; LSS, Lumbar spinal stenosis; MEP, Motor evoked potential; MRI, Magnetic resonance imaging; NASS, North American Spine Society; SSEP, Somatosensory evoked potential; VAS, Visual analog scale; WFNS, World Federation of Neurosurgical Societies
Introduction
The syndrome of lumbar spinal stenosis (LSS) was not widely diagnosed until Verbiest's clinical description in 1954.1 The cardinal symptom is neurogenic claudication (spinal claudication), defined as diffuse buttock and leg pain, paresthesia, and cramping of 1 or both lower extremities induced by walking, which is relieved when sitting and forward bending.
Walking ability can become very limited due to neurogenic claudication, causing patients to seek medical help. Typically, a neurologic examination of the lower limbs does not reveal any major deficit.
LSS is defined as a degenerative disorder showing a narrowing of the lumbar spinal canal. It is often combined with instability in 1 or several segments of the lumbar spine. It can be classified based on the anatomical location of the narrowing of the spinal cord (central spinal stenosis, lateral spinal stenosis, foraminal stenosis), or based on the etiology (primary or acquired). The stenosis usually results from degenerative changes such as facet joint degeneration, hypertrophic ligamentum flavum, degenerative spondylolisthesis or lumbar intervertebral disc protrusion, or a combination of these conditions. The diagnosis is straightforward in cases with typical neurogenic claudication symptoms and unequivocal imaging findings. However, not all patients present with typical symptoms and, unfortunately, there is no correlation between the severity of stenosis (as assessed by computed tomography [CT] and magnetic resonance imaging [MRI]) and clinical complaints. In fact, recent MRI studies have shown that mild-to-moderate stenosis can also be found in asymptomatic individuals.
The value of electrophysiological tests, although widely ordered in lumbar radiculopathies, on the diagnosis of LSS and estimating the treatment outcomes are not well known.
Scientific literature on LSS diagnosis and treatment is wide and not homogeneous in treatment decision. A value brief is presented by the North American Spine Society (NASS), in its 2011 guidelines on the diagnosis and treatment of LSS2 although cannot be considered definitive.
This review aims to precipitate our knowledge regarding the diagnosis of LSS with both imaging techniques and electrophysiology, endeavoring to set up recommendations for the diagnosis of the disease.
Methods
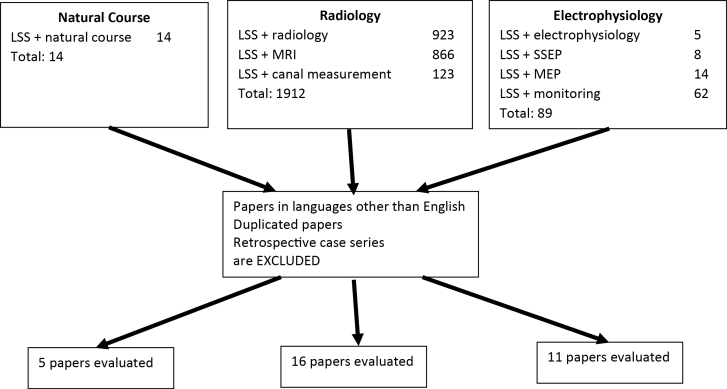
A search was performed for peer-reviewed papers, published in English, over the last 10 years (2008–2018). Review of the literature was done using PubMed, Ovid Medline, the Cochrane Central Register of Controlled Trials, and Cochrane Database of Systematic Reviews. The search items were “Lumbar spinal stenosis and natural course,” “Lumbar spinal stenosis and radiology,” “Lumbar spinal stenosis and magnetic resonance imaging,” “Lumbar spinal stenosis and canal measurement,” “Lumbar spinal stenosis and electrophysiology,” “Lumbar spinal stenosis and somatosensory evoked potentials,” “Lumbar spinal stenosis and motor evoked potentials,” and “Lumbar spinal stenosis and monitoring.” The flowchart of the searched items is shown in Figure 1.
Figure 1.
Flowchart for manuscript selection of the last 10 years. LSS, lumbar spinal stenosis; MEP, motor evoked potential; MRI, magnetic resonance imaging; SSEP, somatosensory evoked potential.
On the basis of the most significant literature, we drafted statements and presented them in Milan in November 2018. After a preliminary voting session, some statements were excluded because of the very low evidence of existing literature. The remaining statements were then presented and voted on in Belgrade in March 2019. The method is described in detail in the introductive paper of this special issue.
This paper is summarized in 3 sessions: Natural course, Radiologic diagnosis, and Electrophysiological diagnosis.
Results
Natural Course of Lumbar Spinal Stenosis
There is a recent trend for more surgeries being carried out involving LSS. The increased number of spinal surgeries, fusion surgeries, and surgeries in older patients in a recent cohort are noteworthy. A Korean study examined the national health insurance database between 2003 and 20083 and found a 2.54-fold increase in surgical volume in the 2008 cohort. The proportion of fusion surgeries also increased (20.3% in 2003, 37.0% in 2008). A major critic for the trend is that a conservative treatment or simple follow-up (wait and see) may be deemed sufficient for those patients.
There are 2 main questions that need to be answered: 1) What percentage of patients with spinal stenosis worsens without surgery? 2) Are there predictive signs/symptoms that patients will worsen?
