Abstract
Background:
Type 2 diabetes and cardiometabolic comorbidities manifesting as the metabolic syndrome (MetS) are highly prevalent in coronary heart disease (CHD) patients attending cardiac rehabilitation (CR). The study aimed to determine the prevalence of cardiometabolic derangements and MetS, and compare post-CR clinical responses in a large cohort of CHD patients with and without diabetes.
Methods:
Analyses were conducted on 3953 CHD patients [age: 61.1 ± 10.5 years; 741 (18.7%) with diabetes] that completed a representative 12-week CR program. A propensity model was used to match patients with diabetes (n = 731) to those without diabetes (n = 731) on baseline and clinical characteristics.
Results:
Diabetic patients experienced smaller improvements in metabolic parameters after completing CR, including abdominal obesity, and lipid profiles (all P ≤ .002), compared to non-diabetic patients. For both groups, there were similar improvement rates in peak metabolic equivalents ([METs]; P < .001); however, peak METs remained lower at 12-weeks in patients with diabetes than without diabetes. At baseline, the combined prevalence of insulin resistance (IR) and diabetes was 57.3%, whereas IR was present in 48.2% of non-diabetic patients, of which rates were reduced to 48.2% and 32.8% after CR, respectively. Accordingly, MetS prevalence decreased from 25.5% to 22.3% in diabetic versus 20.0% to 13.4% in non-diabetic patients (all P ≤ .004).
Conclusions:
Completing CR appears to provide comprehensive risk reduction in cardio-metabolic parameters associated with diabetes and MetS; however, CHD patients with diabetes may require additional and more aggressive attention towards all MetS criteria over the course of CR in order to prevent future cardiovascular events.
Keywords: Diabetes mellitus, Cardiac rehabilitation, Coronary artery disease, Metabolic syndrome, Cardiorespiratory fitness
1. Introduction
The global prevalence of type 2 diabetes (hereinafter referred to as diabetes) has nearly tripled in recent decades [1,2], reaching epidemic proportions in westernized countries including the US and Canada. Diabetes is recognized as a potent risk factor of cardiovascular disease (CVD) [3,4] such that individuals with diabetes have a 2–4-fold increase in their risk for myocardial infarction and stroke compared to their healthy counterparts [4,5]. Adults with diabetes who have suffered a major cardiovascular event have a poorer prognosis relative to adults without diabetes, including a higher risk of cardiovascular-related mortality [3,5,6]. Cardiometabolic comorbidities that collectively manifest as the metabolic syndrome ([MetS]; i.e., hypertension, dyslipidemia, impaired fasting glucose control, and abdominal obesity), are also highly prevalent among adults with coronary heart disease (CHD) and when aggregated with diabetes, may further amplify cardiovascular morbidity and mortality [7,8].
Exercise-based cardiac rehabilitation (CR) programs are recognized as a standard of care for patients with CVD, with prevention efforts focused on reducing or preventing secondary coronary events including death and cardiovascular-related morbidity, and improving risk factor profile, functional capacity and quality of life [9-11]. Despite the high and increasing prevalence of metabolic dysfunction in patients attending CR, there is limited evidence to suggest that patients with diabetes receive equivalent cardiometabolic benefit compared to patients without diabetes [3,12]. Further, given that the metabolic syndrome is closely related to diabetes, greater clinical attention is needed to understand the effects of CR on individual cardiometabolic derangements in the context of the MetS, which may provide some indication of targeted strategies for intervention in this multimorbid population. The objectives of this study were to: 1) determine the prevalence of cardiometabolic risk factors and MetS prior to CR participation in a large, well described cohort of CHD patients; and 2) compare post-CR clinical responses in those with and without diabetes. We hypothesized that patients with diabetes would have an overall higher burden of cardiometabolic risk factors and MetS at baseline, but would experience larger improvements in cardiometabolic profiles after completing CR.
2. Methods
2.1. Study population
A retrospective analysis was conducted on the data of patients who were referred to outpatient CR with the TotalCardiology™ Rehabilitation [TCR] program, in Calgary, Alberta, Canada between January 1996 and March 2016 [13]. The reason for referral, provincial health number (PHN), and baseline demographics were prospectively recorded for all referrals received, regardless of attendance. All patients were offered a multidisciplinary 12-week CR program, consistent with the Canadian Association of Cardiovascular Prevention and Rehabilitation guidelines [14]. Additional information on patient co-morbidities, coronary anatomy, vital statistics, therapeutic interventions, and adjudicated hospital readmissions, were obtained through a merge with the Alberta Provincial Project for Outcomes Assessment in Coronary Heart Disease (APPROACH) database [15]. Briefly, the APPROACH database captured all patients who underwent a cardiac catheterization and/or revascularization procedure in Alberta, Canada since 1995 [15]. The TCR and APPROACH databases were linked using PHNs, which are unique identifiers. This study was performed in accordance with the Helsinki Declaration and was approved by the Conjoint Health Ethics Review Board of the University of Calgary. All study participants gave written informed consent after receiving oral and written information about the study.
2.2. Patient selection
The APPROACH study design and eligibility criteria have been published elsewhere [13]. All patients included in the present analyses were over the age of 18 years and had completed the 12-week CR program, including a graded exercise test (GXT) and cardiometabolic risk profiles, at program start and completion. Patients were considered to have completed CR if they attended both their baseline and 12-week post-rehabilitation assessment [16]. Diabetic patients were considered those who reported a history of type 2 diabetes diagnosed or treated by a physician, or had diabetes indicated in their hospital record. Additional cross-checks were conducted in the APPROACH database, including extensive data audits in which records were merged with administrative databases, in order to verify diabetes diagnoses based on hospital coding in the Discharge Abstract Database [17].
