Abstract
Background
Although many species of mycoplasmas regard as normal flora, but some species causes serious genital disease. In Iran several epidemiological studies have documented the prevalence of Mycoplasma hominis, M. genitalium and Ureaplasma urealyticum in genital disorders. This meta-analysis is going to represent the prevalence of M. hominis, M. genitalium and U. urealyticum among Iranian couples and the correlation between mycoplasmas infection and infertility.
Methods
We search online databases from January 2000 to June 2019. We used following MeSH keywords (Prevalence, M. hominis, M. genitalium, U. urealyticum, male, female, fertility, Infertility, genitourinary tract infection and Iran) with all possible combinations with “OR” and “AND”. Finally, forty-four articles from 2670 were chosen for data extraction and analysis by software using STATA version 14.0.
Results
This meta-analysis revealed that the prevalence of U. urealyticum was 17.53% in Iran and the prevalence of M. genitalium and M. hominis were 11.33 and 9.68% respectively. The rate of M. genitalium, M. hominis and U. urealyticum infection in women with symptoms of genitourinary tract infection was higher than men with genitourinary tract infection (6.46% vs 5.4, 7.67% vs 5.88 and 21.04% vs 12.13%, respectively). As expected, the prevalence of M. genitalium, U. urealyticum and M. hominis among infertile women (12.73, 19.58 and 10.81%) were higher than fertile women (3%, 10. 85% and 4. 35%). Similarly, the prevalence of M. hominis and U. urealyticum among infertile men (14 and 21.18%) were higher than fertile men (4 and 3%). Based on this analysis, the rate of U. urealyticum was higher than M. genitalium and M. hominis among infertile men and women compared to the fertile group. The prevalence rate of M. genitalium, M. hominis and U. urealyticum in central provinces is higher than other parts of Iran.
Conclusions
This meta-analysis reemphasizes a significant relationship between the infertility rate and U. urealyticum, M. genitalium and M. hominis infections. Our finding help to plan the prevalence map of M. hominis, M. genitalium and U. urealyticum in Iran but further studies are needed to suggest routine screening of the pathogens.
Keywords: Mycoplasma hominis, M. Genitalium, Ureaplasma urealyticum, Infertility, Iran
Background
Mycoplasma and Ureaplasma geniuses are the smallest self-replicating organism that belong to the Mollicutes class [1–4]. They live as external parasites of the human, animal, bird, insect and plant cells. Some species have a free-living existence in soil and water [5]. Since Dienes and Edsall isolated first mycoplasma from human in a Bartholin’s gland abscess in 1937, seventeen species of human mycoplasmas species have been identified [6, 7]. As a new derivative genus Ureaplasma is divided in to 14 known serotypes and two biovars: U. parvum and U. urealyticum. U. urealyticum can be transmitted in different ways, including directly by sexual transmission, vertically from mother to offspring, or through transplanted tissues [8–13]. Generally, genital mycoplasmas such as M. hominis, M. genitalium and U. urealyticum are important emerging sexually transmitted bacterial pathogens capable to cause asymptomatic, long-term and chronic infection in genitourinary tract which is considered to be a threat to community health [14, 15]. In a clinical study, about 40% of infants born from infected mothers with genital Mycoplasma infection had symptomatic infection such as neonatal conjunctivitis and meningitis by an ascending route or by crossing the placenta from the mother’s blood via delivery through a colonized birth canal [16].
Despite the worldwide incidence of genital mycoplasmas infections, there are no accurate reports of prevalence, common types, common routes of transmission and antibiotic resistance patterns of M. genitalium, M. hominis and U. urealyticum in Iran [17]. There are some studies about the presence of genital mycoplasmas among men, women, pregnant, newborns, infertile and etc in Iran. In this systematic review and meta-analysis, we are going to present an illustration of prevalence of M. hominis, M. genitalium, and U. urealyticum in Iran and the correlation between mycoplasmas infection and infertility in Iranian couples.
Methods
Search strategy
We search online databases including Pubmed, Scopus, Science Direct, IranMedex, SID (Scientific Information Database), and Google Scholar for the papers that were performed in Iran from January 2000 to June 2019. We used following MeSH keywords (Prevalence, M. hominis, M. genitalium, U. urealyticum, male, female, fertility, Infertility, genitourinary tract infection and Iran) with all possible combinations with “OR” and “AND”. Then the titles of the articles were entered into Mendeley software to find similar articles. Difinition of terms were considered as WHO recommended. One of the limitations of this study is the lack of data in some part of Iran. Since different researchers worked on different samples and conditions, the data was categorized in six groups: 1. Fertile men 2. Infertile men 3. Men with urinary tract infection or prostatitis 4. Fertile women 5. Infertile women 6. Women with urogenital infection or abortion or pregnant.
Inclusion and exclusion criteria
Inclusion criteria of this study consisted of a reference to the prevalence of M. genitalium, M. hominis, and U. urealyticum in Iranian men and women by culture and PCR. Exclusion criteria were irrelevance or limited information, countries other than Iran, review articles, methods other than culture and PCR. At the end, 44 articles, which met our inclusion criteria, were conducted for meta- analysis.
Data extraction
The data were extraction by a pre-prepared checklist from all included articles. The checklist included the author’s name, year of the study, the location, sample volume, type of specimen and the prevalence of M. genitalium, M. hominis, and U. urealyticum. The studies on each Mycoplasma species were further categorized into subgroups, considering (1) study population according to gender (men and women) fertile, infertile and urogenital tract infection; (2) Analytical method (including PCR, and culture); (3) geographical region of sampling (including Eastern provinces: Kerman, North Khorasan, Razavi Khorasan, South Khorasan, Sistan and Baluchestan, and Yazd Provinces; Middle provinces (Northern, Central & Southern): Alborz, Golestan, Mazandaran, Qazvin, Qom, Semnan, Tehran, Bushehr, Chaharmahal and Bakhtiari, Fars, Hormozgan, Isfahan, Kohgiluyeh and Boyer-Ahmad Provinces; Western provinces: Ardabil, East Azerbaijan, Gilan, Kordestan, West Azerbaijan, Zanjan, Hamadan, Ilam, Kermanshah, Khuzestan, Lorestan and Markazi Provinces).
Analytic approach
The ratio of positive samples to total samples was defined as prevalence. Meta-analysis was conducted by STATA version 14 for prevalence of each bacterium on available data. Chi-squared (Q) and I-squared tests were used to assess heterogeneity among the studies. Since the heterogeneity was statistically significant (p-value of Q test < 0.1 and I2 index > 75%), a random-effects model was used; The outcome was estimated as prevalence and 95% confidence intervals (CI).