A literature search in PubMed using “Lumbar spinal stenosis” and “natural course” produced 14 papers. Of those, only 8 studies were relevant. Three studies were removed because they had been published more than 10 years ago. The remaining 5 studies were examined.4, 5, 6, 7, 8
In a series of 34 conservatively treated patients, with a 10-year follow-up,6 the symptoms improved in 30% of the patients, whereas they remained unchanged in 30%, and became worse in the remaining 30%. The authors also measured canal diameters and found that the clinical course may deteriorate with conservative treatment in patients with a dural sac cross-sectional area <50 mm2.6
Another series involving 56 conservatively treated patients and a 7.3-year follow-up5 showed that a satisfactory outcome could be achieved in approximately 61% of the patients. Electrophysiological abnormalities, namely the presence of pluriradicular involvement and abnormalities of the soleus H-reflex, were reported to be predictive of a deterioration in clinical status.5
The so-called Verbiest trial to evaluate the effectiveness of prolonged conservative treatment compared with decompressive surgery had a 5-year follow-up.8 The authors concluded that the overall observed clinical improvement after surgery was disappointing and that a substantial proportion of surgical candidates managed conservatively may still report improvement.8
A relatively new study from 2016 involved 274 conservatively treated patients and a 3-year follow-up.4 In 30% of patients, conservative treatment led to a subjective improvement in the symptoms, whereas in 70% of patients, the symptoms remained unchanged, worsened, or required surgical treatment. The predictive factors for subjective symptom improvement were found: 1) presence of only radicular symptoms, 2) absence of degenerative spondylolisthesis/scoliosis, and 3) symptom duration of <1 year.4
In a Japanese study with 1080 participants and 1-year follow-up,7 more than 50% of the subjects with LSS-positive showed an LSS-negative at the end of the 1-year follow-up (i.e., symptoms disappeared). On the other hand, 10% of those who were LSS-negative in the initial analysis developed LSS-positive within 1 year (i.e., new symptoms developed).7
According to the literature review, the World Federation of Neurosurgical Societies (WFNS) Spine Committee has proposed and voted the statements as follows.
Statement 1: approximately 30% of patients with LSS are expected to worsen, but 30% may improve with conservative measures. This statement reached a strong positive consensus (67% voted grade 5 of Likert scale [LS], 22% voted grade 4, and 11% voted grade 3).
Statement 2: there are predictive signs/symptoms that they will worsen: dural sac cross-sectional area <50 mm2, presence of radicular symptoms and back pain, presence of degenerative spondylolisthesis and/or scoliosis, symptom duration >1 year. This statement also reached a strong positive consensus (11% voted grade 5 of LS, 22% voted grade 4, 56% voted grade 3, and 11% voted grade 2).
Radiology in Diagnosis of Lumbar Spinal Stenosis
The radiologic diagnosis of LSS may be seen as an easy task at first sight. However, there are many confusing points. In particular, the main drawback is considered to be the lack of correlation between symptoms and degree of stenosis. Furthermore, the type of quantitative measurements, radiologic classification, and estimation of outcome are all matters to be addressed.
We tried to answer the following questions:
-
1.
What is the best diagnostic test for the diagnosis of LSS?
-
2.
Which radiologic criteria (qualitative and quantitative) best describe LSS?
-
3.
Which radiologic criteria do we have to define instability in LSS?
Regarding the first question (what is the best diagnostic test for the diagnosis of LSS), the summary presented by the NASS is quite useful.2 This is a publication of the NASS on evidence-based clinical guidelines for the diagnosis and treatment of degenerative LSS. They provide recommendations to address key clinical questions surrounding the diagnosis and treatment of degenerative LSS. Their conclusions are as follows:
-
1.
In patients with history and physical examination findings consistent with LSS, MRI is suggested as the most appropriate noninvasive test to confirm the presence of anatomic narrowing of the spinal canal or the presence of nerve root impingement (grade of recommendation B).
-
2.
In patients for whom MRI is either contraindicated or inconclusive, CT myelography and, if contraindicated, CT are suggested as the most appropriate tests to confirm the presence of anatomical narrowing of the spinal canal or nerve root impingement (grade of recommendation B).
-
3.
About imaging correlation with clinical findings there is insufficient evidence to make a recommendation for or against a correlation between clinical symptoms with the presence of anatomical narrowing of the spinal canal on MRI, CT myelography, or CT.2
Definition
The definition of LSS is as follows: “a condition in which there is diminished space available for the neural and vascular elements in the lumbar spine secondary to degenerative changes in the spinal canal.”2
Literature is full of different recommendation studies. In 2016, Cowley9 published a review analyzing the circumstances underlying the physical narrowing of the spinal canal, the pathophysiology of clinical syndromes associated with stenosis, the assessment of the strengths and weaknesses of different imaging strategies, the different observational sings and objective criteria that have been proposed in neuroimaging literature, and clinical-radiologic correlation.
Radiologic Criteria for LSS
Andreisek et al10 presented the results of a consensus conference regarding core radiologic parameters to describe the lumbar stenosis. The aims of the meeting were as follows: 1) to define radiologic criteria and parameters that should be used as a minimum standard in a structured radiologic report for suspected LSS; 2) to identify radiologic criteria and parameters that might be used for research purposes. In this meeting, all available criteria for LSS were identified by 15 internationally renowned experts from different countries using systematic literature reviews and Delphi survey. They did not consider any of the quantitative parameters to be an essential part of standard clinical reports because of the lack of evidence correlation between parameters and symptoms, difficult acquisition during clinical routine, time-consuming measurements, and moderate reproducibility and reliability.10 However, they defined a core set of 5 qualitative radiologic criteria that should be included in a radiologic report for patients referred with suspected LSS: 1) compromise of the central zone and relation between fluid and cauda equina for the central stenosis; 2) nerve root compression in the lateral recess for the lateral stenosis; and 3) nerve root impingement and compromise of the foraminal zone for foraminal stenosis. In order to grade and define these criteria, the reference papers were compromising of the central zone with classification of mild, moderate, and severe; relation between fluid and cauda equina with grading of severity of LSS; nerve root compression in the lateral recess for the lateral stenosis; and nerve root impingement and compromise of the foraminal zone for foraminal stenosis11, 12, 13, 14 (Table 1). The experts also defined some other quantitative parameters that are better suited for research as anteroposterior diameter; cross-sectional area of the dural sac; compression of the dural sac in percentage; and lateral recess height.
Table 1.