2.3. Cardiac rehabilitation program
The 12-week multidisciplinary TCR program included one-hour supervised exercise sessions 2 times per week, with participants encouraged to engage in additional exercise sessions on their own. All the patients underwent a baseline assessment that included a complete cardiovascular examination, anthropometric measurements, a GXT following the Bruce or Modified Bruce protocol, and phlebotomy to assess lipid profile. Patients performed a symptom-limited maximal GXT to determine peak metabolic equivalents (METs) to assess CRF, to optimize patient safety, and to determine the exercise prescription. The peak MET value was calculated from treadmill speed and grade during the final stage of the exercise protocol using an established equation [18]. Each one-hour session was then supervised by a multidisciplinary team of physicians, exercise specialists, and registered nurses, and included a 5 min warm-up, 20–60 min of steady state aerobic training (walking on an indoor track or using a treadmill, elliptical trainer, recumbent cross trainer, or cycle ergometer), performed at an intensity of 45–85% of the heart rate reserve (based on GXT test results), followed by a 5 min cool-down. Patients were provided a target heart rate and an associated MET level, and were further instructed on how to interpret the Borg 6–20 scale and exercise in the 12 to 14 range.
To supplement the exercise prescription, CR participants were offered individualized education, support with risk factor management, and access to a multidisciplinary team of healthcare providers. During the TCR program, patients with diabetes had capillary blood glucose levels measured by program nurses for the first 6 sessions, pre-exercise (2 h post-cibum); in patients with diabetes receiving insulin/secretagogues, additional monitoring occurred after exercise completion. Optimization of medical management was done by program physicians or by consulting primary care providers. Medication changes were made under physician direction. All the patients underwent a repeat assessment upon program completion (12-weeks) and were given instructions for ongoing lifestyle changes at home, including an exercise program and dietary advice.
2.4. Outcomes
Outcomes of interest included the MetS and cardiometabolic markers. Baseline and 12-week CR lipid profiles were analyzed within one month of starting and completing CR. Blood samples were collected under non-fasting conditions by trained phlebotomists and were analyzed by the Alberta Public Laboratories (Calgary, AB, Canada).
The presence of the MetS was determined using the recommended guidelines [19,20], defined as presence of at least three of the five following criteria: 1) waist circumference of >102 and >88 cm in White men and women, respectively [19]; and >90 and >80 cm in South Asian men and women, respectively [20]; 2) HDL cholesterol <1.03 and <1.29 mmol/L in men and women, respectively; 3) systolic blood pressure >130 or diastolic blood pressure >85 mm Hg, measured at rest with the patient in supine position; 4) triglycerides (TG) ≥1.7 mmol/L; and 5) insulin resistance using the ratio of TG to HDL ratio (TG/HDL) ≥1.2 mmol/L. Previous studies have found the TG/HDL ratio to be a valid indicator of insulin resistance (IR) [21] with a sensitivity and specificity comparable to criteria proposed by the Adult Treatment Panel III (ATP III) to diagnose MetS [19,22,23].
Additional cardiometabolic markers included LDL cholesterol (ideal LDL < 2.6 mmol/L by National Cholesterol Education Program [NCEP] ATP III goals [19]; <2.0 mmol/L by Canadian Cardiovascular Society [CCS] [24]), and peak METS performed before CR initiation (baseline) and at the end of the 12-week CR program. BMI was calculated from clinically measured weight (kg)/height (m)2.
2.5. Study covariates
Baseline demographic and clinical characteristics were recorded in the TCR database. The APPROACH database linkage was used to obtain additional clinical covariates, including diagnosed hypertension, diabetes, chronic obstructive pulmonary disease (COPD), cerebrovascular disease (CEVD), congestive heart failure (HF), peripheral vascular disease (PVD), and coronary interventions [percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG)], and results of coronary catheterization, including coronary anatomy (summarized by the Duke Jeopardy Score) [25]. Clinical covariates were captured only at the time of the initial catheterization; thus, the present analyses included only patients who were referred for CR within 1 year of their first catheterization to ensure that the covariate data reasonably reflected the state of the patient at CR, and thus eliminate potential bias introduced by any change in patient comorbidity status.
3. Statistical analysis
3.1. Propensity matching methods
Similar to our previously published methods [13], a nonparsimonious regression model was conducted to match and compare CHD patients with and without diabetes based on observed characteristics using logistic regression to produce a propensity score for CR treatment effect [13]. Propensity matching methods were applied to account for potential systematic differences in baseline characteristics between diabetic and nondiabetic patients that may arise when estimating the effect of CR on MetS and cardiometabolic comorbidities [26]. Propensity matched models included the following patient characteristics: 1) age; 2) sex; 3) ethnicity 4) baseline BMI; 5) coronary disease severity (defined by Duke Jeopardy score) [25]; 5) current or former smoker; 6) presence of malignancy; 7) dialysis; 8) HF; 9) COPD; 10) liver or gastrointestinal disease; 11) PVD; 12) CEVD; 13) prior CABG; 14) prior MI; 15) prior PCI; and, 16) prior ST/elevation MI. The model produced a propensity score for the probability of a CR treatment effect that was then used to match diabetics versus non-diabetic patients in a greedy 1-to-1 manner using PS matching with R-plug in within SPSS (Chicago, IL, USA). Balance in the matched groups was evaluated by ensuring that there was no more than a 10% difference present between the groups.