Results
Description of included and excluded studies
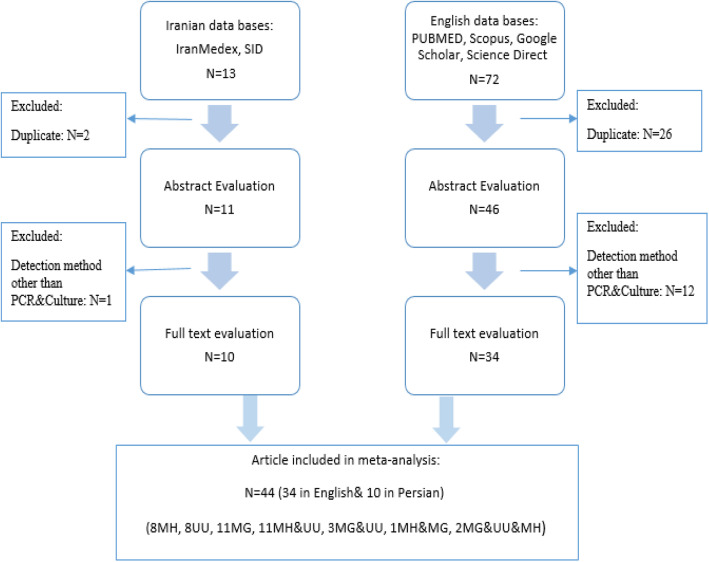
Initially 11,345 articles were identified through database searching. About 2670 articles were remained after discarding duplicate papers based on title and abstract. From 2670 articles, we excluded further 1606 papers based on exclusion criteria (489 papers on M. genitalium, 595 papers on M. hominis, and 522 papers on U. urealyticum were excluded). Forty-four original articles (full texts) related to prevalence of M. genitalium, M. hominis, and U. urealyticum in Iranian men and women in our literature review remained for reviewing and assessing for eligibility criteria. The final 44 articles were included: M. genitalium [17], M. hominis [18], U. urealyticum [19] with some of them contains two [15] or three [2] of these bacteria (Fig. 1). Table 1 provides an overview of the eligible studies.
Fig. 1.
Flow chart of the literature search, systematic review and study selection
Table 1.
Prior studies concerning prevalence of Mycoplasma hominis, Mycoplasma genitalium and Ureaplasma urealyticum in Iran
| no: | Location | Year | Author | Number & kind of sample | Method | Prevalence (%) | Comment | Ref |
|---|---|---|---|---|---|---|---|---|
| 1 | Tehran | 2001 | Badami |
n = 375 cervical swab |
Culture |
Fertile women: MH = 18(7.2) & UU = 48(19.2) Infertile women: MH = 32(25.6) & UU = 41(32.8) |
Fertile women = 250 Infertile women = 125 |
[20] |
| 2 | Tehran | 2003 | Salari |
n = 125 swab urethral |
PCR |
MG = 9(7.2) UU = 24(19.2) MH = 3(2.4) |
Men with NGU | [21] |
| 3 | Tehran | 2005 | AleYasin |
n = 312 cervical swab |
Culture PCR |
Culture: MH = 20(6.4) PCR:MH = 50(16) Culture &PCR = 16(5) |
Infertile women | [22] |
| 4 | Tehran | 2005 | Najar Peerayeh |
n = 312 cervical swab |
Culture PCR |
Culture: UU = 18(5.7) PCR: UU = 32(10.25) |
Infertile women | [23] |
| 5 | Tehran | 2006 | Najar Peerayeh |
n = 377 cervical swab |
PCR |
MH = 31(8.2) UU = 60(15.9) MH & UU = 25(6.6) |
Infertile women | [18] |
| 6 | Tehran | 2007 | Golshani |
n = 200 semen samples |
Multiplex PCR |
MH = 22(11) UU = 6(3) MH&UU = 2(1) |
Infertile men | [24] |
| 7 | Tehran | 2007 | Zeighami |
n = 200 semen samples |
PCR |
Fertile men: UU = 3(3) Infertile men: UU = 12(12) |
Fertile men = 100 Infertile men = 100 |
[19] |
| 8 | Tehran | 2007 | soleimani rahbar |
n = 100 semen samples |
PCR |
MH = 3(3) UU = 17(17) |
Infertile men | [25] |
| 9 | Tehran | 2007 | Najar Peerayeh |
n = 377 cervical swab |
PCR | UU = 85(22.5) | Infertile women | [26] |
| 10 | Tehran | 2008 | NajarPeerayeh |
n = 312 cervical swab |
Culture PCR |
Culture: MH = 12(4) & UU = 39(12) PCR: MH = 28(9) & UU = 54(17) |
Infertile women | [27] |
| 11 | Tehran | 2008 | Ghazisaidi |
n = 75 urethral secretion samples after prostatic massage & First void urine |
PCR |
urethral secretion: MH = 11(15) & UU = 19(25) First void urine: MH = 9 (12)& UU = 17(23) |
Men suffering from nongonococcal urethritis and non-specific urethritis | [28] |
| 12 | Tehran | 2008 | Najar Peerayeh |
n = 246 semen samples |
PCR |
Fertile men: UU = 3(3) Infertile men: UU = 23(15.7) |
Fertile men = 100 Infertile men = 146 |
[29] |
| 13 | Tehran | 2009 | Amirmozafari |
n = 210 cervical swab |
Culture PCR |
Culture: UU = 69(32.8) PCR: UU = 67(31.9) |
Women with urogenital infection | [30] |
| 14 | Tehran | 2010 | Ahmadi |
n = 220 semen sample |
PCR |
MH = 34 (15) UU = 89(40) MH&UU = 25(11) |
Infertile men | [31] |
| 15 | Tehran | 2011 | Mirnejad |
n = 210 genital samples |
PCR |
UU = 89(42.4) MG = 7(3.3) |
Women with urogenital infection | [32] |
| 16 | Sabzevar | 2011 | Haghighi Hasanabad |
n = 196 urine |
PCR | MG = 2(1) | Pregnant women | [33] |
| 17 | Ahwaz | 2011 | Moosavian |
n = 265 urine = 110 cervical swab = 155 |
Culture Multiplex PCR |
Culture: MH = 5 (1.8)& UU = 0 Multiplex PCR: MH = 11(4)&UU = 13(5) |
Women with urogenital infection | [34] |
| 18 | Kerman | 2013 | Vosooghi |
n = 58 semen sample |
PCR | MH = 13(22) | Infertile men | [35] |
| 19 | Tehran | 2013 | irajian |
n = 200 paraffin blocks |
PCR | MG = 4(2) | Men with prostatitis | [36] |
| 20 | Ahvaz | 2013 | Maleki |
n = 265 urine = 110 cervical swab = 155 |
Multiplex PCR |
Urine: MH = 11(10) & UU = 13(12) cervical swab: MH = 7 (4)& UU = 15(10) |
Women with urogenital infection | [37] |
| 21 | Tehran | 2013 | Yeganeh |
n = 200 urine |
PCR | MG = 14(7) | Men refer to urology clinic | [38] |
| 22 | Tehran | 2013 | Sadrpour |
n = 120 semen samples |
PCR | MG = 12(10) | Infertile men | [39] |
| 23 | Mazandaran | 2013 | mohseni |
n = 44 genital samples |
PCR | MG = 10(22.7) | Pregnant women | [40] |
| 24 | Tehran | 2014 | Seifoleslami |
n = 350 cervical swab |
PCR |
Infertile women: MH = 8(5.3) & UU = 10(6.6) MH&UU = 4(2.6) Fertile women: MH = 3(1.5) & UU = 5(2.5) MH&UU = 1 |
Infertile women = 150 Fertile women = 200 |
[17] |
| 25 | Kurdistan | 2014 | Ahmadi |
n = 218 cervical swab |
PCR |
Pregnant women: UU = 8(7.3) Spontaneous abortion: UU = 18(16.5) |