Parameters Suggested for Radiologic Diagnosis of Lumbar Spinal Stenosis in a Consensus Conference10
| Qualitative | Quantitative | |
|---|---|---|
| Central stenosis | Hypertrophy of the ligamentum flavum Disc pathology Relation between fluid and cauda equina∗ Compromise of the central zone∗ Reduction of posterior epidural fat Epidural lipomatosis Redundant nerve roots of the cauda equina Sedimentation sign |
AP diameter of the spinal canal AP diameter of the contrast column (myelography) AP diameter of the thecal sac Compression of the thecal sac area in percent of normal mid-sagittal diameter Cross-sectional area of the dural sac Ligamentous interfacet distance Transverse diameter of the spinal canal |
| Lateral stenosis | Compression of the subarticular area Nerve root compression in the lateral recess∗ |
Lateral recess height Depth of lateral recess Lateral recess angle |
| Foraminal stenosis | Foraminal nerve root impingement∗ Size and shape of the foramen Hypertrophic facet joint degeneration Compromise of the foraminal zone∗ Perineural intraforaminal fat |
Foraminal diameter |
Only the qualitative parameters with ∗ are accepted as reliable and reached a consensus.
AP, anteroposterior.
The reference paper for distinction in central, lateral, and foraminal stenosis is the one by Fardon et al,15 reported in the guidelines of the NASS, American Society of Spine Radiology, and American Society of Neuroradiology. The authors describe the central zone as the zone within the vertebral canal between sagittal planes through the medial edges of each facet; the lateral recess as that portion of the subarticular zone that is medial to the medial border of the pedicle; and the foraminal zone as the zone between planes passing through the medial and lateral edges of the pedicles.15
For a definition of radiologic criteria, a panel containing 21 radiologists (musculoskeletal and neuroradiologist mixed from Europe and the USA) discussed the issue in a 3-round Delphi survey. The purpose was to develop a list of radiologic criteria for describing LSS and to assess the strength of agreement among experts on the most relevant criteria. In general, the panel did not accept quantitative measurements; the highest rated quantitative criterion was the anteroposterior diameter of the bony canal (cutoff 12 mm at the level of the end plate). Accepted qualitative criteria, with the highest rated, were: 1) disc protrusion, 2) lack of perineural intraforaminal fat, 3) hypertrophic facet joint degeneration, 4) absent fluid around the cauda equina, and 5) hypertrophy of the ligamentum flavum.16
Steurer et al17 published a review identifying papers reporting on radiologic criteria to describe LSS; they identified 25 studies reporting on radiologic signs of LSS; 10 different parameters were identified to quantify LSS. The most frequent measures reported for central stenosis were the anteroposterior diameter (<10–12 mm) and cross-sectional area (<70 mm2) of the spinal canal. For lateral stenosis height and depth of the lateral recess, and for foraminal stenosis, the foraminal diameters were typically used. Only 4 of 63 primary studies included in the systematic reviews reported on quantitative measures for defining inclusion criteria of patients in prognostic studies. In conclusion, there is no consensus on well-defined, unambiguous quantitative radiologic criteria to define LSS to improve diagnostic accuracy and to formulate reliable inclusion criteria for clinical studies.17
However, one of the most apparent drawbacks in quantitative radiologic definitions of LSS is represented by the reproducibility.18,19
Barz et al20 stressed different signs to describe LSS, such as redundant nerve root and sedimentation signs particularly evident in the MRI study.
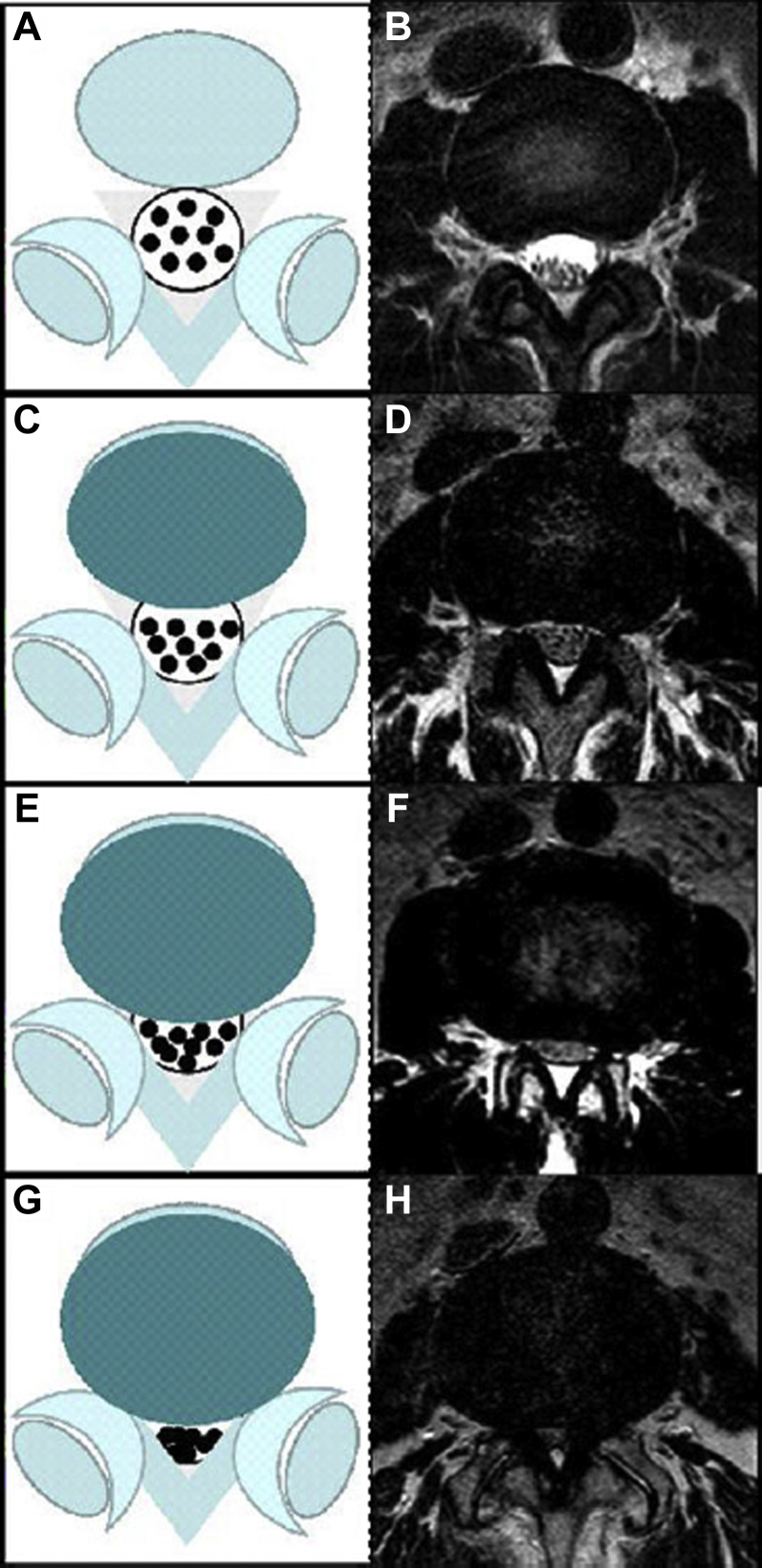
Guen et al21 proposed a qualitative grading system for central canal stenosis according to axial MRs on T2-weighted images. After defining the amount of cerebrospinal fluid space obliterations, the canal stenosis is called mild, moderate, and severe stenosis. Grade 0, no stenosis; grade 1, mild stenosis with clear separation of each cauda equina; grade 2, moderate stenosis with some cauda equina aggregation; grade 3, severe stenosis with the entire cauda equina as a bundle (Figure 2).