Baseline demographic and health characteristics were computed for the total sample and compared across diabetic and non-diabetic subgroups using chi (χ2) or F-test statistics for categorical variables and independent (student’s) t-test for continuous variables. Baseline and post (12-week) CR differences in each cardiometabolic comorbidity were compared using the paired-t-test and mean 12-week changes were computed as post-CR minus baseline values. A two-tailed value of P < .05 was defined as statistically significant. Statistical analyses were conducted using SPSS (Version 24.0; Chicago, IL, USA).
4. Results
A total of 22,978 patients were referred to CR between 1996 and 2016. Among these, 15,522 patients enrolled and started CR, of whom 11,850 (76.3%) had completed the 12-week CR program. Of these, 3953 patients [750 (19.0%) female; mean ± SD age: 61.1 ± 10.5 years] had no missing covariate data at baseline and 12-week assessments. Among this sample, 18.7% were identified as having diabetes, and propensity matching analysis yielded a final analytic sample size of 1462 patients (731 with diabetes and 731 without diabetes). Within propensity matched groups, men represented most of the study population (~80%). Patients with diabetes were more likely to be diagnosed with hypertension, hyperlipidemia, and a family history of CHD, and had more extensive CHD according to their Duke Jeopardy scores (Table 1). Overall, patients included in the study appeared to have a lower prevalence of comorbidity burden and prior cardiovascular events, and had a less adverse cardiometabolic risk profile at baseline compared to patients who were excluded from the analyses due to non-compliance or who were non-responsive during the 12-week CR program (Supplemental Table 1).
Table 1.
Baseline Differences in Demographics and Clinical Characteristics in Cardiac Rehabilitation Patients with and without Type 2 Diabetes
| Propensity Matched | ||||
|---|---|---|---|---|
| Entire Population* (n=3953) |
Diabetic patients (n=731) |
Non-Diabetic patients (n=731) |
P- value† |
|
| Male, n (%) | 3203 (81.0) | 595 (81.4) | 585 (80.0) | 0.51 |
| Age, mean, y ± SD | 61.1 ± 10.5 | 62.6 ± 9.4 | 62.7 ± 10.7 | 0.94 |
| Age categories, n (%) | ||||
| <40yrs | 87 (2.0) | 4 (0.5) | 16 (2.2) | 0.32 |
| 40 to <50yrs | 482 (12.2) | 58 (7.9) | 80 (10.9) | 0.01 |
| 50 to <60yrs | 1259 (31.8) | 223 (30.5) | 187 (25.6) | 0.05 |
| 60 to <70yrs | 1310 (33.1) | 285 (39.0) | 259 (35.4) | 0.04 |
| 70 to <80yrs | 676 (17.1) | 139 (19.0) | 156 (21.3) | 0.27 |
| ≥ 80yrs | 139 (3.5) | 22 (3.0) | 33 (4.5) | 0.16 |
| Weight (kg) | 84.9 ± 16.8 | 87.7 ± 17.3 | 87.9 ± 17.3 | 0.83 |
| BMI categories (kg/m2), n (%) | ||||
| <18.5 | 19 (0.5) | 3 (0.4) | 2 (0.3) | 0.65 |
| 18.5-24.9 | 1047 (26.5) | 150 (20.5) | 145 (19.8) | 0.75 |
| 25-29.9 | 1821 (46.1) | 299 (40.9) | 320 (43.8) | 0.27 |
| 30-34.9 | 785 (19.9) | 197 (26.9) | 191 (26.1) | 0.72 |
| 35-39.9 | 207 (5.2) | 60 (8.2) | 53 (7.3) | 0.49 |
| ≥40 | 74 (1.9) | 22 (3.0) | 20 (2.7) | 0.75 |
| Ejection fraction | 15.9 ± 34.8 | 16.2 ± 35.2 | 16.1 ± 35.1 | 0.95 |
| Hypertension, n (%) | 2407 (60.9) | 582 (79.6) | 440 (60.2) | 0.0001 |
| Hyperlipidemia, n (%) | 2592 (65.6) | 550 (75.2) | 505 (69.1) | 0.009 |
| Congestive Heart Failure | 233 (5.9) | 63 (8.6) | 66 (9.0) | 0.43 |
| COPD | 401 (10.1) | 91 (12.4) | 94 (12.9) | 0.81 |
| Liver Disease | 38 (1.0) | 10 (1.4) | 12 (1.6) | 0.67 |
| Liver/GI Disease | 287 (7.3) | 49 (6.7) | 58 (7.9) | 0.37 |
| GI Disease | 252 (6.4) | 41 (5.6) | 47 (6.4) | 0.51 |
| Malignancy | 139 (3.5) | 31 (4.2) | 32 (4.4) | 0.90 |
| Peripheral vascular disease | 159 (4.0) | 22 (3.0) | 19 (2.6) | 0.64 |
| Cerebrovascular disease | 1332 (3.3) | 25 (3.4) | 22 (3.0) | 0.66 |
| Present Smoker | 778 (19.7) | 126 (17.2) | 121 (16.6) | 0.73 |
| Former Smoker | 1117 (28.3) | 241 (33.0) | 246 (33.7) | 0.78 |
| Renal Disease | 54 (1.4) | 17 (2.3) | 13 (1.8) | 0.46 |
| Dialysis | 10 (0.3) | 2 (0.3) | 3 (0.4) | 0.65 |
| Family History of Heart Disease | 1395 (35.3) | 267 (36.5) | 227 (31.1) | 0.03 |
| Previous MI, n(%) | 324 (8.2) | 81 (11.1) | 85 (11.6) | 0.74 |
| Prior PCI, n(%) | 228 (5.8) | 58 (7.9) | 55 (7.5) | 0.77 |
| Prior CABG, n(%) | 254 (6.4) | 61 (8.3) | 61 (8.3) | N/A |
| Indication for Catheterization, n(%) | ||||
| Stable angina | 884 (22.4) | 217 (29.7) | 196 (26.8) | 0.22 |
| Myocardial infarction | 2110 (53.4) | 327 (44.7) | 364 (49.8) | 0.05 |
| Unstable angina | 517 (13.1) | 95 (13.0) | 92 (12.6) | 0.81 |
| Other | 442 (11.2) | 92 (12.6) | 79 (10.8) | 0.29 |
| ST/Elevation MI, n (%) | 1289 (32.6) | 196 (26.8) | 193 (26.4) | 0.86 |
| Duke Jeopardy Score n (%) | 0.028 | |||
| Normal | 31 (2.1) | 9 (1.2) | 22 (3.0) | 0.678 |
| <50% | 54 (3.7) | 29 (4.0) | 25 (3.4) | 0.046 |
| Low risk | 665 (45.5) | 313 (42.8) | 352 (48.2) | 0.003 |
| High risk | 560 (38.3) | 308 (42.1) | 252 (34.5) | 0.606 |
| Left main | 151 (10.3) | 72 (9.8) | 79 (10.8) | N/A |
| missing | 1 (0.1) | 0 (0.0) | 1 (0.1) | |
Notes: Data are presented as mean (SD), or % as appropriate.