Pregnant women = 109 Spontaneous abortion = 109 |
[41] |
| 26 | Sanandaj | 2014 | Mousavi |
n = 104 cervical swab |
Multiplex PCR |
MH = 3(3) MG = 3(3) UU = 39(37) MH&UU = 1(1) MH&MG = 1(1) MG&UU = 1(1) MG&UU&MH = 1(1) |
Infertile women | [42] |
| 27 | Kerman | 2014 | JamalizadehBahaabadi |
n = 200 semen sample = 100 cervical swab = 100 |
PCR |
Semen sample: MH = 15(7) cervical swab: MH = 18(18) |
Infertile men & Infertile women |
[43] |
| 28 | Kerman | 2014 | Mohseni Moghadam |
n = 200 semen samples = 100 cervical swab = 100 |
PCR |
Semen samples: MG = 13(13) cervical swab: MG = 10(10) |
Infertile men Infertile women |
[11] |
| 29 | Tehran | 2014 | Sobouti |
n = 330 cervical swab their baby after delivery |
Multiplex PCR |
Pregnant women: MH = 25(15.1) & UU = 25(15.1) their baby after delivery: MH = 15(9) & UU = 18(10.9) |
Pregnant women = 165 their baby after delivery = 165 |
[44] |
| 30 | Tehran | 2015 | Dadashi |
n = 124 sample from ovarian cancer |
PCR | MG = 9(7.2) |
Ovarian Cancer = 62 Benign Ovarian Cancer =62 |
[45] |
| 31 | Tehran | 2015 | Eslami |
n = 124 paraffin blocks |
PCR |
MG = 0 UU = 1(0.8) |
From men who undergo prostatectomy | [46] |
| 32 | Tehran | 2015 | Safavifar |
n = 45 semen samples |
PCR |
Infertile men: MG = 6(40) Fertile men: MG = 11(37) |
Infertile men = 15 Fertile men = 30 |
[47] |
| 33 | Kerman | 2015 | EftekhariMoghadam |
n = 50 urine |
PCR | MH = 3(6) | UTI patients | [14] |
| 34 | Kashan | 2015 | Safari |
n = 864 urine |
PCR | MH = 1(0.1) | UTI patients | [48] |
| 35 | Sanandaj | 2016 | Ramazanzadeh |
n = 218 genital swab |
PCR |
Normal pregnant: MG = 4(3.6) Spontaneous abortion: MG = 2(1.8) |
Normal pregnant = 109 Spontaneous abortion = 109 |
[49] |
| 36 | Tehran | 2016 | Ahmadi |
n = 330 semen samples |
Real-time PCR |
Infertile men = MH = 24(14) Fertile men: MH = 6(4) |
Infertile men = 165 Fertile men = 165 |
[50] |
| 37 | Qazvin | 2016 | Bahrami |
n = 232 cervical swab |
culture | UU = 87(37.5) | married females(20–50 years) | [51] |
| 38 | Qom | 2016 | Asgari |
n = 187 semen samples |
PCR | MH = 71(39) | Infertile men | [52] |
| 39 | Tehran | 2016 | Irajian |
n = 200 prostatitis tissues with paraffin |
PCR | UU = 7(3.5) | men suffering from prostatitis | [53] |
| 40 | Tehran | 2017 | sameni |
n = 65 cervical swab |
PCR | MG = 11(16.9) | Infertile women | [54] |
| 41 | Tehran | 2017 | Javadinia |
n = 194 urine |
PCR |
UU = 22(11.3) MG = 11(5.6) UU&MG = 5(2.6) |
Pregnant women | [55] |
| 42 | Mashhad | 2017 | Makari golkhatmi |
n = 200 vaginal swab |
PCR-ELISA |
Infertile women: MG = 21(21) Fertile women: MG = 3(3) |
Infertile women = 100 Fertile women = 100 |
[56] |
| 43 | Hamedan | 2018 | Moradi |
n = 234 cervical swab |
Culture PCR |
Culture: MH = 14(5.9) PCR: MH = 30(12.8) |
married females(20–50 years) | [57] |
| 44 | Mashhad | 2018 | Moridi |
n = 100 semen samples |
Culture PCR |
Culture: MH = 7(7) PCR: MH = 8(8) Culture: MG = 0(0) PCR: MG = 0(0) |
Infertile men | a |
aThe data is under publication
Prevalence of M. genitalium
The overall prevalence of M. genitalium was 16.60% (CI 95%; 12.01–21.18%) and 8.26%(CI 95%; 6.33–10.19%) in male and female respectively (Table 2).
Table 2.
The prevalence of M. genitalium in Iran based of meta-analysis
| Study Population | studies | sample | prevalence, 95% CI | Model |
|---|---|---|---|---|
| Men | 8 | 1114 | 16.60, 12.01–21.18 | Random |
| Fertile | 1 | 30 | 37.00, 36.83–37.17 | Random |
| Infertile | 4 | 435 | 21.00, 13.18–28.82 | Random |
| Symptomatic1 | 4 | 649 | 5.40, 1.55–9.25 | Random |
| Women | 11 | 1455 | 8.26, 6.33–10.19 | Random |
| Fertile | 1 | 100 | 3.00, 2.97–3.03 | Random |
| Infertile | 4 | 369 | 12.73, 4.44–21.01 | Random |
| Symptomatic2 | 7 | 986 | 6.46, 4.62–8.29 | Random |
| Women and men | 18 | 2569 | 11.33, 9.58–13.08 | Random |
1. Men with urinary tract infection or prostatitis; 2. Women with urogenital infection or abortion or pregnant
Prevalence of M. hominis
The overall prevalence of M. hominis was 10.73% (CI 95%; 6.77–14.69%) and 8.83% (CI 95%; 6.67–10.98%), among male and female respectively (Table 3).
Table 3.
The prevalence of M. hominis in Iran based of meta-analysis
| Study Population | studies | sample | prevalence, 95% CI | Model |
|---|---|---|---|---|
| Men | 12 | 2344 | 10.73, 6.77–14.69 | Random |
| Fertile | 1 | 165 | 4.00, 3.97–4.03 | Random |
| Infertile | 7 | 1130 | 14.00, 7.45–20.55 | Random |
| Symptomatic1 | 4 | 1049 | 5.88, 2.18–9.57 | Random |
| Women | 12 | 3670 | 8.83, 6.67–10.98 | Random |
| Fertile | 2 | 450 | 4.35, −1.24 - 9.94 | Random |
| Infertile | 7 | 1480 | 10.81, 7.18–14.45 | Random |
| Symptomatic2 | 3 | 1740 | 7.67, 4.34–10.99 | Random |
| Women and men | 22 | 6014 | 9.68, 7.75–11.61 | Random |
1. Men with urinary tract infection or prostatitis; 2. Women with urogenital infection or abortion or pregnant
Prevalence of U. urealyticum
The prevalence of U. urealyticum was 13.92% (CI 95%; 7.58–20.26%) and 19.43% (CI 95%; 11.56–27.30%), in male and female respectively (Table 4).