Figure 2.
Lumbar central canal stenosis (LCCS) is defined when anterior cerebrospinal fluid space is obliterated and is divided into 4 grades: grade 0, no LCCS (A, B); grade 1, mild stenosis with clear separation of each cauda equine (C, D); grade 2, moderate stenosis with some cauda equina aggregation (E, F); grade 3, severe stenosis with the entire cauda equina as a bundle (G, H). Diagrams on left and T2-weighted axial images on right side of each LCCS grade are illustrated.
(From Guen et al.21)
Instability Signs
Instability is defined as “an abnormal response to applied loads, characterized by movement in the motion segment beyond normal constraints” (Academy of Orthopedic Surgeons). There are many papers regarding the predictive signs of instability in radiologic imaging. However, although degenerative spondylolisthesis has an unequivocal radiologic definition, regarding “instability” there are no unquestionable and currently applicable clinical or radiologic criteria available. Moreover, the relationship between radiologic evidence of instability and its symptoms remains controversial.
Current literature suggests numerous radiologic parameters and cutoff values to diagnose spinal instability for both translation and angulations, demonstrating the lack of consensus as far as the diagnosis of spinal instability is concerned. These criteria are further defined as sagittal plane translation >3–4.5 mm, abnormal alignment of segments greater than 8%, as well as sagittal plane rotations of 15% at L1–L2, L2–L3, and L3–L4; 20% at L4–L5; and 25% at L5-S1 in a flexion-extension radiograph.
Direct signs of instability are defined as translation and rotation, whereas indirect signs of instability are Modic changes, end-plate edema, traction spurs, extended discal vacuum, synovial cysts, annular tears, spondylolisthesis, retrolisthesis, facets gaping with joint effusion or vacuum, and “facet fluid sign.”22,23
Tarpada et al24 underlined that relaxed supine position (as during MRI and CT studies) can facilitate the reduction of the anterolysthetic segment by decreasing paraspinal tension and increasing vertebral segment mobility.
The reference paper for the definition of lumbar instability is the one by Matz et al,25 reported in the guidelines of the NASS, American Society of Spine Radiology, and American Society of Neuroradiology. The authors state that the lateral radiograph is the most appropriate, noninvasive test for detecting degenerative lumbar spondylolisthesis (grade of recommendation B), and in the absence of reliable evidence, it is the work group's opinion that the lateral radiograph should be obtained in the standing position whenever possible. They underlined that there is insufficient evidence to make a recommendation on the most appropriate diagnostic or physical examination test consistent with fixed or dynamic deformity in degenerative lumbar spondylolisthesis patients because of the lack of uniform reference standards that define instability. In fact, there is no universally accepted standard to diagnose fixed versus dynamic spondylolisthesis. To evaluate instability, many studies employ the use of lateral flexion extension radiographs, which may be done in the standing or recumbent position; however, there is a broad variation in the definition of instability.25
According to these literature reviews, the WFNS Spine Committee has proposed and voted the statements as follows.
Statement 3: MRI is the most appropriate noninvasive test and the second is CT scan. CT myelography is appropriated if MRI is contraindicated or inconclusive. This statement reached a strong positive consensus; all expressed a positive vote (67% voted grade 5 of LS, 22% voted grade 4, and 11% grade 3).
Statement 4: there is no correlation between clinical symptoms or function and the presence of anatomic narrowing of the spinal canal on MRI, CT, or myelo-CT. All expressed a positive vote with a strong consensus (33% voted grade 5, 33% voted grade 4, and 34% grade 3).
Statement 5: qualitative radiologic criteria describe adequately spinal stenosis in central, lateral, or foraminal stenosis. This statement has reached a strong positive consensus (50% voted grade 5, 10% grade 4, 20% grade 3, and 20% grade 2).
Statement 6: there are some radiologic signs that describe instability. Direct signs on functional radiograms are translation and/or rotation. Indirect signs on MRI and CT are Modic changes, end-plate edema, extended discal vacuum, traction spurs, synovial cysts, annular tears, spondylolisthesis, and “facet fluid sign.” This statement also reached a strong positive consensus (38% voted grade 5, 25% grade 4, 25% grade 3, and 12% grade 2).
Two fundamental conditions are necessary for the diagnosis of LSS: 1) radiologic evidence of reduction of the section of the spinal canal (this evidence alone does not require surgical treatment) and 2) clinical symptoms such as neurogenic claudication or radicular deficits. The first condition is defined by radiologic examinations. One of the most accepted descriptions of spinal stenosis is that of the NASS: “a condition in which there is diminished space available for the neural and vascular elements in the lumbar spine secondary to degenerative changes in the spinal canal.”
Although it is stated that MRI is the best radiologic imaging for the diagnosis of LSS and that an adequate description is provided by qualitative parameters, there are still some open questions that require further discussion.
The lack of correlation between radiologic imaging and clinical symptoms leaves 2 issues open: 1) there is no globally accepted radiologic definition between normal and abnormal imaging; and 2) the need to define some criteria to correlate imaging and clinical onset.