CABG, coronary artery bypass grafting; CR, cardiac rehabilitation; COPD, chronic obstructive pulmonary disease; MI, myocardial infarction; PCI, percutaneous coronary intervention
Entire population based on unmatched patient population who completed Cardiac Rehabilitation.
Overall difference across groups as determined by paired t-test or X2 test.
At baseline, the MetS was prevalent in 25% of diabetic patients and 20% of non-diabetic patients (Fig. 1). IR was the most prevalent cardio-metabolic characteristic among diabetic (57.3%) and non-diabetic (48.2%) patients at the start of CR, followed by abdominal obesity, low HDL, high TG, and high BP. After completing CR, the percentage of patients with IR decreased by 16% in diabetic patients and 32% in non-diabetic patients. Further, a significant percentage of diabetic patients were no longer classified as having high TG (26%) or low HDL (6%), but 17% more diabetic patients experienced high BP (Supplemental Table 2, all Ppre-post < .0001), and the overall magnitude in prevalence change was considerably lower than “cure-rates” observed in nondiabetic patients (TG, 40%; HDL, 19%, P for between groups difference < .03). Accordingly, the proportion with MetS decreased by 3.3% (relative improvement of 13%) among patients with diabetes, and by 6.6% absolute (relative improvement of 33%) in non-diabetic patients (both groups Ppre-post < .05; P for between groups difference < .01).
Fig. 1.
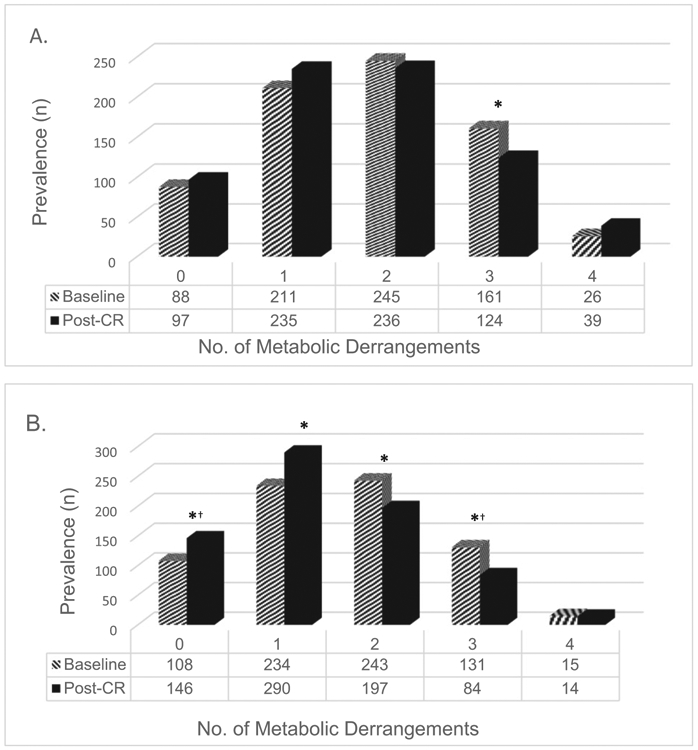
Metabolic syndrome components at baseline and after cardiac rehabilitation completion in A) diabetic patients (n = 731) and B) non-diabetic patients (n = 731). CR, cardiac rehabilitation.
*Significant differences between baseline and 12-weeks (post-CR) metabolic markers; all P ≤ .004 by paired sample t-test
†Significance at P< .0001 bypaired sample t-test.
Clinical profile of individual cardiometabolic measures among diabetic and non-diabetic patients are described in Table 2. Compared to non-diabetics, patients with diabetes had higher waist circumference, TG, IR levels, and lower HDL and initial peak METs, but also had lower LDL and diastolic BP (all P < .05). After CR completion, overall improvements in cardiometabolic comorbidities in diabetic patients were modest compared to those reported in non-diabetic patients (all P for between groups difference ≤ .002). Specifically, IR reduced by 13% in diabetic patients and 21% non-diabetic patients. Though peak METs were significantly lower at baseline in diabetic patients, both diabetic and non-diabetic patients showed similar improvements in peak METs (both groups, 13% increase in peak METs). Both groups also demonstrated marked improvements in lipid profiles, particularly LDL-cholesterol, contributing to a greater proportion of diabetic and non-diabetic patients meeting ideal LDL thresholds established by CCS [24], and by the NCEP ATP III goals [19] (Fig. 2). However, the percentage of diabetic patients who attained lipid goals after completing CR was considerably lower for HDL and TG, compared to non-diabetic patients (P for between groups difference < .024). No significant improvement in systolic or diastolic BP was observed in either group after completing CR.