Table 4.
The prevalence of U. urealyticum in Iran based of meta-analysis
| Study Population | studies | sample | prevalence, 95% CI | Model |
|---|---|---|---|---|
| Men | 8 | 1290 | 13.92, 7.58–20.26 | Random |
| Fertile | 2 | 200 | 3.00, 2.98–3.02 | Random |
| Infertile | 4 | 766 | 21.18, 8.61–33.74 | Random |
| Symptomatic1 | 4 | 324 | 12.13, 3.23–21.02 | Random |
| Women | 14 | 4441 | 19.43, 11.56–27.30 | Random |
| Fertile | 2 | 450 | 10.85, −5.52 - 27.22 | Random |
| Infertile | 7 | 1757 | 19.58, 13.59–25.57 | Random |
| Symptomatic2 | 5 | 2234 | 21.04, 8.95–33.13 | Random |
| Women and men | 24 | 5731 | 17.53, 11.40–23.66 | Random |
1. Men with urinary tract infection or prostatitis; 2. Women with urogenital infection or abortion or pregnant
The prevalence rates of genital mycoplasma infection are due to U. urealyticum, M. genitalium and M. hominis respectively, in Iran. This study shows that the rate of U. urealyticum, M. genitalium and M. hominis infection in women with symptoms of genitourinary tract infection was higher than men with genitourinary tract infection. The result indicated that the prevalence of U. urealyticum, M. genitalium and M. hominis in infertile women were higher than fertile women. However, the prevalence of U. urealyticum and M. hominis in infertile men were higher than fertile men.
Geographical distribution of M. hominis, M. genitalium and U. urealyticum in Iran
In Eastern provinces of Iran, the prevalence of M. genitalium and M. hominis were 9.60 and 9.73% respectively based of meta-analysis (CI 95%). There is no documented study on U. urealyticum in Eastern provinces. In Middle provinces, the prevalence of M. genitalium, M. hominis and U. urealyticum were 13.39, 11.17 and 17.94% respectively. While in Western provinces of Iran, the prevalence of M. genitalium, M. hominis and U. urealyticum were 3.3, 5.65 and 14.98% respectively (Table 5).
Table 5.
The prevalence and 95% CI of Mycoplasma hominis, Mycoplasma genitalium and Ureaplasma urealyticum in different regions of Iran based of meta-analysis
| Study Location | M. genitalium | M. hominis | U. urealyticum | |||
|---|---|---|---|---|---|---|
| provinces (studies) | prevalence, 95% CI | provinces (studies) | prevalence, 95% CI | provinces (studies) | prevalence, 95% CI | |
| Eastern provinces1 | 2 (4) | 9.60, 4.01–15.19 | 2 (5) | 9.73, 4.49–14.96 | 0 | – |
| Middle provinces2 | 2 (12) | 13.39, 11.14–15.64 | 2 (11) | 11.17, 8.10–14.24 | 2 (17) | 17.94, 11.08–24.80 |
| Western provinces3 | 1 (2) | 3.30, 2.71–3.89 | 3 (4) | 5.65, 3.09–8.22 | 2 (4) | 14.98, 6.83–23.12 |
(1) Eastern provinces: Kerman, North Khorasan, Razavi Khorasan, Sistan and Baluchestan, South Khorasan and Yazd Provinces; (2) Middle provinces: (Northern Central & southern): Alborz, Golestan, Mazandaran, Qazvin, Qom, Semnan, Tehran, Bushehr, Chaharmahal and Bakhtiari, Fars, Hormozgan, Isfahan, Kohgiluyeh and Boyer-Ahmad Provinces; (3) Western provinces: Ardabil, East Azerbaijan, Gilan, Kordestan, West Azerbaijan, Zanjan, Hamadan, Ilam, Kermanshah, Khuzestan, Lorestan and Markazi Provinces
Analytical method
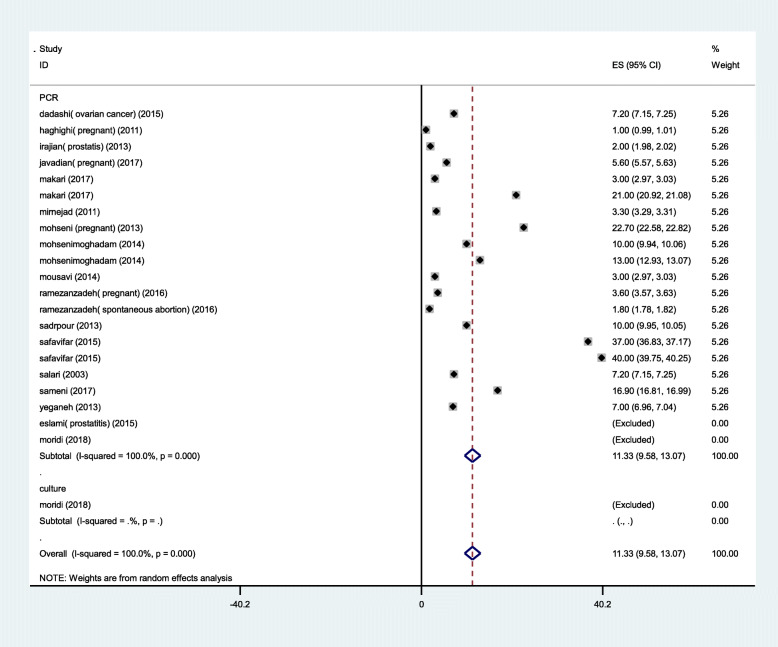
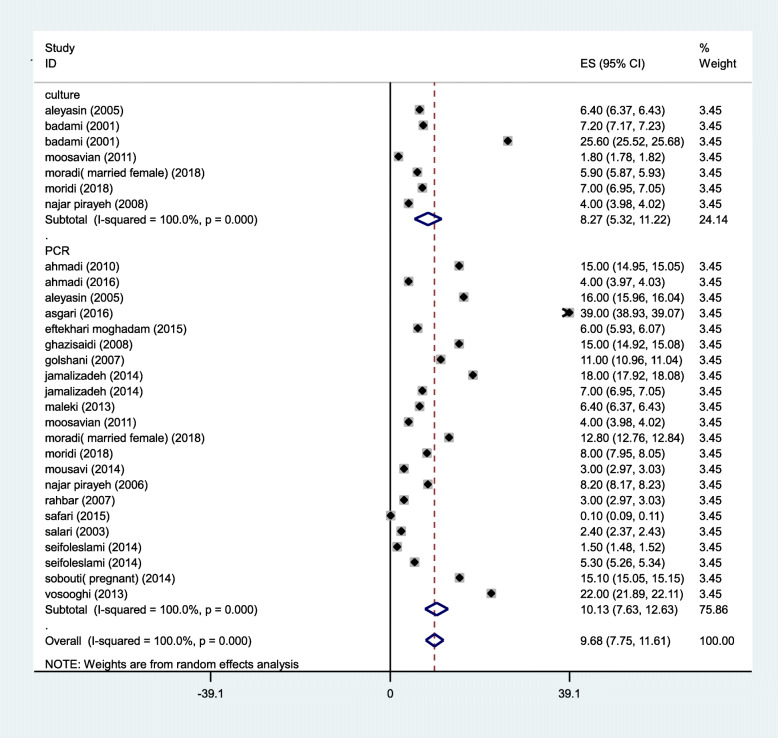
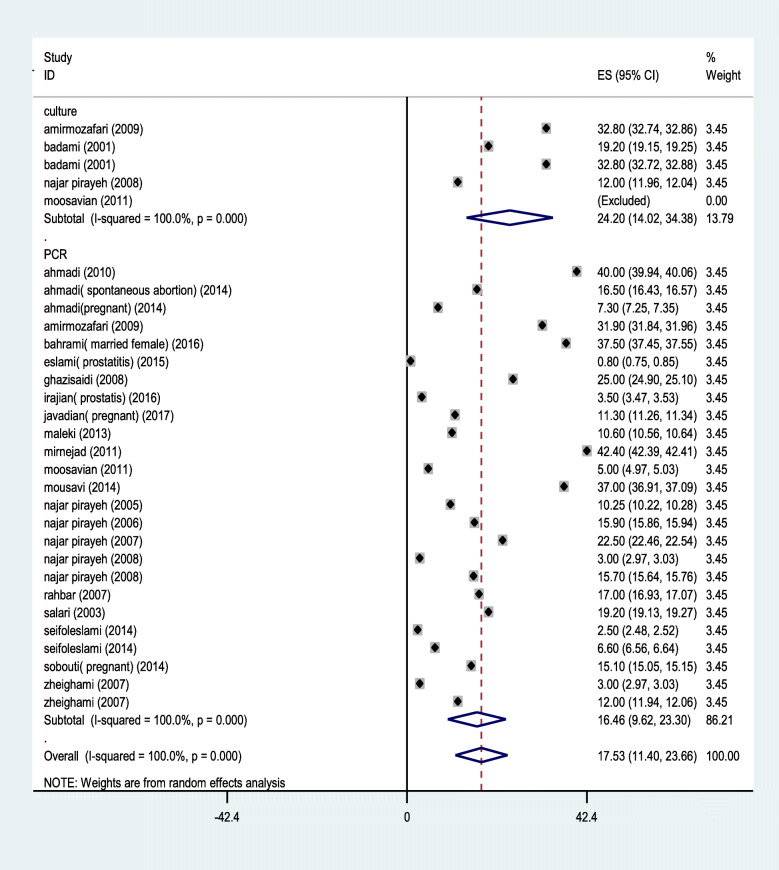
The forty- four selected articles which met our inclusion criteria were analyzed according to the culture and PCR methods. The prevalence rate of M. hominis and M. genitalium base on PCR (10.13%&11.33%) was higher than culture method (8.27%& 0%), whereas that was contrary in U. urealyticum (Figs. 2, 3 and 4).
Fig. 2.
Forest plot of the meta-analysis of the prevalence of M.genitalium in Iran based on analytical method
Fig. 3.
Forest plot of the meta-analysis of the prevalence of M. hominis in Iran based on analytical method
Fig. 4.
Forest plot of the meta-analysis of the prevalence of U. urealyticum in Iran based on analytical method
Discussion