Another open question is the choice of the best diagnostic method; it should be easy, cheap, readily available, and reproducible.
Lastly, the most difficult question to discuss is the definition of instability; it is still unclear which examination is the best for the diagnosis, especially concerning the utility of dynamic X-ray, and how to perform it, and which indirect signs can correlate with instability.
Electrophysiology in Diagnosis of the Lumbar Spinal Stenosis
As there is still no consensus in scientific literature for a “golden standard” in LSS neurophysiological investigation, the following tests have been proposed and used.
Electromyographic Paraspinal Mapping
Yagci et al26 reported a prospective comparative study of 62 nonconsecutive patients evaluating the utility of lumbar paraspinous mapping in the diagnosis of LSS. Clinical criteria assessed were pain that improves with sitting and is exacerbated with standing, thigh pain with 30 seconds of sustained lumbar extension, the presence of neurogenic claudication, and the presence of paresthesia.
The midline anteroposterior diameter of the dural sac was assessed, along with nerve conduction studies and electromyography (EMG) of the lower extremities. Paraspinous mapping showed fibrillation potentials and positive sharp waves in at least 2 levels in 92.8% of the patients with clinical and radiologic LSS, whereas it was normal in 93.8% of patients in the radiologic spinal stenosis group. In the control group, 6 of 14 patients had high paraspinous mapping scores, mostly secondary to acute monoradiculopathy caused by disc herniation. If the cutoff value is set at 9, the sensitivity and specificity would be 96.8% and 92.3%, respectively.
The authors concluded that the paraspinous mapping technique is a sensitive method in diagnosis and reflects the physiology of nerve roots better than the limb EMG.26 Lumbar paraspinous mapping may be useful in the presurgical evaluation of patients with equivocal clinical and MRI findings (Level III evidence).
Haig et al27 reported a prospective comparative study evaluating the sensitivity and specificity of electrodiagnostic testing, specifically paraspinal mapping. Paraspinous mapping EMG of >4 muscles had a 100% specificity and 30% sensitivity for stenosis compared with either back pain or asymptomatic patients.
A composite limb and paraspinal fibrillation score had a specificity of 87.5% and a sensitivity of 47.8%; H-wave had a specificity of 91.3% and a sensitivity of 36.4%. Seven subjects with previously undiagnosed neuromuscular disease were diagnosed. Their conclusion was that electrodiagnostic testing has statistically significant and clinically meaningful specificity for spinal stenosis and detects neuromuscular diseases that may mimic stenosis.27
This study provides Level III diagnostic evidence that paraspinal mapping is useful in diagnosing polyneuropathy and myopathy in both patients with stenosis and controls, and that paraspinous mapping, a composite limb and paraspinous fibrillation score, and absence of H-waves had a high specificity and low sensitivity for LSS compared with asymptomatic controls.
F-Wave
F-waves are often used to measure nerve conduction velocity and are particularly useful for evaluating conduction problems in the proximal region of nerves.
H-Reflex
The H-reflex (or Hoffmann's reflex) is a reflex reaction of muscles after electrical stimulation of sensory fibers in their innervating nerves. H-reflex is analogous to the mechanically induced spinal stretch reflex.
Somatosensory Evoked Potentials (SSEPs)
SSEPs indicate a lumbar nerve involvement complementary to a neurologic examination and can provide confirmatory information in a less obvious clinical examination. Egli et al28 presented a prospective case series investigating the relationship between electrophysiological recordings and clinical as well as radiologic findings in patients with LSS. Of the 54 patients included in the study, 68% indicated suffering from a severe reduction of walking distance limited to 500 m or less. In 70% of patients, the motor and/or sensory (pin prick and light touch) scores were normal. 87% of patients showed pathologic electrophysiological recordings. Abnormal tibial SSEP was in 78% of patients, delayed F-wave responses was in 15%, and abnormal H-reflex was in 52% of patients.
This study provides Level III evidence that electrophysiological studies, in particular the SSEP and nerve conduction velocity, are abnormal in patients more often than the clinical examination. The results of these studies do not correlate with radiologic findings.
Liu et al29 described a retrospective case series evaluating the clinical usefulness of assessing lumbar SSEPs in central LSS. Of the patients included in the study, 40 had MRI and clinical examination findings consistent with LSS, whereas 39 patients with cervical myelopathy served as controls.
The latencies of lumbar SSEPs in patients with LSS and in the control group were 23.0 ± 2.0 and 21.6 ± 1.9 milliseconds, respectively. There was a statistically significant difference between the LSS and control groups (P < 0.05). The latency of lumbar SSEPs in LSS was clearly delayed when the visual analog scale (VAS) score of leg numbness was 0.8 (P < 0.05).
The authors concluded that lumbar SSEPs are able to detect neurologic deficit in the lumbar area effectively, and they can reflect part of the subjective severity of sensory disturbance (numbness). Both lumbar SSEPs and VAS scores of leg numbness may be useful for clinical evaluation in patients with LSS.
Motor Evoked Potentials (MEPs)
Liu et al30 discussed results of a retrospective case series evaluating the clinical usefulness of assessing MEPs in 23 patients with LSS. MEP latency was related to the walking distance, limb symptoms, and the VAS for numbness. MEP latency was significantly delayed in patients who described a walking distance less than 500 m. MEP latency showed no correlation with duration of symptoms and VAS for back or leg pain.
The authors concluded that MEP is useful in LSS assessment. It can reflect the subjective severity of motor disturbance and predict the neurologic deficit before appearance.
Similarly, electrical root stimulation revealed more abnormalities in patients with LSS in comparison with needle EMG.31 However, both methods complement each other to show additional pathology in a given patient.
Motor Conduction Studies
Senocak et al32 described a retrospective case control study evaluating delays in the motor conduction time in the cauda equina of 15 patients with LSS compared with 20 controls.
The mean conduction time along the cauda equine was significantly prolonged in patients with LSS (3.57 ± 2.22 milliseconds) compared with controls (1.97 ± 0.67 milliseconds).
The absolute latency values were significantly prolonged from the L1 level to both the tibialis anterior and the gastrocnemius-soleus muscles, and from the L5 level to the tibialis anterior muscle. However, the latency values from the L5 level to the gastrocnemius-soleus muscle were not significantly different from controls.
