Abstract
Recently a G‐protein‐coupled receptor, MAS Related GPR Family Member X2 (MRGPRX2), was identified as a specific receptor on human mast cells responsible for IgE independent adverse drug reactions (ADR). Although a murine homologue, Mrgprb2, has been identified for this receptor, its affinity for many ADR‐causing drugs is poor making it difficult to undertake in vivo studies to examine mechanisms of ADR and to develop therapeutic strategies. Here, we have created humanized mice capable of generating MRGPRX2‐expressing human MCs allowing for the study of MRGPRX2 MCs‐mediated ADR in vitro as well as in vivo. Humanized mice were generated by hydrodynamic‐injection of plasmids expressing human GM‐CSF and IL‐3 into NOD‐scid IL2R‐γ−/− strain of mice that had been transplanted with human hematopoietic stem cells. These GM/IL‐3 humice expressed high numbers of tissue human MCs but the MRGPRX2 receptor expressed in MCs were limited to few body sites including the skin. Importantly, large numbers of MRGPRX2‐expressing human MCs could be cultured from the bone marrow of GM/IL‐3 humice revealing these mice to be an important source of human MCs for in vitro studies of MRGPRX2‐related MCs activities. When GM/IL‐3 humice were exposed to known ADR causing contrast agents (meglumine and gadobutrol), the humice were found to experience anaphylaxis analogous to the clinical situation. Thus, GM/IL‐3 humice represent a valuable model for investigating in vivo interactions of ADR‐causing drugs and human MCs and their sequelae, and these mice are also a source of human MRGPRX2‐expressing MCs for in vitro studies.
Keywords: contrast agents, mast cells, MRGPRX2 receptor, non‐immune adverse drug reactions, pseudoallergy
Study shows a humanized mice model capable of generating MRGPRX2‐expressing human MCs; this model allows for the study of MRGPRX2 MCs‐mediated ADR in vitro as well as in vivo.
Abbreviations
- ADR
adverse drug reactions
- CB
cord blood
- HSCs
hematopoietic stem cells
- HSPCs
hematopoietic stem and progenitor cells
- Hu‐CB
human cord blood
- mBM‐MCs
mouse bone marrow cultured MCs
- MCs
mast cells
- MRGPRX2
MAS‐related GPR family member X2
- NSAIDs
nonsteroidal anti‐inflammatory drugs
- NSG
NOD‐scid IL2Rγ−/−/NOD scid gamma
- SC
subcutaneously
1. INTRODUCTION
Adverse drug reactions (ADR) can range from mild skin reactions to severe life threating reactions involving multiple organs.1 In the United States, 3–7% of all hospitalizations are due to adverse drug reactions, which can be classified as predictable or unpredictable.2 The predictable reactions are related to the mechanism of action of the drug, its known side effects and that they are generally dose dependent. However, the unpredictable reactions are generally dose independent and are unrelated to the pharmacologic actions of the drug. Based on their rapid onset and the nature of reactions, much of the unpredictable ADRs are believed to be mediated by MCs and they are further subdivided into non‐immune and immune‐mediated hypersensitivity reactions to drugs.3, 4 Immune‐mediated ADR typically occurs when a previously sensitized individual is re‐exposed to an allergen and involves drug‐specific IgE molecules that are bound to MCs and which upon contact with the drug, triggers MC degranulation and release of inflammatory mediators inducing anaphylaxis and other systemic reactions. Non‐immune mediated ADR, which occur much more frequently,4 are caused by direct binding of the drug to MCs triggering their activation and resulting in anaphylaxis. Since these reactions are IgE independent, they are sometimes referred to as pseudoallergic reactions. A large variety of drugs cause non‐immune ADR and they include nonsteroidal anti‐inflammatory drugs (NSAIDs), opiates, vancomycin, ciprofloxacin, and radiocontrast media.5
Recently, the receptor on MCs that specifically binds non‐immune ADR‐causing drugs was reported to be MRGPRX2, a G protein‐coupled receptor found almost exclusively on MCs.6 The mouse analogue of this receptor is Mrgprb2, which is found mostly on connective tissue MCs.7, 8 Although several drugs have been reported to cause adverse reactions in humans, only few have been shown to evoke a similar reaction in mice.9 This is because the binding affinity of Mrbprb2 receptor analogue on mouse MCs for ADR causing drugs is much lower than that of the MRGPRX2 receptor on human MCs.6, 7 This species‐specific disparity in binding affinities exhibited by many drugs causing ADR, has severely impacted our ability to study the underlying mechanisms of ADR and to develop appropriate therapies as in vivo studies in animals cannot be performed. This species‐disparity in inflammatory responses has also curtailed our ability to reliably survey the in vivo toxicity of newly developed drugs that have the potential to trigger ADR in subjects. One approach to overcoming this hurdle is to utilize humanized mice (hereon referred to as humice) that display, at least partially, a functional human immune system. Humice are classically generated by the transplantation of human hematopoietic stem cells (HSCs) into immunodeficient mice, such as the NOD‐scid IL2Rγ−/− (NSG) strain of mice.10 A frequent limitation with humice is inadequate development of certain immune cell subsets, which is attributed to the inability of certain mouse cytokines to interact with human immune cells.11, 12 Here, we have sought to employ the above‐mentioned strategy to generate human MC producing humice that will allow us to investigate in vivo MC activation by non‐immune ADR‐causing drugs. Since a recent humice model employing transgenic expression of human GM‐CSF and human IL‐3 was shown to specifically improve levels of human MCs in various body sites,13 we hydrodynamically injected plasmids encoding these cytokines to create our humice (GM/IL‐3 humice). We found that adult GM/IL‐3 humice generated in this technique were highly enriched in human MCs at various body sites including the skin. Furthermore, human MCs could readily be cultured from the bone marrow of these GM/IL‐3 humice, which upon exposure to ADR causing drugs evoked a robust degranulation response. When we examined the effect of human‐specific ADR causing drugs on these GM/IL‐3 humice, we observed significant cutaneous anaphylaxis. Thus, GM/IL‐3 humice provides a valuable platform for preclinical screening of drugs for ADR as well as for identifying putative inhibitors of ADR.
2. METHODS
2.1. Hematopoietic stem cell isolation
Human fetal liver samples from male and female donors, 16–23 weeks of age were obtained from Kandang Kerbau Women's and Children's Hospital (KKH) with written consent from donors. Fetal liver processing was performed by disassociating the liver, digesting it with collagenase VI (2 mg/mL in DMEM; Thermo Fisher Scientific, USA) with constant rotation for 15 min at 37°C. Digested tissue was passed through a 100 µm mesh to obtain single‐cell suspension that was isolated for CD34+ cells with a CD34+ selection kit (STEMCELL™ Technologies, USA) as per manufacturer's instructions.