The epidemiology and role of M. hominis, M. genitalium and U. urealyticum in infertility has been less discussed in Iran [14]. The different reports documented in other countries around the world. M. genitalium has been identified as a causative agent of 10–35% nongonococcal-nonchlamydia urethritis [58–62]. According to the community-based prospective cohort study from Oakeshott (2010) M. genitalium is found in 0.7 to 3.3% of women in general populations, while the prevalence in high-risk groups such as sex workers and STD clinic attendees is 7–22% in London [5, 63]. However, M. hominis resides commensally on the mucosal surfaces of the cervix or vagina. It’s colonization values ranges between 20 and 30% around the world [48, 64]. M. hominis was detected in 21–53% of women without genitourinary tract infection and at a lower percentage in the urethra of male [1]. Several studies have proposed that M. hominis is potentially pathogenic and sometimes associated with a variety of disorders including bacterial vaginosis, pyelonephritis, pelvic inflammatory disease, chorioamnionitis, endometritis, preterm birth, low birth, spontaneous abortion, stillbirth, premature birth, postpartum fever, perinatal mortality and infertility overtime [65, 66]. The positive rates of M. hominis, M. genitalium and U. urealyticum are controversial and diverse in the world [67]. Recently, Ghadiri (2019) in Iran (Ahwaz) detected U. urealyticum (28%) and M. hominis (10%) in semen specimens of infertile men by PCR and isolated 22% of U. urealyticum and 2% of M. hominis in the same samples by culture. While, U. urealyticum and M. hominis were detected in 50% & 26% by PCR of endocervical swabs specimens of infertile women and 8% & 4% by culture [68]. Christian Leli (2018) was detected U. urealyticum in 4.7%, M. hominis in 3.4% and M.genitalium in 0% of 232 cervical swab specimens of infertile women by real-time PCR in Italy [69]. Xiaofei Zhu (2016) showed that the prevalence of U. urealyticum and M. hominis were 42.3 and 0.4% among 7374 infertile men by culture [70]. Mahlangu (2019) was determined M. genitalium in 8.9% of urine and 10.6% of endocervical swab specimens which collected from males and females with genital discharge syndrome [71].
Baumann (2017) performed a meta-analysis on prevalence of M. genitalium and found that: the prevalence among women is similar to men and was 1.4% in developed countries and 3.9% in developing countries among general population. He showed that the prevalence among pregnant women were 0.9%, and the prevalence among men who have sex with men in the community was 3.2%, and among female commercial sex workers was 15.9% in the world [72]. Huang performed meta-analysis study (2015) and investigated the association between U. urealyticum, and M. hominis positive rate (5.2% & 14.9%) and risk of male infertility. While the M. genitalium prevalence did not showed any correlation to male infertility [73]. Kasprzykowska (2018) indicated that the prevalence of Ureaplasma spp in women (14.4%) and men (3.9%) is higher than M. hominis in women (0.2%) and men (0.2%) with urogenital tract infection in Poland [74]. Cassell estimated that the U. urealyticum can be found in 40 to 80% of cervicovaginal samples from sexually mature women [74, 75]. Zinzendorf (2008) investigated M. hominis in 23.8% of infertile men in Africa [76]. Taken (2016) could determine M. hominis in 3% of infertile men in Turkey [77]. Abusarah (2013) detected U. urealyticum in 10.8% versus 5.7% and M. genitalium in 3.2% versus 1.4% among infertile and fertile men respectively in Jordan [78]. Jensen indicated M. genitalium in 17% of male patient with urogenital tract infection in Denmark [79]. Al- Sweih (2012) in Kuwait detected M. hominis in 17.1% & 32.4%, M. genitalium in 4.7% & 3.2% and U. urealyticum in 24.4% & 26.1%, among infertile and fertile men respectively [80]. Lee (2013) displayed U.urealyticum in 48% & 25%, M. hominis in 14% & 6.3% of infertile and fertile men, while, U. urealyticum in 40% & 22.9% and M. hominis in 8% & 4.2% of infertile and fertile women in Korea [81]. Andersen reported that the prevalence of infection due to M. genitalium in general population was 2.3% in women and 1.1% in men whereas that was about 19% in men with urethritis and 11% in women with cervicitis in Denmark [82]. Also Grześko indicated M. genitalium from 19.6% of specimens obtained from cervical canal of infertile women, whereas it was 4.4% in control group (women with proven fertility) in Poland [83].