Table 2:
Effect of Cardiac Rehabilitation on cardiometabolic risk factors in patients with and without Type 2 Diabetes
| Diabetic Patients (n=731) | Non-Diabetic patients (n=731) | |||||
|---|---|---|---|---|---|---|
| Baseline | Post-CR* | Mean 12 week difference (%Δ)† |
Baseline | Post-CR‡,§ | Mean 12 week difference (%Δ)† |
|
| BMI (kg/m2) | 29.1 ± 4.9 | 29.1 ± 4.9 | 0.03 ± 1.1 (0.1%) | 28.9 ± 4.8 | 28.7 ± 4.9 | −0.2 ± 1.1 (0.7%) |
| Waist Circumference (cm) | 104.5 ± 13.3 | 104.1 ± 13.3 | −0.5 ± 4.0 (−0.44) | 102.9 ± 12.7 | 101.8 ± 12.7 | −1.1 ± 3.9 (−1.1%) |
| Cardiorespiratory fitness (Peak METs) | 6.7 ± 1.9 | 7.6 ± 2.0 | 0.9 ± 0.9 (13.0%) | 7.2 ± 2.1 | 8.2 ± 2.1 | 1.0 ± 1.0 (13.2%) |
| Systolic BP (mmHg) | 117.5 ± 18.6 | 118.7 ± 16.4 | 1.2± 17.5 (1.0%) | 116.4 ± 18.1 | 116.7 ± 16.3 | 0.2 ± 17.0 (0.2%) |
| Diastolic BP (mmHg) | 69.6 ± 9.8 | 69.9 ± 9.1 | 0.3 ± 10.0 (−0.45%) | 71.1 ± 9.6 | 71.3 ± 9.2 | 0.1 ± 9.7 (0.18%) |
| HDL (mmol/L)∥ | 1.06 ± 0.28 | 1.08 ± 0.27 | 0.03 ± 0.18 (1.9%) | 1.14 ± 0.30 | 1.19 ± 0.31 | 0.06 ±0.20 (5.3%) |
| LDL (mmol/L)∥ | 1.91 ± 0.85 | 1.56 ± 0.67 | −0.35 ± 0.82 (−18.3%) | 2.28 ± 1.00 | 1.74 ± 0.70 | −0.55 ± 0.95 (−23.7%) |
| TG (mmol/L)¶ | 1.55 ± 0.78 | 1.39 ± 0.67 | −0.16 ± 0.69 (−10.3%) | 1.46 ± 0.72 | 1.22 ± 0.57 | −0.24 ± 0.62 (−16.4%) |
| TG/HDL (mmol/L) | 1.63 ± 1.06 | 1.41 ± 0.89 | −0.22 ± 0.85 (−13.5%) | 1.43 ± 0.92 | 1.12 ± 0.71 | −0.30 ± 0.72 (−21.0%) |
mean 12week difference calculated as the difference of baseline and 12-week for each variable, and % change calculated relative to baseline (pre-CR), based on non-rounded values
significant improvement in diabetic patients between baseline and post-CR metabolic markers (except SBP, DBP, and BMI), all P≤.002 by paired sample t-test
significant improvement in non-diabetic patients between baseline and post-CR metabolic markers (except SBP, DBP), all P<.0001 by paired sample t-test
significant difference between diabetic and non-diabetic groups in all metabolic risk factors (except for SBP) at baseline, all P≤0.03 and in all metabolic factors (except BMI) post CR, all P≤.02 as determined by independent t-test
To convert HDL-C or LDL-C concentration to mg/dl, divide concentration in mmol/L by 0.02586.
To convert triglyceride concentration to mg/dl, divide concentration in mmol/L by 0.01129.
Fig. 2.
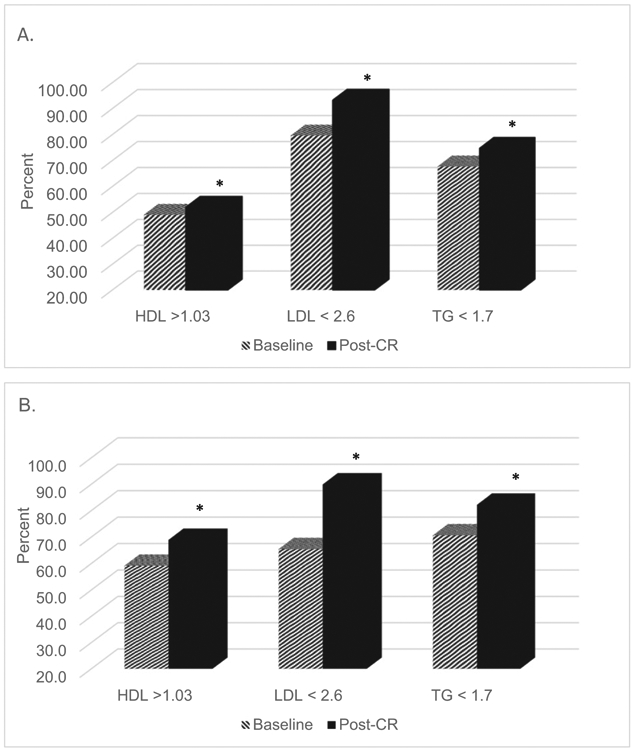
Percent of patients meeting the NCET ATP III cholesterol guidelines at baseline and after cardiac rehabilitation completion in A) diabetic patients (n = 731) and B) non-diabetic patients (n = 731).