The authors concluded that determining the motor conduction time along the cauda equina using L1 and L5 magnetic stimulation provides an effective alternative method for evaluating the lumbar motor roots in patients with LSS. As a critic, this was a small study of nonconsecutive patients and should be considered Level IV diagnostic evidence.
Dermatomal Somatosensory Evoked Potentials (DSEPs)
Shen33 reported results from a retrospective case control study evaluating the clinical significance of DSEP, assessing the degree of nerve root injury after LSS in 47 nonconsecutive patients compared with 50 controls. The sensitivity and diagnostic concurrence with surgery of nerve root injury after LSS evaluated by DSEP was 95.7%.
Nerve root injury was categorized according to DSEP latency as follows: severe damage (disappearance of the P40 wave in 103 dermatomes), moderate damage (prolongation of the P40 peak latency ≥3.0 times the standard deviation of the normal mean in 60 dermatomes), and mild damage (prolongation of the P40 peak latency ≥2.5 times the standard deviation of the normal mean in 31 dermatomes).
In conclusion, DSEP can be used to determine the severity of nerve root injury after LSS with high sensitivity and specificity (Level III).
Intraoperative Neurophysiological Monitoring (IONM) During LSS Surgery
To receive real-time feedback, neurophysiological assessments during surgery were introduced and have developed into a useful tool.34,35 IONM has clearly been shown to be effective in spinal cord tumors. Its use is nevertheless not widely accepted.
Sharan et al36 could not find any evidence in the literature that IONM can help in preventing nerve root injuries during pedicle screw fixation. Similarly, not all neurologic incidents had been recognized by IONM in a study by Alemo and Sayadipour.37 There is not always a distinction in literature reviews between the different modalities in particular between SSEPs and MEPs that differ in their prognostic value with SSEPs being regarded as less sensitive.
Little is known so far about the possible positive effect of surgical decompression procedures to the electrophysiological response and functional outcome. Piasecki et al38 found that immediate neurophysiological response in IONM after decompressive surgery for LSS is correlated with positive effects on clinical outcomes after a mean 8-month follow-up, but at late follow-up (more than 28 months), the beneficial effects of surgery decline gently and no significant correlation could be found between the MEP response and patient clinical condition. Piasecki et al38 suggest that the intraoperative neurophysiological improvement during decompressive surgery may predict clinical outcome at 6 months after surgery. Unfortunately, the initial improvement in functional outcome diminishes after time making the relation between function and neurophysiological changes less meaningful. Initial neurophysiological changes could be helpful in predicting short-term failures.
According to these literature reviews, the WFNS Spine Committee has proposed and voted the statements as follows.
Statement 7: routine electrophysiological tests (EMG, nerve conduction study, F-wave response, H-reflex, SSEP, MEP) have no diagnostic value for LSS. All expressed a positive vote to this statement with a strong positive consensus (38% voted grade 5 of LS, 25% voted grade 4, and 37% voted grade 3).
Statement 8: electrophysiological tests do not predict outcome of patients with LSS. This statement also reached a strong positive consensus (75% voted grade 5 of LS, 13% voted grade 4, and 12% voted grade3).
Discussion and Conclusions
There are very few studies dedicated to evaluating the utility of standard electrodiagnostic studies in LSS. NASS guidelines published in 20112 suggest that electrodiagnostic studies are helpful for the evaluation of patients in which stenosis alone may not account for the neurologic symptoms. However, in the absence of reliable evidence, the opinion of the NASS work group is that imaging studies should be considered as a first-line diagnostic test in the diagnosis of degenerative LSS. The NASS group conclusions are as follows.
1. Electromyographic paraspinal mapping is suggested to confirm the diagnosis of degenerative LSS in patients with mild or moderate symptoms and radiographic evidence of stenosis.
2. There is insufficient evidence to make a recommendation for or against the use of F-wave, H-reflex, MEP, motor nerve conduction studies, SSEP, DSEP, and lower extremity EMG in the confirmation of LSS. These studies may be used to help identify other comorbidities.
The NASS work group identified the following potential studies that would generate meaningful evidence to assist in further defining the appropriate diagnostic tests for LSS 2: perform additional prospective studies addressing the utility of paraspinous mapping and electrodiagnostic testing in the evaluation of patients with clinical and radiologic degenerative LSS. Future studies should also address the value of these tests in the evaluation of patients with equivocal clinical signs and symptoms, and patients with confounding diagnoses such as diabetes. Future studies should focus on the ability of paraspinous mapping and electrodiagnostic testing to improve outcomes with surgical decompression.
The WFNS Spine Committee Recommendations on natural course and diagnosis of LSS are summarized in Table 2.
Table 2.
WFNS Spine Committee Recommendations on Natural Course and Diagnosis of Lumbar Spinal Stenosis
| Natural course Approximately 30% of patients with lumbar spinal stenosis (LSS) are expected to worsen, but 30% may improve with conservative measures There are predictive signs/symptoms that they will worsen: • Dural sac cross-sectional area <50 mm2 • Presence of radicular symptoms and back pain • Presence of degenerative spondylolisthesis and/or scoliosis • Symptom duration >1 year |
| Radiologic diagnosis MRI is the most appropriate noninvasive test for the diagnosis of LSS and the second is CT scan. CT myelography is appropriate if MRI is contraindicated or inconclusive There is no correlation between clinical symptoms or function with the presence of anatomic narrowing of the spinal canal on MRI, CT, or myelo-CT Qualitative radiologic criteria describe adequately spinal stenosis in central, lateral, or foraminal stenosis There are some radiologic signs that describe instability: • Direct signs on functional radiograms • Indirect signs on MRI and CT such as Modic changes, end-plate edema, extended discal vacuum, traction spurs, synovial cysts, annular tears, spondylolisthesis, and “facet fluid sign” |
| Electrophysiological diagnosis Routine electrophysiological tests (EMG, nerve conduction study, F-wave response, H-reflex, SSEP, MEP) have no diagnostic value for LSS Electrophysiological tests do not predict the outcome of patients with LSS |
WFNS, World Federation of Neurosurgical Societies; MRI, magnetic resonance imaging; CT, computed tomography; EMG, electromyography; SSEP, somatosensory evoked potential; MEP, motor evoked potential.