2.2. Construction of humice
NOD‐scid IL2Rγ−/−/NOD scid gamma (NSG) mice (Stock #005557) were obtained from Jackson Laboratory and bred in a specific‐pathogen free animal facility at A*STAR, Biological Resource Centre (BRC). Newborn mice were sub‐lethally irradiated (100 rads) within 72 h of birth and intra‐hepatically injected with 1 × 105 human CD34+ hematopoietic stem cells (HSCs). Flow cytometric analysis of mouse PBMCs was used to determine the reconstitution levels of human immune cells in humice at 10–12 weeks posttransplantation. Less than 6% of the total humice population displayed a human reconstitution level <10% and were excluded from the study. A total of 18 (8 males and 10 females) NSG and 82 (37 males and 45 females) humice (engrafted from 5 different HSC donors) were used in this study. Experimental mice were chosen randomly, regardless of sex and reconstitution level.
2.3. Hydrodynamic gene delivery
Human GM‐CSF and IL‐3 genes were cloned separately into pcDNA3.1(+) vector (Invitrogen, USA). Plasmid deoxyribonucleic acid was purified by NucleoBond Xtra kit (Macherey‐Nagel, USA). For hydrodynamic gene delivery, 10–12‐week‐old humice were injected with 50 µg of each plasmid in a total of 1.5 mL phosphate buffered saline (PBS) through the tail vein using a 27‐gauge needle within 7 s.
2.4. Organ processing and cell isolation
Mice were cheek bled and euthanized by carbon dioxide. Various organs such as the liver, lung, spleen, and skin (ear) were harvested and digested by incubating samples at 37°C for 20 min in RPMI medium 1640 (Thermo Fisher Scientific, USA) containing 0.05% collagenase IV (Gibco, USA) and 0.01% DNase I (Sigma–Aldrich, USA). Post‐digestion of organs, the liver, lung, and ear (skin) were mashed through a 100 µm filter (Thermo Fisher Scientific, USA) in RPMI 1640 media (Thermo Fisher Scientific, USA) and centrifuged at 50 × g for 5 min to remove debris. For liver cells, supernatant containing mononuclear cells (MNCs) were collected, washed in PBS, and resuspended in 40% Percoll (Sigma–Aldrich, USA) in media. The cell suspension was gently overlaid onto 70% Percoll and centrifuged at 750 × g for 20 min. Mononuclear cells were collected from the interphase, washed twice in PBS, and resuspended in media supplemented with 10% FBS (Gibco, USA). Spleen tissues were mashed through a 100 µm filter (Thermo Fisher Scientific, USA) in media. The single‐cell suspension was washed and re‐suspended in media supplemented with 10% FBS (Gibco, USA). RBCs in these samples were lysed with (Ammonium‐Chloride‐Potassium) ACK lysis buffer (Gibco, USA). Cell viability was assessed with trypan blue (Sigma–Aldrich, USA).
2.5. Flow cytometry
Single cell suspensions from blood and organs were stained with 200 µL of LIVE/DEAD fixable blue dead cell stain kit (Life Technologies, USA) for 30 min at 4°C and washed in PBS containing 0.2% BSA (GE Healthcare, USA) and 0.05% sodium azide (Merck, Germany) (FACS buffer). CountBright™ (Thermo Fisher Scientific, USA) Absolute Counting Beads were used for quantification of absolute cell numbers, according to manufacturer's instructions. Surface immunostaining of human MCs were carried out with fluorescent conjugated Abs, specific for mouse CD45.1 (A20; BD Biosciences, USA) and human CD11c (B‐ly6; BD Biosciences, USA), CD45 (HI30, BD Biosciences, USA), CD66b (G10F5; BioLegend, USA), CD117 (104D2; BioLegend, USA), CD123 (6H6; BioLegend, USA), CD203c (NP4D6; BioLegend, USA), and FcεRI (CRA1; Miltenyi Biotech, USA). Cells were stained with Abs, in 100 µL FACS buffer for 30 min on ice. To detect maturation status of MCs, culture suspension was resuspended in PBS without calcium (Ca++) and magnesium (Mg++) containing EDTA 2 mM, 2% FBS (Gibco, USA), and stained with human CD45 (HI30, BD Biosciences, USA), CD203c (NP4D6; BioLegend, USA), FcεR1 (CRA1; Miltenyi Biotech, USA), and CD117 (104D2; BioLegend, USA). Controls were setup with msIgG1 PE‐Cy7 (MOPC‐21, BioLegend, USA) and msIgG1 APC (MOPC‐21, BioLegend, USA) and msIgG1 PE (MOPC‐21, BioLegend, USA). In another set of analysis, human CD203c (NP4D6; BioLegend, USA) was replaced with MRGPRX2 (K125H4, BioLegend, USA). After surface staining the membrane markers, cells were fixed in 4% PFA (Sigma–Aldrich, USA) for 30 min at room temperature, incubated overnight at 4°C with purified anti‐huChymase (Polyclonal, rabbit‐IgG, 1:300; Abcam, UK) and anti‐huTryptase (EPR8476, goat‐IgG, 1:300; Abcam, UK) diluted in blocking buffer. The next day, cells were washed twice for 5 min each in blocking buffer and incubated with chicken anti‐rabbit IgG FITC (Invitrogen, USA) and donkey anti‐goat IgG AF405 (Invitrogen, USA) diluted (1:300) in blocking buffer for 1 h at room temperature. At the end of the incubation, cells were washed and resuspended for analysis. For expression analysis of MRGPRX2 in tissue MCs, FITC conjugated Abs to mouse CD45.1 (A20; BD Biosciences, USA), human CD3 (OKT3; BioLegend, USA), CD11c (B‐ly6; BD Biosciences, USA), CD14 (HCD14; BioLegend, USA), CD19 (HIB19, BioLegend, USA), CD45 (HI30, BD Biosciences, USA), CD56 (NCAM; BioLegend, USA), CD117 (104D2; BioLegend, USA), MRGPRX2 (K125H4, BioLegend, USA), and huFcɛRI (CRA1; Miltenyi Biotech, USA) were used. Mouse IgG2b‐PE (MPC‐11, BioLegend) was used as isotype control. Flow cytometry was performed on an LSRII flow cytometer using the FACSDiva software (BD Biosciences, USA) and analyzed using the Flowjo software version 10 (Treestar, Ashland, USA). Relative FcεRI expression level was measured in Staining Index, where the formula is [(MFI (Mean Fluorescence Intensity)(pos)/MFI(neg)*2s.d(neg)].
2.6. Peptides and drugs
Compound 48/80 (Sigma–Aldrich), levofloxacin (Sigma–Aldrich), meglumine diatrizoate (Sigma–Aldrich), gadobutrol (Sigma–Aldrich), (R)‐ZINC‐3573 (Sigma–Aldrich), human IgE (CalbioChem, USA), and rabbit polyclonal anti‐human IgE (Dako, USA) were obtained from respective companies.