*Significant improvement in the percentage of patients meeting lipid guidelines from baseline to 12-week (post-CR) observed in diabetic and nondiabetic groups, respectively, all P ≤ .0001, by paired sample t-test
Significant difference between percentages of diabetic versus non-diabetic patients meeting lipid guidelines after CR completion, all P ≤ .024, by independent t-test.
5. Discussion
In this large CR cohort, patients with and without diabetes demonstrated a reduction in the MetS prevalence, in addition to comprehensive cardiometabolic risk reduction after completing a 12-week CR program. However, overall improvements in the cardiometabolic risk profiles among patients with diabetes were smaller than improvements reported in patients without diabetes. Reductions in IR and increases in peak METs were among the most notable cardiometabolic benefits observed regardless of diabetes status, though, peak METs achieved after CR completion in diabetic patients remained lower than non-diabetic counterparts. Improvements in lipid profiles translated to a greater percentage of patients meeting national lipid guidelines [19,24]; while, non-diabetic patients were more effective at meeting HDL and TG goals than diabetic patients.
Our findings represent the first systematic attempt to examine the changes in MetS, diabetes and other cardiometabolic risk factors during CR participation in a large population of CHD patients who completed CR. Our analyses adjusted for systematic differences in baseline patient characteristics, which may otherwise confound the observed CR “treatment” effect, by applying propensity matching methods to identify and uniquely balance diabetic and non-diabetic groups based on group similarities in various demographic, behavioral, and clinical covariates. As expected, the MetS burdened a higher proportion of diabetic (25.5%) than non-diabetic patients (20%). IR, which affected 57% diabetic patients and 48% non-diabetic patients, along with abdominal obesity, and low HDL levels were the three most common comorbidities that explained MetS prevalence in diabetic and non-diabetic groups. Contrary to our hypothesis, CR completion appeared less effective at improving MetS and metabolic derangements in diabetic patients than in nondiabetic patients. After CR completion, nearly 3.3% of diabetic patients no longer had MetS, an improvement rate of 13% that was clinically significant, albeit, modest compared to the 33% improvement rate noted in non-diabetic patients. The decrease in MetS prevalence observed in both groups was primarily explained by the change (% decrease) in the prevalence of patients who met the risk criteria for high TG (26%, 40%), IR (16%, 32%) and low HDL (6%, 19%) after 12-weeks.
It is important to highlight that the modest changes in individual MetS markers observed in both groups may have clinically meaningful health benefits. Both diabetic and non-diabetic patients, on average, experienced a 3% and 5% improvement (respectively) in HDL levels, which has been suggested to attenuate cardiac disease progression by 2%−3% [27]. More substantial improvements occurred in TG levels, which may translate into reduced risk of secondary cardiovascular events [28]. CR had the greatest effect on LDL cholesterol levels (diabetic patients, 18% decrease; non-diabetic patients, 24% decrease), consistent with previous studies [12,29,30] and the lipid lowering goals of traditional CR [9]. The net benefit of CR on lipid profiles resulted in a greater percentage of patients achieving national NCEP ATP III and CCS guidelines [19,24]. Despite appreciable improvements in lipid profiles, these findings corroborate with other studies [3,12,29,30] suggesting the need for more tailored lipid management approaches during CR, especially those that target HDL and TG levels. Further, our study showed that the IR levels favorably changed in patients with and without diabetes by 13% and 21%, respectively, indicating that all patients benefited from the exercise training and lifestyle modifications facilitated by CR.
CRF, measured in this study by peak METs, is considered to be one of the most important clinical vital signs for cardiovascular health [31]. We have previously reported in our CR patient sample, that those with the lowest baseline peak MET level achieved greater improvements in CRF after CR completion; however, diabetes was a significant and negative determinant of CRF [32]. In the present study, diabetic patients experienced similar improvements in peak METS relative to non-diabetic patients (~1 MET), translating to a 13% increase over 12 weeks, despite diabetic patients having a lower CRF at baseline and after CR compared to non-diabetic patients at baseline (6.7 versus 7.2 peak METs at baseline and 7.6 vs. 8.2 peak METs at 12 weeks). For reasons that remain unknown, the 13% peak MET gain was considerably smaller than fitness gains observed in previous studies (range 22%–38%) [3,12,30,33]; though, the smaller sample of diabetic patients included in earlier studies, in addition to differences in disease severity, prevalence of comorbidity, medication use, and volume/intensity of exercise training are likely factors explaining this discrepancy [30]. Nonetheless, the average improvement of 1 MET increase is clinically meaningful, as our group has previously shown that each 1 MET increase translates to a 13% reduction in mortality [34].
Interestingly, improvements in peak METs and other cardiometabolic risk factors occurred without any change in BMI. Given the negative impacts of body weight on endurance, which often parallels a greater cardiometabolic risk burden, it is possible that greater weight reduction may have facilitated larger improvements in peak METs [7]. These data further emphasize critical gaps in CR concerning weight reduction strategies [7,12,33], and warrant the need for greater research to examine the added value of comprehensive weight loss interventions in combination with standard exercise-based CR for patients with and without diabetes.