DECLARATION OF COMPETING INTEREST
The authors declare that the article content was composed in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
CRediT authorship contribution statement
Mehmet Zileli: Conceptualization, Methodology, Writing - review & editing. Marco Crostelli: Data curation, Writing - original draft. Marco Grimaldi: Data curation, Writing - original draft. Osvaldo Mazza: Data curation, Writing - original draft. Carla Anania: Writing - original draft, Validation. Maurizio Fornari: Writing - review & editing. Francesco Costa: Methodology, Formal analysis, Validation, Writing - review & editing.
References
- 1.Verbiest H. A radicular syndrome from developmental narrowing of the lumbar vertebral canal. J Bone Joint Surg Br. 1954;36B:230–237. doi: 10.1302/0301-620X.36B2.230. [DOI] [PubMed] [Google Scholar]
- 2.Kreiner D.S., Shaffer W.O., Baisden J.L. An evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spinal stenosis (update) Spine J. 2013;13:734–743. doi: 10.1016/j.spinee.2012.11.059. [DOI] [PubMed] [Google Scholar]
- 3.Kim C.H., Chung C.K., Kim M.J. Increased volume of surgery for lumbar spinal stenosis and changes in surgical methods and outcomes: a nationwide cohort study with a 5-year follow-up. World Neurosurg. 2018;119:e313–e322. doi: 10.1016/j.wneu.2018.07.139. [DOI] [PubMed] [Google Scholar]
- 4.Matsudaira K., Hara N., Oka H. Predictive factors for subjective improvement in lumbar spinal stenosis patients with nonsurgical treatment: a 3- year prospective cohort study. PLoS One. 2016;11:e0148584. doi: 10.1371/journal.pone.0148584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Micankova Adamova B., Vohanka S., Dusek L., Jarkovsky J., Bednarik J. Prediction of long-term clinical outcome in patients with lumbar spinal stenosis. Eur Spine J. 2012;21:2611–2619. doi: 10.1007/s00586-012-2424-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minamide A., Yoshida M., Maio K. The natural clinical course of lumbar spinal stenosis: a longitudinal cohort study over a minimum of 10 years. J Orthop Sci. 2013;18:693–698. doi: 10.1007/s00776-013-0435-9. [DOI] [PubMed] [Google Scholar]
- 7.Otani K., Kikuchi S.I., Yabuki S. Prospective one-year follow-up of lumbar spinal stenosis in a regional community. J Pain Res. 2018;11:455–464. doi: 10.2147/JPR.S148402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Overdevest G.M., Luijsterburg P.A., Brand R. Design of the Verbiest trial: cost-effectiveness of surgery versus prolonged conservative treatment in patients with lumbar stenosis. BMC Musculoskelet Disord. 2011;12:57. doi: 10.1186/1471-2474-12-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cowley P. Neuroimaging of spinal canal stenosis. Magn Reson Imaging Clin N Am. 2016;24:523–539. doi: 10.1016/j.mric.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 10.Andreisek G., Deyo R.A., Jarvik J.G. Consensus conference on core radiological parameters to describe lumbar stenosis-an initiative for structured reporting. Eur Radiol. 2014;24:3224–3232. doi: 10.1007/s00330-014-3346-z. [DOI] [PubMed] [Google Scholar]
- 11.Bartynski W.S., Lin L. Lumbar root compression in the lateral recess: MR imaging, conventional myelography, and CT myelography comparison with surgical confirmation. AJNR Am J Neuroradiol. 2003;24:348–360. [PMC free article] [PubMed] [Google Scholar]
- 12.Lee G.Y., Lee J.W., Choi H.S., Oh K.J., Kang H.S. A new grading system of lumbar central canal stenosis on MRI: an easy and reliable method. Skeletal Radiol. 2011;40:1033–1039. doi: 10.1007/s00256-011-1102-x. [DOI] [PubMed] [Google Scholar]
- 13.Pfirrmann C.W., Dora C., Schmid M.R., Zanetti M., Hodler J., Boos N. MR image-based grading of lumbar nerve root compromise due to disk herniation: reliability study with surgical correlation. Radiology. 2004;230:583–588. doi: 10.1148/radiol.2302021289. [DOI] [PubMed] [Google Scholar]
- 14.Schizas C., Theumann N., Burn A. Qualitative grading of severity of lumbar spinal stenosis based on the morphology of the dural sac on magnetic resonance images. Spine (Phila Pa 1976) 2010;35:1919–1924. doi: 10.1097/BRS.0b013e3181d359bd. [DOI] [PubMed] [Google Scholar]
- 15.Fardon D.F., Williams A.L., Dohring E.J., Murtagh F.R., Gabriel Rothman S.L., Sze G.K. Lumbar disc nomenclature: version 2.0: recommendations of the combined task forces of the North American Spine Society, the American Society of Spine Radiology and the American Society of Neuroradiology. Spine J. 2014;14:2525–2545. doi: 10.1016/j.spinee.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 16.Mamisch N., Brumann M., Hodler J. Radiologic criteria for the diagnosis of spinal stenosis: results of a Delphi survey. Radiology. 2012;264:174–179. doi: 10.1148/radiol.12111930. [DOI] [PubMed] [Google Scholar]
- 17.Steurer J., Roner S., Gnannt R., Hodler J., LumbSten Research Collaboration Quantitative radiologic criteria for the diagnosis of lumbar spinal stenosis: a systematic literature review. BMC Musculoskelet Disord. 2011;12:175. doi: 10.1186/1471-2474-12-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lurie J.D., Tosteson A.N., Tosteson T.D. Reliability of readings of magnetic resonance imaging features of lumbar spinal stenosis. Spine (Phila Pa 1976) 2008;33:1605–1610. doi: 10.1097/BRS.0b013e3181791af3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winklhofer S., Held U., Burgstaller J.M. Degenerative lumbar spinal canal stenosis: intra- and inter-reader agreement for magnetic resonance imaging parameters. Eur Spine J. 2017;26:353–361. doi: 10.1007/s00586-016-4667-1. [DOI] [PubMed] [Google Scholar]