The prevalence rates of Mycoplasma and Ureaplasma are not well established and varies from one study to another. The heterogeneity of prevalence of mycoplasma urinary tract infection in different reports can be probably caused by differences in the geographic areas, the sensitivity of the identification method, the condition of the group (fertile/infertile), other infection accompanied agents, the sample size, and the operator proficiency.
Based on present meta-analysis study the prevalence rates of genital Mycoplasma infection are due to U. urealyticum (17.53%), M. genitalium (11.33%) and M. hominis (9.68%) respectively in Iran which is parallel to Christian Leli (Italy) and Xiaofei (China) results. According to other researcher results, this study shows that the rate of M. genitalium, M. hominis and U. urealyticum infections in women with symptoms of genitourinary tract infection is higher than men with genitourinary tract infection (6.46% Vs 5.4, 7.67% Vs 5.88 and 21.04% Vs 12.13%, respectively). That is in line with kasprzykowska and Mahlangu results. Iranian researches indicated that the prevalence of M. genitalium, U. urealyticum and M. hominis among infertile women (12.73, 19.58 and 10.81%) are higher than fertile women (3%, 10. 85% and 4. 35%), which is similar to Lee (2013) report. However, the prevalence of M. hominis and U. urealyticum in infertile men (14 and 21.18%) is higher than fertile men (4 and 3%), which is like Lee (2013) result.
According to analysis result, the prevalence of M. genitalium, M. hominis and U. urealyticum in Middle provinces is higher than other provinces in Iran. This may be due to the presence of infertility centers and specialized STD clinics in the capital of the country; Tehran. There are some different diagnostic equipment and facilities to research and attract infertile couples for treatment all over Iran.
Conclusions
Based on our meta-analysis, the most common Mycoplasma in Iran, in descending order are: U. urealyticum, M. genitalium, and M. hominis. There is statistically significant relationship between couple infertility and U. urealyticum, M. genitalium and M. hominis infections. There is the higher rate of mycoplasmas infection in women than men and their correlated infertilities.
However, obstetricians should consider mycoplasmas infection as a major agent of infertility. Since mycoplasmas are resistant to common antibiotics and the high prevalence of some mycoplasmas like U. urealyticum, Iranian physicians should be careful in the treatment of genitourinary tract infections to the possible presence of mycoplasmas agent and the sensitive antibiotics.
Further epidemiological and phylogenetic studies in different provinces will be needed to clarify the exact prevalence and distribution pattern of U. urealyticum, M. genitalium and M. hominis in Iran, and proposed routine screening of the pathogens in patients with infertility.
Acknowledgements
We gratefully acknowledge the help provided by Branch Razi Vaccine and Serum Research Institute, Agricultural Research, Education and Extension Organization (AREEO), Mashhad, Iran; and Department of Microbiology and Virology, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
Abbreviations
- M
Mycoplasma
- U
Ureaplasma
- PCR
polymerase chain reaction
Authors’ contributions
MK & HM collected information from database and writing the article. FMH and KAH performed Meta-analysis and interpretation. HM and AA and GK contributed to the design of the study and supervised the research. The author(s) read and approved the final manuscript.
Funding
No funding was obtained for this study.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
No conflicts of interest are declared.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jironkin A, Brown RJ, Underwood A, Chalker VJ, Spiller OB. Genomic determination of minimum multi-locus sequence typing schemas to represent the genomic phylogeny of Mycoplasma hominis. BMC Genomics. 2016;17(1):16. doi: 10.1186/s12864-016-3284-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bébéar CM, Pereyre S. Infections à « Mycoplasma hominis ». EMC - Mal Infect. 2004;1(1):1–6. [Google Scholar]
- 3.Wolf M, Müller T, Dandekar T, Pollack JD. Phylogeny of Firmicutes with special reference to Mycoplasma (Mollicutes) as inferred from phosphoglycerate kinase amino acid sequence data. Int J Syst Evol Microbiol. 2004;54(3):871–875. doi: 10.1099/ijs.0.02868-0. [DOI] [PubMed] [Google Scholar]
- 4.Weisburg WG, Tully JG, Rose DL, Petzel JP, Oyaizu H, Yang D, et al. A phylogenetic analysis of the mycoplasmas: basis for their classification. J Bacteriol. 1989;171(12):6455–6467. doi: 10.1128/jb.171.12.6455-6467.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lokken E. Recent bacterial vaginosis is associated with the acquisition of Mycoplasma genitalium: University of Washington; ProQuest. 2015. [Google Scholar]
- 6.Zhang S, Tsai S, Wu TT, Li B, Shih JW-K, Lo S-C. Mycoplasma fermentans infection promotes immortalization of human peripheral blood mononuclear cells in culture. Blood. 2004;104(13):4252–4259. doi: 10.1182/blood-2004-04-1245. [DOI] [PubMed] [Google Scholar]
- 7.Hayflick L, Chanock RM. Mycoplasma species of man. Bacteriol Rev. 1965;29(2):185–221. doi: 10.1128/br.29.2.185-221.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sweeney EL, Dando SJ, Kallapur SG, Knox CL. The human Ureaplasma species as causative agents of chorioamnionitis. Clin Microbiol Rev. 2017;30(1):349–379. doi: 10.1128/CMR.00091-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zarei O, Rezania S, Mousavi A. MINI-REVIEW Mycoplasma genitalium and Cancer : a brief review. Asian Pac J Cancer Prev. 2013;14:3425–3428. doi: 10.7314/apjcp.2013.14.6.3425. [DOI] [PubMed] [Google Scholar]
- 10.Ahmadi MH, Mirsalehian A, Bahador A. Prevalence of Urogenital Mycoplasmas in iran and their effects on fertility potential. Syst Rev Meta-Anal. 2016;45(4):409–422. [PMC free article] [PubMed] [Google Scholar]
- 11.Moghadam NM, Kheirkhah B, Mirshekari TR, Harandi F, Tafsiri E. Isolation and molecular identification of Mycoplasma genitalium from the secretion of genital tract in infertile male and female. Iran J Reprod Med. 2014;12(9):601–608. [PMC free article] [PubMed] [Google Scholar]