2.7. Isolation and differentiation of human hematopoietic progenitor cells to mast cells
A CD34+ selection kit (STEMCELL™ Technologies, USA) was used to isolate CD34+ cells from the cord blood (CB) of a donor as per manufacturer's instructions. Isolated CB hematopoietic stem and progenitor cells were frozen down in vials of 1 × 106 cells resuspended in RPMI 1640 media (Thermo Fisher Scientific) containing 10% human albumin (Lonza, USA) and 10% DMSO (Sigma). In different batches of humice constructed from 4 separate donors, human CD34+ cells were isolated by FACS Aria II (BD Biosciences, USA). Bone marrow cell suspensions were stained with mouse CD45.1 (A20; BioLegend, USA), human CD45 (HI30, BD Biosciences), and CD34 (AC136; Miltenyi Biotec, USA). Human CD45.1−CD45+CD34+ population of cells from the bone marrow of mice were cultured in serum‐free conditions, in StemPro‐34 media, supplemented with StemPro‐34 Nutrient, rhIL‐3 (20 ng/mL), rhIL‐6 (50 ng/mL), rhSCF (100 ng/mL), l‐glutamine (2 mM; Life Technologies, USA), Penicillin (100 U/mL)/Streptomycin (100 µg/mL) (Lonza, USA), HEPES (10 mM; Lonza), and 1× non‐essential amino acids (Lonza). As a positive control for phenotypic and functional analysis, human cord blood‐derived CD34+ progenitor cells (Hu‐CB) were cultured under the same conditions. After 2 weeks of culture, the concentration of rIL‐6 was increased to 100 ng/mL while rhIL‐3 was excluded from the media.14 By the sixth week of culture, when Mϕs were undetectable in the culture, the media was supplemented with FBS to promote the expression of FcεRI.15 At 6 weeks, the absence of myeloid cells was confirmed via flow cytometric analysis of cells from the culture, stained with human CD45 (HI30, BD Biosciences) and CD14 (HCD14; BioLegend, USA). After confirming the absence of myeloid populations, the cell culture was supplemented with a medium containing 15% FBS (Gibco, USA) and cultured for an additional 4 to 6 weeks. To determine the maturation status of MCs in culture, a flow cytometric analysis (LSR II, BD Biosciences) was performed on cells stained with FcεRI (CRA1; Miltenyi Biotech, USA) and CD117 (104D2; BioLegend) Abs.
2.8. In vitro studies
To generate mouse BM‐MCs for in vitro and in vivo studies, cells obtained from flushed mouse femurs were cultured in RPMI medium 1640 (Thermo Fisher Scientific) containing 10% FBS (Gibco), Penicillin (100 U/mL)/Streptomycin (100 µg/mL) (Lonza, USA), HEPES (10 mM; Lonza, USA), Sodium pyruvate (Thermo Fisher Scientific, USA), non‐essential amino acids (Lonza, USA), rhIL‐3 (30 ng/ml; ImmunoTools, Germany), and Stem Cell Factor (SCF; 3 ng/mL, ImmunoTools, Germany). For β‐hexosaminidase secretion assay, MCs were resuspended in PBS containing Ca++, Mg++, 2% FBS (Gibco, USA), and HEPES (10 mM; Lonza), and plated in 96‐well plates (2 × 105 cells/well). After stimulating the cells with indicated peptides and drugs for 30 min, cells and supernatant were collected to quantify amounts of β‐hexosaminidase released from cells. To quantify the release of Prostaglandin D2 (PGD2) (Cayman Chemical Company, USA) and histamine (Enzo Life Sciences, USA), human MCs were seeded at a concentration of 3 × 105/well/200 µL. Calcium flux levels in human BM‐MCs were determined by the Fluo‐4 NW assay (Thermo Fisher Scientific, USA).
2.9. Passive cutaneous anaphylaxis assay
Mice were anesthetized through an i.p. injection of Ketamine (80 mg/kg; Ceva, USA). Human IgE stimulation was performed by s.c. injecting human IgE mAb (60 ng/mouse; Calbiochem, USA) at the base of the ear. Twenty‐four hours after sensitization, mice were injected IV with 50 µL of 12.5 mg/mL Evans blue (Sigma–Aldrich). After 10 min, mice were i.p. injected with anti‐human IgE (50 µg in 100 µL/mouse; Dako, USA). After 15 min, dorsal skin and ears were collected. To stimulate MRGPRX2 receptor after Evans blue injection, 10 µL of a solution containing 1 µM of meglumine diatrizoate (Sigma–Aldrich), gadobutrol (Sigma–Aldrich), and (R)‐ZINC‐3573 (Sigma–Aldrich) were administered by intraplantar injection in one paw and the vehicle was administered in the other paw. Evans blue extravasation was visualized 15–20 min later and paws were collected. Samples were dried for 24 h at 55–60°C, weighed, Evans blue was extracted by a 24‐h incubation in formamide at 55–60°C and quantified by the absorbance at OD620nm (Tecan, USA).
2.10. Statistical analysis
All statistical analyses were performed using GraphPad Prism 5.0 software (GraphPad Software Inc., USA). Two‐tailed unpaired nonparametric tests were performed with Mann‐Whitney U test was used to compare 2 groups of data. One‐way ANOVA was used for comparisons of >2 groups, followed by Dunnett's multiple comparisons test. Standard error of mean (SEM) are indicated by error bars and P‐values < 0.05 are considered statistically significant.
2.11. Study approval
Fetal liver specimens were obtained with written consent from donors and approval from SingHealth and National Health Care Group Research Ethics Committees Singapore (CIRB Ref: 2012/064/B). The International Animal Care and Use Committee, A*STAR approved this study and assigned a protocol number (BRC #151039). All animal experimental procedures carried out were in accordance to the protocol's guidelines and regulations.
2.12. Data availability
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files).