5.1. Strengths & limitations
The availability of multiple clinical characteristics, including medical history, and existing comorbidities in this large dataset evaluating the effects of CR in a diabetic population are clear strengths of this analysis. Though we acknowledge that women were underrepresented in this study, our findings are likely generalizable to other CR centers as they describe a patient population that closely mimics contemporary CR programs. However, this study was restricted to those who completed CR, and thus, results may not be generalized to patients who do not enroll and/or do not complete CR. As such, differences observed between patients with and without diabetes may be partly attributable to lower exercise attendance and lower adherence to the self-management behaviors during CR, which we were unable to measure in this study. Further, while we confirm that patients in our study were correctly identified has having T2DM, it is possible that patients with undiagnosed type 2 diabetes were misclassified as non-diabetic patients and thus, we cannot rule out potential bias of these findings. Additionally, direct assessment of IR using HbA1c data were not available for most patients, however, TG/HDL, an established surrogate of IR that has been used in prior CR studies [3,35] was used to estimate the change in glycemic control after completing CR. Nonetheless, we acknowledge that more research is needed to verify the utility of the TG/HDL as a reliable indicator of IR in patients who are taking lipid-altering medications (e.g., statins) [8]. Accordingly, details of medication use (e.g., antihypertensive, lipid-lowering or antihyperglycemic therapies) were unavailable for this analysis. It therefore remains unclear whether cardiometabolic improvements observed after CR completion were due to the exercise intervention alone or due to other CR components (e.g., medication adherence, dietary change, psychosocial treatment) or stringent adjunctive therapies. Lastly, this study lacked detailed information regarding the number of CR sessions attended, perceived exertion at peak exercise, and follow-up measures on CHD status, which may have provided additional insight regarding differences in baseline and 12-week clinical profiles observed in diabetic and non-diabetic patients in this population.
6. Conclusion
The present study demonstrated that the MetS, and particularly IR, were common among CHD patients with and without diabetes enrolled in a comprehensive CR program. Completion of CR was associated with reduced MetS prevalence and improvements in various cardio-metabolic derangements, however, improvements were generally modest among diabetic patients compared non-diabetic patients. Given the rising prevalence of MetS and diabetes seen across populations with CVD, improvement in the delivery of evidence-based CR practices, including more routine screening of the MetS prior to CR initiation will be necessary in order to optimally reduce risk of secondary CHD events and mortality. CHD patients with diabetes may benefit from additional more aggressive weight reduction strategies (i.e., behavioral weight loss therapy) to ideally address clinical shortcomings in IR, and better manage BP, which together may lead to a better clinical trajectory.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the TotalCardiology investigators, staff, and APPROACH study participants for their valuable contributions.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Declaration of competing interest
The authors report no relationships that could be construed as a conflict of interest. There are no relationships with industry to report, or relationships relevant to the contents of this paper to disclose by any of the author.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcard.2019.11.134.
References
- [1].Mensah GA, Wei GS, Sorlie PD, Fine LJ, Rosenberg Y, Kaufmann PG, et al. , Decline in cardiovascular mortality: possible causes and implications, J. Circ. Res 120 (2017) 366–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cho N, Shaw J, Karuranga S, Huang Y, da Rocha Fernandes J, Ohlrogge A, et al. , IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045, Diabetes Res. Clin. Pract 138 (2018) 271–281. [DOI] [PubMed] [Google Scholar]
- [3].Lavie CJ, Milani RV, Cardiac rehabilitation and exercise training programs in metabolic syndrome and diabetes, J. Cardiopulm. Rehabil. Prev 25 (2005) 59–66. [DOI] [PubMed] [Google Scholar]
- [4].Einarson TR, Acs A, Ludwig C, Panton UH, Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017, Cardiovascular diabetology 17 (2018) 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fox CS, Cardiovascular disease risk factors, type 2 diabetes mellitus, and the Framingham Heart Study, Trends Cardiovasc. Med 20 (2010) 90–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Spencer EA, Pirie KL, Stevens RJ, Beral V, Brown A, Liu B, et al. , Diabetes and modifiable risk factors for cardiovascular disease: the prospective Million Women Study, Eur. J. Epidemiol 23 (2008) 793. [DOI] [PubMed] [Google Scholar]
- [7].Khadanga S, Savage PD, Ades PA, Insulin resistance and diabetes mellitus in contemporary cardiac rehabilitation, J. Cardiopulm. Rehabil. Prev 36 (2016) 331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Grundy SM, Pre-diabetes, metabolic syndrome, and cardiovascular risk, J. Am. Coll. Cardiol 59 (2012) 635–643. [DOI] [PubMed] [Google Scholar]
- [9].Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, et al. , ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, Journal of the American College of Cardiology 62 (2013) e147–e239. [DOI] [PubMed] [Google Scholar]
- [10].Kwan G, Balady GJ, Cardiac rehabilitation 2012: advancing the field through emerging science, Circulation. 125 (2012) e369–e373. [DOI] [PubMed] [Google Scholar]