- 12.Haghighi Hasanabad M, Mohammadzadeh M, Bahador A, Fazel N, Rakhshani H, Majnooni A. Prevalence of Chlamydia trachomatis and Mycoplasma genitalium in pregnant women of Sabzevar-Iran. Iran J Microbiol. 2011;3(3):123–128. [PMC free article] [PubMed] [Google Scholar]
- 13.Jensen JS, Cusini M, Gomberg M, Moi H. Background review for the 2016 European guideline on Mycoplasma genitalium infections. J Eur Acad Dermatology Venereol. 2016;30(10):1686–1693. doi: 10.1111/jdv.13850. [DOI] [PubMed] [Google Scholar]
- 14.Moghaddam HE, Kheirkhah B, Amirheidari B. A comparison between the molecular identity of Mycoplasma hominis in urine samples of patients with urinary tract infections and similar strains available in GenBank. J Babol Univ Med Sci. 2015;17(10):67–73. [Google Scholar]
- 15.Manhart LE, Holmes KK, Hughes JP, Houston LS, Totten PA. Mycoplasma genitalium among young adults in the United States: an emerging sexually transmitted infection. Am J Public Health. 2007;97(6):1118–1125. doi: 10.2105/AJPH.2005.074062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stellrecht KA, Woron AM, Mishrik NG, Venezia RA. Comparison of multiplex PCR assay with culture for detection of genital Mycoplasmas. J Clin Microbiol. 2004;42(4):1528–1533. doi: 10.1128/JCM.42.4.1528-1533.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seifoleslami M, Safari A, Khayyat KM. Prevalence of Ureaplasma urealyticum and Mycoplasma hominis in high vaginal swab samples of infertile females. Iran Red Crescent Med J. 2015;17(12):0–4. doi: 10.5812/ircmj.16823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sh NP, Sattari M. Detection of Ureaplasma urealyticum and Mycoplasma hominis in endocervical specimens from infertile women by polymerase chain reaction. Middle East Fertil Soc J. 2006;11(2):104–108. [Google Scholar]
- 19.Zeighami H, Sh NP, Safarlu M. Detection of Ureaplasma urealyticum in semen of infertile men by PCR. Pak J Biol Sci. 2007;10(21):3960–3963. doi: 10.3923/pjbs.2007.3960.3963. [DOI] [PubMed] [Google Scholar]
- 20.Badami N. Rate of Chlamydia trachomatis, Mycoplasma hominis and Ureaplasma urealyticum in infertile females and control group. Iran J Publ Heal. 2001;30(1–2):57–60. [Google Scholar]
- 21.Salari MH. Prevalence of Ureaplasma urealyticum and Mycoplasma genitalium in men with non-gonococcal urethritis. East Mediterr Heal J. 2003;9(3):291–295. [PubMed] [Google Scholar]
- 22.Ale YA. Comparison of PCR with culture for detection of Mycoplasma hominis in infertile women. Kowsar Med J. 2005;10(3):183–190. [Google Scholar]
- 23.Peerayeh SN, Mirdamadi R. Comparison of culture with polymerase chain reaction for detection of Ureaplasma urealyticum in endocervical specimens. Med J Islam Repub Iran. 2005;19(2):175–179. [Google Scholar]
- 24.Golshani M, Eslami G, Mohhammadzadeh Ghobadloo S, Fallah F, Goudarzi H, Soleimani Rahbar AA, et al. Detection of Chlamydia trachomatis, mycoplasma hominis and Ureaplasma urealyticum by multiplex PCR in semen sample of infertile men. Iran J Public Health. 2007;36(2):50–57. [Google Scholar]
- 25.Soleimani Rahbar A, Golshani M, Fayyaz F, Rafiee Tabatabaei SMA. Detection of Mycoplasma DNA from the sperm specimens of infertile men by PCR. Iran J Med Microbiol. 2007;1(1):47–53. [Google Scholar]
- 26.Najar Peerayeh S. Samimi R. Detection of Ureaplasma urealyticum in clinical samples from infertile women by polymerase chain reaction. Iran J Pharmacol Ther. 2007;6(1):23–26. [Google Scholar]
- 27.Peerayeh SN, Samimi R. Comparison of culture with the polymerase chain reaction for detection of genital mycoplasma. Eur J Gen Med. 2008;5(2):107–111. [Google Scholar]
- 28.Ghazisaidi K, Fateminasab F. Prostatic massage method versus first -void urine samples to isolate Mycoplasma hominis and Ureaplasma urealyticum from urinary tract infection. Spring Summer. 2008;2(1):69. [Google Scholar]
- 29.Sh NP, Yazdi RS, Zeighami H. Association of Ureaplasma urealyticum infection with Varicocele-related infertility. J Infect Dev Ctries. 2008;2(2):116–119. [PubMed] [Google Scholar]
- 30.Amirmozafari N, Mirnejad R, Kazemi B, Sariri E. Comparison of polymerase chain reaction and culture for detection of genital mycoplasma in clinical samples from patients with genital infections. Saudi Med J. 2009;30(11):1401–1405. [PubMed] [Google Scholar]
- 31.Ahmadi MH, Amirmozafari N, Kazemi B, Gilani MAS, Jazi FM. Use of PCR to detect Mycoplasma hominis and Ureaplasma urealyticum from semen samples of infertile men who referred to royan institute in 2009. Yakhteh Med J. 2010;12(3):371–380. [Google Scholar]
- 32.Mirnejad R, Amirmozafari N, Kazemi B. Simultaneous and rapid differential diagnosis of Mycoplasma genitalium and Ureaplasma urealyticum based on a polymerase chain reaction-restriction fragment length polymorphism. Indian J Med Microbiol. 2011;29:33–36. doi: 10.4103/0255-0857.76521. [DOI] [PubMed] [Google Scholar]
- 33.Hasanabad MH, Mohammadzadeh M, Bahador A, Fazel N, Rakhshani H, Majnooni A. Prevalence of Chlamydia trachomatis and Mycoplasma genitalium in pregnant women of Sabzevar-Iran. Iran J Microbiol. 2011;3(3):123–128. [PMC free article] [PubMed] [Google Scholar]
- 34.Moosavian SM, Motamedi H, Maleki S, Shahbazian N. Comparison between prevalence of Mycoplasma Hominis and Ureaplasma urealyticum in women with urogenital infections by multiplex PCR and culture methods. Med J Tab Univ Med Sci. 2011;33(5):33. [Google Scholar]
- 35.Vosooghi S, Karimi B, Kheirkhah B, Mirshekari T. Molecular detection of Mycoplasma hominis from genital secretions of infertile men referred to the Kerman infertility center. J Microb World. 2013;6(1):14–22. [Google Scholar]
- 36.Irajian GR, Mirnejad R, Jalili Nidishan MR. Determing the prevalence rate of Mycoplasma genitalium in patients with prostatitis by PCR-RFLP technique. J Ardabil Univ Med Sci. 2013;13(1):86–92. [Google Scholar]
- 37.Maleki S, Motamedi H, Moosavian SM, Shahbaziyan N. Frequency of Mycoplasma hominis and Ureaplasma urealyticum in females with urogenital infections and habitual abortion history in Ahvaz, Iran; using multiplex PCR. Jundishapur J Microbiol. 2013;6(6):e10088. [Google Scholar]