3. RESULTS
3.1. Populating humice with functionally active human MCs through increased expression of GM‐CSF and IL‐3
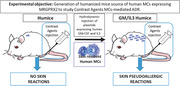
At 12 weeks of age, successful engraftment of human CD34+ HSCs in NSG mice was determined by flow cytometry. As previously described,16 more than 90% of the animals harbored 20–60% human leukocytes in their blood, which is indicative of successful engraftment of human cells. Humice with peripheral blood human CD45+ cell levels below 20% were excluded from the study.16 Thereafter, we split these animals into 2 groups, with 1 group receiving a single hydrodynamic injection of plasmids encoding IL‐3 and GM‐CSF, designated “GM/IL‐3 humice” and the second group was untreated designated untreated ”humice control.” The single hydrodynamic injection of human cytokine‐encoding genes was previously shown to ensure a systemic presence of human cytokines lasting at least 2–3 weeks in mouse.11 The result of the systemic presence of human IL‐3 and GM‐CSF was a significant increase in the number of circulating human immune cells compared to the control humice group of mice (Supplementary Fig. 1A). After another 8–10 weeks, when we examined for the presence of human and mouse leukocytes in both groups of humice, we found that in the spleens and livers of both control and GM/IL‐3 humice, the majority of CD45+ cells were of human origin (Fig. 1A). However, when we examined the lung or the skin of both groups of mice, only the GM/IL‐3 treated humice exhibited high numbers (70–80%) of human immune cells (Fig. 1A). Indeed, other than the liver and spleen, in each of the main tissue compartments examined, we consistently observed a marked increase in the percentage of human MCs found among human leukocytes in GM/IL‐3 humice compared to control humice (Fig. 1A and B; Supplementary Fig. 2). Since the expression of the IgE receptor (FcεRI) is an important marker of MCs, we examined its presence on these human MCs. We found that the expression of FcεRI in MCs in GM/IL‐3 humice was markedly much higher than in MCs found in humice controls indicating that the human MCs in the former mice were markedly more mature (Fig. 1B). Furthermore, the expression of FcεR1 in the human MCs in GM/IL‐3 humice was, for the most part, comparable regardless of their location in the mouse. Having established that human MCs produced in GM/IL‐3 humice were mature, we examined these cells for the presence of MRGPRX2, the receptor of interest for this study. Flow cytometry analysis of human MCs in the skin of GM/IL‐3 humice revealed high expression of MRGPRX2 levels (Fig. 1C, left panel). Whereas MRGPRX2 in human MCs in the lung, spleen, and liver was poorly expressed suggesting that MRGPRX2 expression is regulated by signal/factors generated in the skin microenvironment (Fig. 1C). This differential expression of this receptor is not surprising since in the mouse, the murine homolog, Mrgprb2 is expressed on connective tissue MCs in the skin but not on mucosal MCs found in lung or spleen.17 Taken together, exposure of humice to hydrodynamically injected cytokines resulted in marked enhancement of the population of mature human MCs but MRGPRX2‐expressing MCs were largely found in the skin. Since basophils potentially can contribute to ADRs,18 we also examined for the presence of human basophils in the peripheral blood of both humice groups. Although we found moderate increment of these cells in the circulation and in some of the organs of GM/IL‐3 humice compared to control humice mice, the amounts of human basophils in the skin of GM/IL‐3 humice was no different from that seen in control humice (Supplementary Fig. 1B and C).
Figure 1.
Humice treated with GM‐CSF and IL‐3 exhibited increased expression of fully differentiated of human mast cells in peripheral tissue and internal organs. (A) Left, Representative dot plots of human (hu)CD45 + and mouse (ms)CD45 + cells gated on lived singlets cells isolated from spleen, lung, liver and skin (ear) obtained from GM/IL‐3 compared to control humice group. Right, amount of tissue human leucocytes, expressed as the relative percentage to total (human and mouse) CD45+ cells isolated. (B) Left, dot plots of human CD117 and FcεRI gated on huCD45+HLA‐DR−CD66b− cells. Right, the frequency of human MCs (FcɛRI+CD117+) as well as the relative FcɛRI expression obtained from control humice and GM/IL‐3 humice organs are indicated. (C) Representative histograms of MRGPRX2 expression in MCs isolated from spleen, liver, lung and ear of GM/IL‐3 humice are shown (left) and the percentage of MCs expressing MRGPRX2 obtained from humice groups are plotted (right). Data represent the means ± se of 2 experiments (n = 2–4 mice/group/experiment). *P < 0.05, **P < 0.005, ***P < 0.001, as indicated.
3.2. Bone marrow derived cultured MCs from GM/IL‐3 humice are a source of human MCs
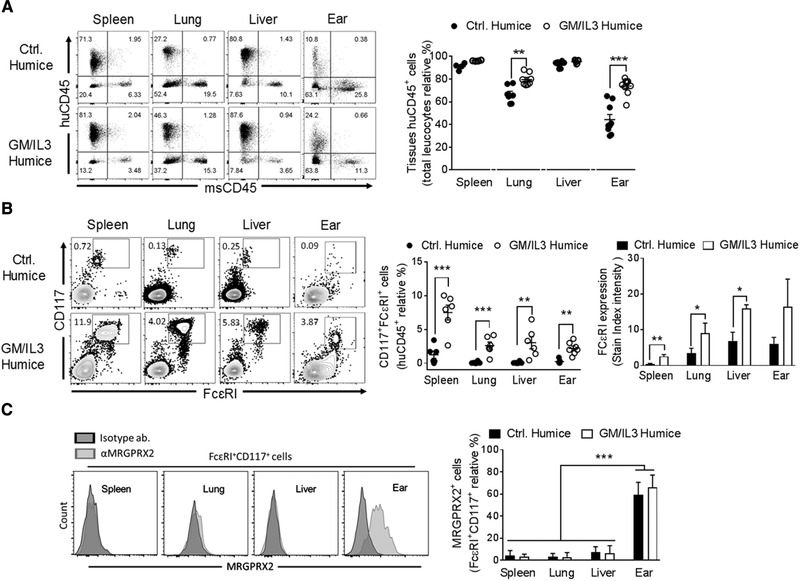
Since these GM/IL‐3 humice possessed a significant number of functional human MCs they potentially could also be a valuable source of human MCs for in vitro studies examining MC mediated ADR. Mature human MCs populating the various body sites in the GM/IL‐3 humice originate from human hematopoietic stem and progenitor cells (HSPCs) residing in the mouse bone marrow, therefore we hypothesized that by collecting the bone marrow cells from these GM/IL‐3 mice (Supplementary Fig. 3), and culturing them in appropriate growth media in vitro, we could generate significant number of human MCs for study. Indeed, this strategy is analogous to how we currently derive human MCs for in vitro studies, where HSPCs derived from peripheral blood or umbilical cord blood of human subjects are cultured for weeks in a range of growth factors and cytokines including SCF, IL‐3, and IL‐6.19 After 6 weeks of differentiation, Mϕs were undetectable as both cultures were negative (Supplementary Fig. 4A). It is noteworthy that compared to the bone marrow derived cells from GM/IL‐3 humice (humice‐BM), the cells derived from human cord blood (Hu‐CB) proliferated at a higher rate (Supplementary Fig. 4B). After 10–12 weeks, cells from both culture conditions were analyzed for cell surface expression of lineage‐specific markers. We observed that over 90% of the cells in each culture expressed CD117 and FcεRI, characteristic markers for mature MCs, however, we also noticed that the expression levels of both markers where markedly higher in humice‐BM cells compared to Hu‐CB cells (Fig. 2A; Supplementary Fig. 4C and D). We examined both human MC cultures for the expression of the MRGPRX2 receptor and found that it was expressed in both human cell cultures but the number of MRGPRX2+ cells in the cell population as well as the level of MRGPRX2 expression on each cell in the humice‐BM culture was 2‐fold higher compared to Hu‐CB culture (Fig. 2A; Supplementary Fig. 4D). Since the human MCs are differentiated based on the tryptase and chymase content of their granules, we examined both human MCs cultures for protease expression and found that some MCs expressed only tryptase (MCT), while others expressed both tryptase and chymase (MCTC). We found that humice‐BM exhibited a relatively greater proportion of MCTC cells compared to Hu‐CB MCs (Fig. 2A). Interestingly and consistent with published data,20, 21 we found that MRGPRX2 expression was mostly in MCTC cells in the cultured human MC population (Supplementary Fig. 4E). Hu‐CB MCs express fewer FcεRI and MRGPRX2 receptors as well as the co‐expression of tryptase and chymase and when activated through FcεRI or with MRGPRX2 agonists (C48/80 and Substance P), they release less of β‐hexosaminidase and histamine (Fig. 2A and B; Supplementary Fig. 4D and E). The apparent superiority of humanized BM‐MCs compared to CB‐MCs, is probably because of their distinct origins, taken together, MCs generated from GM/IL‐3 humice are a potentially valuable source of cultured and functionally competent MRGPRX2 expressing human MCs.