- 20.Barz T., Melloh M., Staub L.P. Nerve root sedimentation sign: evaluation of a new radiological sign in lumbar spinal stenosis. Spine (Phila Pa 1976) 2010;35:892–897. doi: 10.1097/BRS.0b013e3181c7cf4b. [DOI] [PubMed] [Google Scholar]
- 21.Guen Y.L., Joon W.L., Hee S.C., Kyoung-Jin O., Heung S.K. A new grading system of lumbar central canal stenosis on MRI: an easy and reliable method. Skeletal Radiol. 2011;40:1033–1039. doi: 10.1007/s00256-011-1102-x. [DOI] [PubMed] [Google Scholar]
- 22.Leone A., Cassar-Pullicino V.N., Guglielmi G., Bonomo L. Degenerative lumbar intervertebral instability: what is it and how does imaging contribute? Skeletal Radiol. 2009;38:529–533. doi: 10.1007/s00256-009-0646-5. [DOI] [PubMed] [Google Scholar]
- 23.Muto M., Giurazza F., Guarnieri G., Izzo R., Diano A. Neuroimaging of spinal instability. Magn Reson Imaging Clin N Am. 2016;24:485–494. doi: 10.1016/j.mric.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Tarpada S.P., Cho W., Chen F., Amorosa L.F. Utility of supine lateral radiographs for assessment of lumbar segmental instability in degenerative lumbar spondylolisthesis. Spine (Phila Pa 1976) 2018;43:1275–1280. doi: 10.1097/BRS.0000000000002604. [DOI] [PubMed] [Google Scholar]
- 25.Matz P.G., Meagher R.J., Lamer T. Guideline summary review: an evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spondylolisthesis. Spine J. 2016;16:439–448. doi: 10.1016/j.spinee.2015.11.055. [DOI] [PubMed] [Google Scholar]
- 26.Yagci I., Gunduz O.H., Ekinci G., Diracoglu D., Us O., Akyuz G. The utility of lumbar paraspinal mapping in the diagnosis of lumbar spinal stenosis. Am J Phys Med Rehabil. 2009;88:843–851. doi: 10.1097/PHM.0b013e3181b333a9. [DOI] [PubMed] [Google Scholar]
- 27.Haig A.J., Tong H.C., Yamakawa K.S. The sensitivity and specificity of electrodiagnostic testing for the clinical syndrome of lumbar spinal stenosis. Spine. 2005;30:2667–2676. doi: 10.1097/01.brs.0000188400.11490.5f. [DOI] [PubMed] [Google Scholar]
- 28.Egli D., Hausmann O., Schmid M., Boos N., Dietz V., Curt A. Lumbar spinal stenosis: assessment of cauda equine involvement by electrophysiological recordings. J Neurol. 2007;254:741–750. doi: 10.1007/s00415-006-0427-1. [DOI] [PubMed] [Google Scholar]
- 29.Liu X., Konno S., Miyamoto M., Gembun Y., Horiguchi G., Hiromoto I. Clinical usefulness of assessing lumbar somatosensory evoked potentials in lumbar spinal stenosis. J Neurosurg Spine. 2009;11:71–78. doi: 10.3171/2009.3.SPINE08513. [DOI] [PubMed] [Google Scholar]
- 30.Liu X., Konno S., Miyamoto M., Gembun Y., Horiguchi G., Ito H. Clinical value of motor evoked potentials with transcranial magnetic stimulation in the assessment of lumbar spinal stenosis. Int Orthop. 2009;33:1069–1074. doi: 10.1007/s00264-008-0604-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zileli B., Ertekin C., Zileli M., Yünten N. Diagnostic value of electrical stimulation of lumbosacral roots in lumbar spinal stenosis. Acta Neurol Scand. 2002;105:221–227. doi: 10.1034/j.1600-0404.2002.1o143.x. [DOI] [PubMed] [Google Scholar]
- 32.Senocak O., Hurel D.M., Sener U., Ugurel B., Oztura I., Ertekin C. Motor conduction time along the cauda equina in patients with lumbar spinal stenosis. Spine. 2009;34:1410–1414. doi: 10.1097/BRS.0b013e3181a19082. [DOI] [PubMed] [Google Scholar]
- 33.Shen N. Evaluation of degree of nerve root injury by dermatomal somatosensory evoked potential following lumbar spinal stenosis. Neural Regen Res. 2008;3:1249–1252. [Google Scholar]
- 34.Murohashi T., Yoshimoto M., Takebayashi T. Efficacy of intraoperative direct electrical stimulation of the spinal root and measurement of distal motor latency in lumbar spinal stenosis. Eur Spine J. 2017;26:434–440. doi: 10.1007/s00586-016-4772-1. [DOI] [PubMed] [Google Scholar]
- 35.Voulgaris S., Karagiorgiadis D., Alexiou G.A. Continuous intraoperative electromyographic and transcranial motor evoked potential recording in spinal stenosis surgery. J Clin Neurosci. 2010;17:274–276. doi: 10.1016/j.jocn.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 36.Sharan A., Groff M.W., Dailey A.T. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 15: electrophysiological monitoring and lumbar fusion. J Neurosurg Spine. 2014;21:102–105. doi: 10.3171/2014.4.SPINE14324. [DOI] [PubMed] [Google Scholar]
- 37.Alemo S., Sayadipour A. Role of intraoperative neurophysiologic monitoring in lumbosacral spine fusion and instrumentation: a retrospective study. World Neurosurg. 2010;73:72–76. doi: 10.1016/j.surneu.2009.04.024. discussion: e7. [DOI] [PubMed] [Google Scholar]
- 38.Piasecki K., Kulik G., Pierzchala K., Pralong E., Rao J.P., Schizas C. Do intra-operative neurophysiological changes predict functional outcome following decompressive surgery for lumbar stenosis? A prospective study. J Spine Surg. 2018;4:86–92. doi: 10.21037/jss.2018.03.05. [DOI] [PMC free article] [PubMed] [Google Scholar]