- [11].Kachur S, Chongthammakun V, Lavie CJ, De Schutter A, Arena R, Milani RV, et al. , Impact of cardiac rehabilitation and exercise training programs in coronary heart disease, Prog. Cardiovasc. Dis 60 (2017) 103–114. [DOI] [PubMed] [Google Scholar]
- [12].Hindman L, Falko JM, LaLonde M, Snow R, Caulin-Glaser T, Clinical profile and outcomes of diabetic and nondiabetic patients in cardiac rehabilitation, Am. Heart J 150 (2005) 1046–1051. [DOI] [PubMed] [Google Scholar]
- [13].Martin BJ, Hauer T, Arena R, Austford LD, Galbraith PD, Lewin AM, et al. , Cardiac rehabilitation attendance and outcomes in coronary artery disease patients, Circulation. 126 (2012) 677–687. [DOI] [PubMed] [Google Scholar]
- [14].Stone JA, Arthur HM, Canadian guidelines for cardiac rehabilitation and cardiovascular disease prevention, 2004: executive summary, Can. J. Cardiol 21 (2005) 3D–19D. [PubMed] [Google Scholar]
- [15].Ghali WA, Knudtson ML, Overview of the Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease. On behalf of the APPROACH investigators, Can. J. Cardiol 16 (2000) 1225–1230. [PubMed] [Google Scholar]
- [16].Colbert JD, Martin BJ, Haykowsky MJ, L Hauer T, Austford LD, A Arena R, et al. , Cardiac rehabilitation referral, attendance and mortality in women, Eur. J. Prev. Cardiol 22 (2015) 979–986. [DOI] [PubMed] [Google Scholar]
- [17].Humphries K, Norris CM, Quan H, Galbraith P, Southern D, Gao M, et al. , An Adminstrative Data Merging Solution for Dealing With Missing Data in a Clinical Registry: Adaptation to ICD-9 to ICD-10, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].McConnell TR, Clark BA, Prediction of maximal oxygen consumption during handrail-supported treadmill exercise, J. Cardpulm. Rehabil 7 (1987) 324–331. [Google Scholar]
- [19].Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III), JAMA 285 (2001) 2486–2497. [DOI] [PubMed] [Google Scholar]
- [20].Alberti KGMM, Zimmet P, Shaw J, Metabolic syndrome—a new world-wide definition. A consensus statement from the international diabetes federation, 23 (2006) 469–480. [DOI] [PubMed] [Google Scholar]
- [21].Li C, Ford ES, X Meng Y, Mokdad AH, Reaven GM, Does the association of the triglyceride to high-density lipoprotein cholesterol ratio with fasting serum insulin differ by race/ethnicity? Cardiovasc. Diabetol 7 (2008) 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].McLaughlin T, Abbasi F, Cheal K, Chu J, Lamendola C, Reaven G, Use of metabolic markers to identify overweight individuals who are insulin resistant, Ann. Intern. Med 139 (2003) 802–809. [DOI] [PubMed] [Google Scholar]
- [23].McLaughlin T, Reaven G, Abbasi F, Lamendola C, Saad M, Waters D, et al. , Is there a simple way to identify insulin-resistant individuals at increased risk of cardiovascular disease? Am. J. Cardiol 96 (2005) 399–404. [DOI] [PubMed] [Google Scholar]
- [24].Anderson TJ, Gregoire J, Pearson GJ, Barry AR, Couture P, Dawes M, et al. , Canadian Cardiovascular Society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult, Can J Cardiol. 32 (2016) 1263–1282. [DOI] [PubMed] [Google Scholar]
- [25].Smith LR, Harrell FE Jr., Rankin JS, Califf RM, Pryor DB, Muhlbaier LH, et al. , Determinants of early versus late cardiac death in patients undergoing coronary artery bypass graft surgery, Circulation 84 (1991) III245–53. [PubMed] [Google Scholar]
- [26].Austin PC, An introduction to propensity score methods for reducing the effects of confounding in observational studies, 46 (2011) 399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Assmann G, Gotto AM Jr., HDL cholesterol and protective factors in atherosclerosis, Circulation 109 (2004) III8–14. [DOI] [PubMed] [Google Scholar]
- [28].Chapman MJ, Ginsberg HN, Amarenco P, Andreotti F, Boren J, Catapano AL, et al. , Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management, Eur. Heart J 32 (2011) 1345–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].St Clair M, Mehta H, Sacrinty M, Johnson D, Robinson K, Effects of cardiac rehabilitation in diabetic patients: both cardiac and noncardiac factors determine improvement in exercise capacity, Clin. Cardiol 37 (2014) 233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Carroll S, Tsakirides C, Hobkirk J, Moxon JW, Moxon JW, Dudfield M, et al. , Differential improvements in lipid profiles and Framingham recurrent risk score in patients with and without diabetes mellitus undergoing long-term cardiac rehabilitation, Arch. Phys. Med. Rehabil 92 (2011) 1382–1387. [DOI] [PubMed] [Google Scholar]
- [31].Ross R, Blair SN, Arena R, Church TS, Despres JP, Franklin BA, et al. , Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association, Circulation. 134 (2016) e653–e699. [DOI] [PubMed] [Google Scholar]
- [32].Laddu D, Ozemek C, Lamb B, Hauer T, Aggarwal S, JA Stone, et al. , Factors associated with cardiorespiratory fitness at completion of cardiac rehabilitation: identification of specific patient features requiring attention, Can. J. Cardiol 34 (2018) 925–932. [DOI] [PubMed] [Google Scholar]
- [33].Banzer JA, Maguire TE, Kennedy CM, O’Malley CJ, Balady GJ, Results of cardiac rehabilitation in patients with diabetes mellitus, Am. J. Cardiol 93 (2004) 81–84. [DOI] [PubMed] [Google Scholar]
- [34].Martin BJ, Arena R, Haykowsky M, Hauer T, Austford LD, Knudtson M, et al. , Cardiovascular fitness and mortality after contemporary cardiac rehabilitation, Mayo Clin. Proc 88 (2013) 455–463. [DOI] [PubMed] [Google Scholar]
- [35].Milani RV, Lavie CJ, Prevalence and profile of metabolic syndrome in patients following acute coronary events and effects oftherapeutic lifestyle change with cardiac rehabilitation, Am. J. Cardiol 92 (2003) 50–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