Figure 2.

Human MCs cultured from GM/IL‐3 humice express both chymase and tryptase and are functional. (A) Representative flow cytometric analysis of human hematopoietic progenitors (singlets/CD45+) obtained from HuCB and humice‐BM differentiated for 10–12 weeks in huMCs media evaluated by CD117, FcεRI, and MRGPRX2 and for chymase and tryptase gated on CD117+FcεRI+ cells. The frequency of the huMCs is shown. (B) HuCB‐MCs and humice‐BM MCs were sensitized overnight with human‐IgE (1 µg/mL) and challenged with αIgE (2 µg/mL), or C48/80 (20 µg/mL) or Substance P (20 µM), 30 min later supernatants and cells were collected for the quantification of β‐hexosaminidase released and histamine. All data represent the means ± se of 3 experiments performed in triplicate *P < 0.05, **P < 0.01, ***P < 0.001, as indicated.
3.3. Humice‐BM MCs exhibit a degranulation response to ADR causing reagents
Non‐immune ADR causing drugs are believed to directly bind MC membranes triggering MC degranulation and anaphylaxis. Although mouse MCs and mice have been the cell type and animal of choice when investigating the in vitro and in vivo roles of MCs, this is not always possible with regard to the study of ADR‐causing drugs and MCs because the mouse Mrgprb2 homologue poorly recognizes several of the key non‐immune ADR causing drugs.22 We investigated if we could use GM/IL‐3 humice generated human MCs to study ADR drug induced MC degranulation.
Accumulating evidence suggests that IgE‐independent activation of mast cells play a critical role in a wide variety of cutaneous inflammatory diseases including ADR. The skin is the most affected organ in immediate reactions to contrast media. The most common symptoms are erythema and urticarial with or without angioedema, which appears in >70% of patients.23 The ADR agents we have chosen to investigate here are meglumine diatrizoate and gadobutrol, 2 contrast agents components used for MRI and known to induce IgE‐independent MC activation resulting in life‐threating allergic reactions.24 Anaphylactoid reactions occur in ∼1% to 3% of patients who receive ionic contrast media and less than 0.5% of patients who receive non‐ionic contrast media.23 Several agonists of MRGPRX2 have been described in the literature and ZINC‐3573 is one of the most selective agonist identified.25 In addition to examining whether meglumine diatrizoate and gadobutrol will bind and activate humice‐BM MC, we will use this agonist to detect and differentiate MC degranulation mediated by MRGPRX2 in our studies (Fig. 3). For comparison, we have also included mouse bone marrow cultured MCs (mBM‐MCs) in these studies. As shown in Fig. 3A and B, both Meglumine diatrizoate and gadobutrol induced substantial degranulation in humice‐BM MCs but not in mBM‐MCs. The specificity of the degranulation response for human MCs evoked by the contrast agents resembles that mediated by ZINC‐3573 (Fig. 3). Since MC degranulation via the MRGPRX2 receptor is typically accompanied by a robust calcium response and secretion of aracadonic acid mediators such as prostaglandin‐2 (PGD2),26 we examined for these indicators of MC activation in humice‐BM MCs following exposure to the contrast agents. We found marked calcium increase and PGD2 production in humice‐BM after ZINC‐3573 treatment as well as with contrast agents. (Fig. 3C). Since the specific binding of meglumine diatrizoate and gadobutrol to MRGPRX2 receptors and subsequent signaling events have not previously been demonstrated, we undertook additional studies to confirm the specificity of these contrast agents for this particular G coupled receptor. Since HEK293T cells do not endogenously express MRGPRX2 receptors,20, 27 we transiently expressed MRGPRX2 in these cells and examined for calcium responses following exposure to each of the 2 contrast agents using a well described NF‐AT luciferase reporter assay28 (Fig. 3D). We observed a robust calcium response with each of the contrast agents but not when these transfected cells were exposed to another powerful MC stimulus, IgE/α‐IgE (Fig. 3D). Taken together, our data show that humice‐BM MCs express MRGPRX2 receptors and evoke a robust degranulation response to ADR drugs known to provoke serious non‐immune allergic reactions in various subjects.
Figure 3.
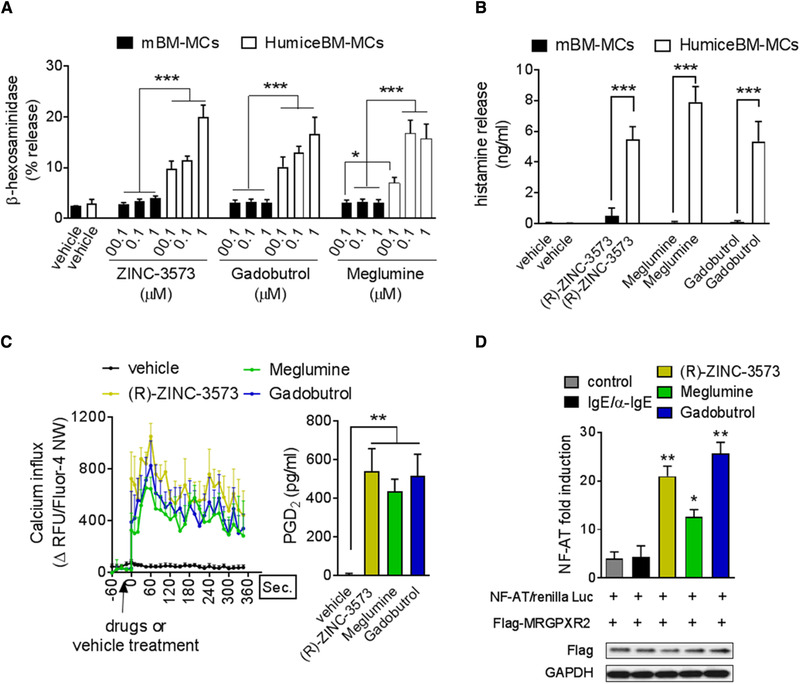
Human MCs cultured from GM/IL‐3 humice are degranulated by specific human ligands. (A) We first confirmed the purity and the functionality of humice‐BM MC compared to mouse (m)BM‐MCs, evaluating the degranulation response (β‐hexosaminidase released levels) after 30 min of challenge with a selective activator of huMCs, (R)‐ZINC‐3573. All data represent the means ± se of 5 experiments. *P < 0.05 and ***P < 0.001. Using the same approach, we identify 2 unknown activators of huMCs degranulation as illustrated by the amount β‐hexosaminidase released in the supernatant after 30 min of a dose response treatment with meglumine and gadobutrol and corroborated by the levels of histamine released (B) after contrast agent treatment and compared to all drugs were used 1 µM for 30 min. All data represent the means ± se of 4 experiments performed in triplicate. ***P < 0.001. (C) The increment of intracellular Ca++ levels within humice‐BM MC after (R)‐ZINC‐3573, meglumine, and gadobutrol treatment (1 µM) was evaluated using Fluo‐4 NW signal and confirmed by PGD22 production levels. Data represent the means ± se of 2 experiments performed in triplicate. **P < 0.05. (D) HEK293T cells were transfected with 100 ng of plasmid encoding MRGPRX2 or NF‐AT or Renilla (10 ng). After 36 h, the cells were stimulated with IgE/anti‐IgE or Zinc or Meglumine or Gadobutrol for 4 h. The lysates were subjected to luciferase assay as described by manufacturers protocol (Promega). NF‐AT fold activation was calculated using NFAT‐luciferase values/Renilla luciferase. Transfection of Flag‐tagged MRGPRX2 into HEK293T cells was confirmed by Western blot. Data represent the means ± se of 2 experiments performed in triplicate. *P < 0.05, **P < 0.01, ***P < 0.001, compared (m)BM‐MCs or control groups
3.4. Anaphylaxis reactions in GM/IL‐3 humice following exposure to ADR causing drugs
Having observed that humice‐BM MCs are capable of evoking a powerful degranulation response to contrast agents in vitro, we investigated if these human MCs were equally capable of evoking degranulation responses in vivo and causing cutaneous anaphylaxis. Since the MRGPRX2 receptor is well expressed in the connective tissue MCs, deposited in the skin of humice, (Fig. 1C), we reasoned that the humice would be capable of triggering cutaneous anaphylaxis. To test this notion, we intradermally injected the contrast agents: meglumine diatrizoate and gadobutrol into the footpads of GM/IL‐3, control humice, and NSG mice that had simultaneously been systemically administered with Evans Blue dye 15 min before. To demonstrate the specificity of the resulting anaphylactic reactions, we challenged both groups of mice with ZINC‐3573, a well‐characterized ligand for human MRGPRX2. The data presented in Fig. 4 upper panel reveals footpad pictures of both humice groups treated with ZINC‐3573 or contrast agents, clearly shown a significant vascular leakage in GM/IL‐3 humice but not in the control humice, confirmed by the amounts of dye extracted (Fig. 4 bottom panel). Thus, the GM/IL‐3 humice can serve as a valuable model for the study of the in vivo interactions of ADR‐causing drugs and human MCs and their harmful sequelae.
Figure 4.
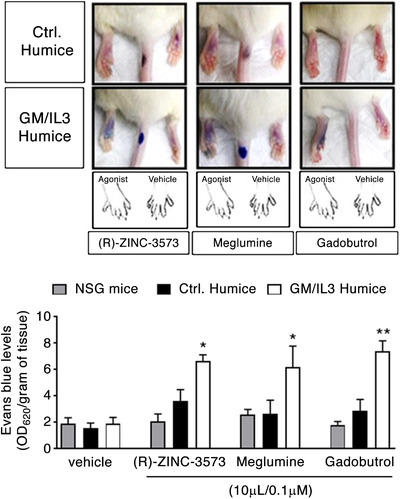
In vivo activation of human MCs in GM/IL‐3 humice by contrast agents. Upper panel, representative images of Evans blue dye extravasation 15–20 min after intraplantar injection the indicated compounds. Bottom panel, quantification of Evans blue leakage into the paw (optical density at 620 nm normalized for the tissue weight) after (R)‐ZINC‐3573, meglumine, and gadobutrol treatment. Data represent the means ± se of 2 experiments (n = 3 mice/group/treatment). *P < 0.05, **P < 0.01, ***P < 0.001. ANOVA test followed by Dunnett's multiple comparisons test
4. DISCUSSION
To date no drugs to ameliorate non‐immune ADR have been forthcoming and this is in part because it has not been possible to mimic these adverse reactions in animal models.
Recently, it was shown that the reason why mice fail to evoke ADR is because Mrgprb2, the mouse analogue for the MRGPRX2 receptor on human MCs, poorly recognizes the ADR causing drugs.6, 8 Here, we have for the first time overcome this limitation by generating human MC‐enriched GM/IL‐3 humice that experience rigorous adverse reactions when exposed to known ADR causing radiocontrast agents as well as specific MRGPRX2 agonist, ZINC‐3573 (Fig. 4). However, not all of the human MCs in the GM/IL‐3 humice expressed the MRGPRX2 receptor, which are targeted by the ADR causing drugs. Indeed, human MCs in the skin of GM/IL‐3 humice but not those in the lung, liver, or spleen expressed this receptor. Presumably only the MRGPRX2 expressing human MCs are responsible for the anaphylactic responses observed in the GM/IL3 humice, this observation is consistent with our in vivo studies that revealed the occurrence of passive cutaneous anaphylaxis in GM/IL3 humice harboring MRGPRX2 expressing human MCs but not in control humice harboring limited number of human MCs (Fig. 1B). Interestingly, these tryptase and chymase expressing MCs were also positive for MRGPRX2 (Fig. 2A; Supplementary Fig. 4E) that is consistent with findings in mice that Mrgprb2 expressing MCs are expressed primarily on connective tissue MCs.17, 29 Flow cytometry of the control humice group of mice showed that <5% of the collected peritoneal cells expressed human CD45 and among these human CD45+ cells, only few of them expressed FcɛRI, which were human Kit+, MCs (Supplementary Fig. 5). Anyway even if the systemic presence of human IL‐3 and GM‐CSF induces in the peritoneal exudate an increment of human immune cells as well as the relative percentage of MCs population, their absolute number was not so elevated to represent an valuable source of human mast cells (Supplementary Fig. 5). Although it has been suggested that radiocontrast agents such as meglumine diatrizoate and gadobutrol targeted the MRGPRX2 receptor on human MCs,24 this binding interaction has yet to be demonstrated. Our in vitro studies employing transfected HEK293T cells expressing the cloned MRGPRX2 receptor revealed that both contrast agents bound specifically to this G coupled receptor triggering a robust calcium flux. Human MC producing humice that have so far been described are based on a NOD‐scid IL2rgnull SCF/GM‐CSF/IL‐3 (NSG‐SGM3) strain of mice engrafted with human umbilical cord blood or thymus, liver, and hematopoietic stem cells and which constitutively express GM‐CSF and IL‐3 have been reported.13, 30 However, long‐term exposure and constitutive expression of human cytokines in humice can be challenging as non‐physiological effects are sometimes observed, including deleterious effects on the human stem cell compartments, and progressive anaemia and polarization of the immune system.31, 32 Therefore, in our model, we performed a hydrodynamic gene delivery of human cytokines into humice, and found that we could markedly enhance the reconstitution and function of human immune cells subsets, without any undesirable side effects.11 Two previously reported transgenic humanized MC expressing mouse strains have both been used to investigate human MC responses to IgE stimulation.15, 31 In this regard, the findings of Ito et al.15 and Bryce et al.31 are very similar to our findings regarding the distribution of human MCs in the mouse tissue and the magnitude of IgE mediated cutaneous anaphylaxis responses observed (Fig. 1; Supplementary Fig. 6). However, our study represent a simpler method to deliver human specific cytokines by hydrodynamic injection and a significant advance over previous work because for the first time we indicate that humice are a valuable model for ex vivo and in vivo investigation of mast cell MRGPRX2‐function such as reactivity to drugs responsible for adverse reactions, which in a large number of cases involve activation of MCs. Although basophils are distinct cells from MCs, they are also important in causing ADR. Basophils complete their differentiation within the bone marrow before entering the blood where they have a lifespan of only about 60 h. Whereas MCs that originate in the bone marrow, circulate in the blood as progenitor cells and enter peripheral tissues as immature cells. In their extravascular location, these cells mature based on the cytokines in the surrounding microenvironment and become long‐lived cells. In the skin, the percentage of MRGPRX2+ MCs isolated from GM/IL‐3 expressing mice remained comparable to control group even though the total number of MRGPRX2+ MCs are at least 10‐fold more (Fig. 1C), supporting the idea that the local development and maturation of MCs is regulated through signals emanating from surrounding tissue microenvironments that are highly conserved across in both humans and mice. Interestingly, the transient secretion of human cytokines observed in GM/IL‐3 humice failed to encourage growth of basophils whereas they supported significant mast cell growth (Fig. 1B; Supplementary Fig. 1B). As a result of the inability of basophils to grow in GM/IL‐3 humice, we were not able to examine the role of basophils in ADR against contrast media reagents.24 Another benefit of the use of GM/IL‐3 humice that we created is that we are able to generate large amounts of human mature MCs for study from in vitro culturing of the bone marrow of these humice. Since the human MCs employed for in vitro studies are from the same source as the MCs employed in vivo, it will greatly minimize phenotypic variation often seen in human MCs of different genetic backgrounds33, 34 and should ease our ability to correlate in vitro with in vivo observations. In addition to its potential utility for testing of effective treatments for ADR, these GM/IL‐3 humice could prove highly valuable for studying the inflammatory cascade associated with ADR as no animal model currently exists. In summary, the GM/IL‐3 humice that we have created here could potentially serve as a powerful platform for the in vivo study of human MC‐mediated allergies and inflammatory disorders as well as in the identification of inhibitors of these MC‐mediated inflammatory conditions, and the screening of novel drugs for their ADR causing potential before initiation of first in man trials.
AUTHORSHIP
A.M. and M.G. contributed equally to this work. A.M. and M.G. designed and performed experiments, analyzed and interpreted data, and prepared the manuscript; K.S.M.Y. analyzed data and prepared manuscript; P.B., W.W.S.T., S.Y.T., and M.L. performed experiments; E.K.H., Y.F., and J.K.Y.C. contributed essential research tools and reagents, and involved in discussion; S.N.A. and Q.C. conceived the study, designed experiments, supervised the project, and prepared the manuscript.
DISCLOSURE
The authors declare no conflict of interest.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
This study was supported by National Medical Research Council Singapore, NMRC/TCR/014‐NUHS/2015, NMRC/CSA‐SI/0013/2017 and NMRC/OFLCG/003/2018 to Q.C. and a block grant award from Duke‐NUS to SNA. National Research Foundation Fellowship Singapore NRF‐NRFF2017‐03 to Q.C. and the Chinese National Nature Science Foundation 81570101 to Y.F.
Mencarelli A, Gunawan M, Yong KSM, et al. A humanized mouse model to study mast cells mediated cutaneous adverse drug reactions. J Leukoc Biol. 2020;107:797–807. 10.1002/JLB.3MA1219-210RR
Contributor Information
Soman N. Abraham, Email: soman.abraham@duke.edu, Email: soman.abraham@duke-nus.edu.sg.
Qingfeng Chen, Email: qchen@imcb.a-star.edu.sg.
REFERENCES
- 1. Ardern‐Jones MR, Friedmann PS. Skin manifestations of drug allergy. Br J Clin Pharmacol. 2011;71:672‐683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Parameswaran Nair N, Chalmers L, Peterson GM, Bereznicki BJ, Castelino RL, Bereznicki LR. Hospitalization in older patients due to adverse drug reactions—the need for a prediction tool. Clin Interv Aging. 2016;11:497‐505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Uetrecht J. Immune‐mediated adverse drug reactions. Chem Res Toxicol. 2009;22:24‐34. [DOI] [PubMed] [Google Scholar]
- 4. Uetrecht J, Naisbitt DJ. Idiosyncratic adverse drug reactions: current concepts. Pharmacol Rev. 2013;65:779‐808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Montañez MI, Mayorga C, Bogas G, Barrionuevo E, Fernandez‐Santamaria R, Martin‐Serrano A, et al. Epidemiology, mechanisms, and diagnosis of drug‐induced anaphylaxis. Front Immunol. 2017;8:614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Subramanian H, Gupta K, Ali H. Roles of MAS‐related G protein coupled receptor‐X2 (MRGPRX2) on mast cell‐mediated host defense, pseudoallergic drug reactions and chronic inflammatory diseases. J Allergy Clin Immunol. 2016;138:700‐710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang T, Che D, Liu R, Han S, Wang N, Zhan Y, et al. Typical antimicrobials induce mast cell degranulation and anaphylactoid reactions via MRGPRX2 and its murine homologue MRGPRB2. Eur J Immunol. 2017;47:1949‐1958. [DOI] [PubMed] [Google Scholar]
- 8. McNeil BD, Pundir P, Meeker S, Han L, Undem BJ, Kulka M, et al. Identification of a mast cell specific receptor crucial for pseudo‐allergic drug reactions. Nature. 2015;519:237‐241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shanks N, Greek R, Greek J. Are animal models predictive for humans. Philos Ethics Humanit Med. 2009;4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shultz LD, Brehm MA, Garcia‐Martinez JV, Greiner DL. Humanized mice for immune system investigation: progress, promise and challenges. Nat Rev Immunol. 2012;12:786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen Q, Khoury M, Chen J. Expression of human cytokines dramatically improves reconstitution of specific human‐blood lineage cells in humanized mice. Proc Natl Acad Sci USA. 2009;106:21783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mestas J, Hughes CCW. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731. [DOI] [PubMed] [Google Scholar]
- 13. Ito R, Takahashi T, Katano I, Kawai K, Kamisako T, Ogura T, et al. Establishment of a human allergy model using human IL‐3/GM‐CSF–transgenic NOG mice. J Immunol. 2013;191:2890. [DOI] [PubMed] [Google Scholar]
- 14. Rådinger M, Jensen BM, Kuehn HS, Kirshenbaum A, Gilfillan AM. Generation, isolation, and maintenance of human mast cells and mast cell lines derived from peripheral blood or cord blood. Curr Protoc Immunol. 2010;90:7.37.1‐7.37.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dahl C, Saito H, Nielsen HV, Schiøtz PO. The establishment of a combined serum‐free and serum‐supplemented culture method of obtaining functional cord blood‐derived human mast cells. J Immunol Methods. 2002;262:137‐143. [DOI] [PubMed] [Google Scholar]
- 16. Gunawan M, Her Z, Liu M, Tan SY, Chan XY, Tan WWS, et al. A novel human Systemic lupus erythematosus model in humanised mice. Sci Rep. 2017;7:16642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gurish Michael F, Austen KF. Developmental origin and functional specialization of mast cell subsets. Immunity. 2012;37:25‐33. [DOI] [PubMed] [Google Scholar]
- 18. Steiner M, Harrer A, Lang R, Schneider M, Ferreira F, Hawranek T, et al. Basophil activation test for investigation of IgE‐mediated mechanisms in drug hypersensitivity. J Vis Exp. 2011;55:3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Saito H, Ebisawa M, Tachimoto H, Shichijo M, Fukagawa K, Matsumoto K, et al. Selective growth of human mast cells induced by Steel factor, IL‐6, and prostaglandin E2 from cord blood mononuclear cells. J Immunol. 1996;157:343. [PubMed] [Google Scholar]
- 20. Tatemoto K, Nozaki Y, Tsuda R, Konno S, Tomura K, Furuno M, et al. Immunoglobulin E‐independent activation of mast cell is mediated by Mrg receptors. Biochem Biophys Res Commun. 2006;349:1322‐1328. [DOI] [PubMed] [Google Scholar]
- 21. Schäfer B, Piliponsky AM, Oka T, Song CH, Gerard NP, Gerard C, et al. Mast cell anaphylatoxin receptor expression can enhance IgE‐dependent skin inflammation in mice. J Allergy Clin Immunol. 2013;131:541‐548.e1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Roth BL, Marshall FH. Studies of a ubiquitous receptor family. Nature. 2012;492:57. [DOI] [PubMed] [Google Scholar]
- 23. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105:259‐273.e78. [DOI] [PubMed] [Google Scholar]
- 24. Boehm I, Nairz K, Morelli J, Silva Hasembank Keller P, Heverhagen JT. General anaesthesia for patients with a history of a contrast medium‐induced anaphylaxis: a useful prophylaxis. Br J Radiol. 2017;90:20160647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lansu K, Karpiak J, Liu J, Huang X‐P, McCorvy JD, Kroeze WK, et al. In silico design of novel probes for the atypical opioid receptor MRGPRX2. Nat Chem Biol. 2017;13:529‐536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fujisawa D, Kashiwakura J‐I, Kita H, Kikukawa Y, Fujitani Y, Sasaki‐Sakamoto T, et al. Expression of Mas‐related gene X2 on mast cells is upregulated in the skin of patients with severe chronic urticaria. J Allergy Clin Immunol. 2014;134:622‐633.e9. [DOI] [PubMed] [Google Scholar]
- 27. Atwood BK, Lopez J, Wager‐Miller J, Mackie K, Straiker A. Expression of G protein‐coupled receptors and related proteins in HEK293, AtT20, BV2, and N18 cell lines as revealed by microarray analysis. BMC Genomics. 2011;12:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Timmerman LA, Clipstone NA, Ho SN, Northrop JP, Crabtree GR. Rapid shuttling of NF‐AT in discrimination of Ca2+ signals and immunosuppression. Nature. 1996;383:837. [DOI] [PubMed] [Google Scholar]
- 29. Shimizu H, Nagakui Y, Tsuchiya K, Horii Y. Demonstration of chymotryptic and tryptic activities in mast cells of rodents: comparison of 17 species of the family Muridae. J Comp Pathol. 2001;125:76‐79. [DOI] [PubMed] [Google Scholar]
- 30. Bryce PJ, Falahati R, Kenney LL, Leung J, Bebbington C, Tomasevic N, et al. Humanized mouse model of mast cell–mediated passive cutaneous anaphylaxis and passive systemic anaphylaxis. J Allergy Clin Immunol. 2016;138:769‐779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yong KSM, Her Z, Chen Q. Humanized mice as unique tools for human‐specific studies. Arch Immunol Ther Exp. 2018;66:245‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Koboziev I, Jones‐Hall Y, Valentine JF, Webb CR, Furr KL, Grisham MB. Use of humanized mice to study the pathogenesis of autoimmune and inflammatory diseases. Inflamm Bowel Dis. 2015;21:1652‐1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bischoff SC. Role of mast cells in allergic and non‐allergic immune responses: comparison of human and murine data. Nat Rev Immunol. 2007;7:93. [DOI] [PubMed] [Google Scholar]
- 34. Reber LL, Sibilano R, Mukai K, Galli SJ. Potential effector and immunoregulatory functions of mast cells in mucosal immunity. Mucosal Immunol. 2015;8:444‐463. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files).