- 38.Yeganeh O, Jeddi-Tehrani M, Yaghmaie F, Kamali K, Heidari-Vala H, Zeraati H, et al. A survey on the prevalence of Chlamydia trachomatis and Mycoplasma genitalium infections in symptomatic and asymptomatic men referring to urology clinic of labbafinejad hospital, Tehran, Iran. Iran Red Crescent Med J. 2013;15(4):340–344. doi: 10.5812/ircmj.8600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sadrpour P, Bahador A, Abbas S, Bagheri R, Chamani-Tabriz L. Detection of Chlamydia trachomatis and Mycoplasma genitaliumin semen samples of infertile men using multiplex PC. Tehran Univ Med J. 2013;70(10):623–629. [Google Scholar]
- 40.Mohseni R, Sadeghi F, Mirinargesi M. A study on the frequency of vaginal species of Mycoplasma genitalium, Gardnerella vaginalisand Neisseria gonorrhoeaeamong pregnant women by PCR technique. Int J Mol Clin Microbiol. 2013;1(3):231–236. [Google Scholar]
- 41.Ahmadi A, Khodabandehloo M, Ramazanzadeh R, Farhadifar F, Nikkhoo B, Soofizade N, et al. Association between Ureaplasma urealyticum endocervical infection and spontaneous abortion. Iran J Microbiol. 2014;6(6):392–397. [PMC free article] [PubMed] [Google Scholar]
- 42.Mousavi A, Farhadifar F, Mirnejad R, Ramazanzadeh R. Detection of genital mycoplasma infections among infertile females by multiplex PCR. Iran J Microbiol. 2014;6(6):398–403. [PMC free article] [PubMed] [Google Scholar]
- 43.Jamalizadeh Bahaabadi S, Mohseni Moghadam N, Kheirkhah B, Farsinejad A, Habibzadeh V. Isolation and molecular identification of Mycoplasma hominis in infertile female and male reproductive system. Nephrourol Mon. 2014;6(6):e22390. doi: 10.5812/numonthly.22390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sobouti B, Fallah S, Mobayen M, Noorbakhsh S, Ghavami Y. Colonization of Mycoplasma hominis and Ureaplasma urealyticum in pregnant women and their transmission to offspring. Iran J Microbiol. 2014;6(4):219–224. [PMC free article] [PubMed] [Google Scholar]
- 45.Dadashi M, Eslami G, Ghalavand Z, Goudarzi H, Fallah F, Owlia P. Prevalence of Chlamydia trachomatis and Mycoplasma genitalium in patients with Benign and malignant ovarian cancer by nested PCR method. Novel Biomed. 2016;4(1):18–23. [Google Scholar]
- 46.Eslami G, Goudarzi H, Baseri N, Ghalavand Z, Taherpour A, Zhaam H. The prevalence of Ureaplasma urealyticum and Mycoplasma genitalium in patients with prostate cancer in Shohada Hospital in Tehran, Iran. Novel Biomed. 2015;3(2):73–78. [Google Scholar]
- 47.Safavifar F, Bandehpour M, Hosseiny SJ, Khorramizadeh MR. Mycoplasma infection in Pyospermic infertile and healthy fertile men. Nov Biomed. 2015;3(1):25–29. [Google Scholar]
- 48.Safari M, Bakhshi A, Erami M, Kheirkhah B, Pourbakhsh A, Pourbabei H. Sequences of Mycoplasma hominis in patients with urinary tract infection in a Hospital in Kashan, Iran. Res J Microbiol. 2015;10(6):260–269. [Google Scholar]
- 49.Ramazanzadeh R, Khodabandehloo M, Farhadifar F, Rouhi S, Ahmadi A, Menbari S, et al. A case–control study on the relationship between Mycoplasma genitalium infection in women with Normal pregnancy and spontaneous abortion using polymerase chain reaction. Osong Public Heal Res Perspect. 2016;7(5):334–338. doi: 10.1016/j.phrp.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahmadi MH, Mirsalehian A, Sadighi Gilani MA, Bahador A, Talebi M. Asymptomatic infection with Mycoplasma hominis negatively affects semen parameters and leads to male infertility as confirmed by improved semen parameters after antibiotic treatment. Urology. 2017;100:97–102. doi: 10.1016/j.urology.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 51.Bahrami H, Farivar TN, Aslanimehr M, Peymani A, Dabaghi T, Ghaleh T, et al. Prevalence of Ureaplasma urealyticum in Endocervical Specimens of Female Patients in Qazvin, Iran. BiotechHealth Sci. 2016;3(4):e39599. [Google Scholar]
- 52.Asgari A, Nazari R, Mohammad S, Razavian H. Investigation of frequency of Mycoplasma hominis and biological parameters in semen sample of men referred to Qom Jahad Daneshgahi infertility treatment center in 2016. Qom Univ Med Sci J. 2018;12(4):81–88. [Google Scholar]
- 53.Irajian G, Sharifi M, Mirkalantari S, Mirnejad R, Jalali Nadoushan MR. Molecular detection of Ureaplasma urealyticum from prostate tissues using PCR-RFLP, Tehran, Iran. Iran J Pathol. 2016;11(2):138–143. [PMC free article] [PubMed] [Google Scholar]
- 54.Sameni F, Zadehmodarres S, Dabiri H. Prevalence of Chlamydia Trachomatis , Mycoplasma genitalium and Neisseria gonorrhea in infertile females referred to Mahdieh hospital in Tehran. Iran J Med Microbiol. 2017;11(5):90–97. [Google Scholar]
- 55.Javadinia S, Movahedi Z, Shokrollahi MR, Naghdalipour M, Tabatabaee A, Asgarian R, et al. Prevalence of Mycoplasma genitalium and Ureaplasma urealyticum in pregnant women of Tehran by duplex PCR. Curr Pediatr Res. 2017;21(4):680–685. [Google Scholar]
- 56.Golkhatmi Mokari A, Farsiani H, Goshayeshi L, Radmanesh H, Jamehdar AS. Development of PCR-ELISA for specific and sensitive detection of Mycoplasma genitalium. Clin Microbiol. 2017;6(1):1. [Google Scholar]
- 57.Moradi F, Yousefi MR. Comparison of PCR and culture methods to determine the prevalence of Mycoplasma hominis in woman’s endocervical samples referred to Infertility Center of Hamadan Fatemieh Hospital in 2016. IJOGI. 2018;20(11):83–92. [Google Scholar]
- 58.Cabral SLI. Antigenic diversity and persistence of infection within a genomically challenged pathogen. ProQuest. 2008. Genetic variation in Mycoplasma genitalium. [Google Scholar]
- 59.Cazanave C, Charron A, Renaudin H, Bébéar C. Method comparison for molecular typing of French and Tunisian Mycoplasma genitalium-positive specimens. J Med Microbiol. 2012;61(4):500–506. doi: 10.1099/jmm.0.037721-0. [DOI] [PubMed] [Google Scholar]
- 60.Jensen JS, Cusini M, Gomberg M, Moi H. 2016 European guideline on Mycoplasma genitalium infections. J Eur Acad Dermatol Venereol. 2016;30(10):1650–1656. doi: 10.1111/jdv.13849. [DOI] [PubMed] [Google Scholar]
- 61.Horner PJ, Blee K, Falk L, van der Meijden W, Moi H. European guideline on the management of non-gonococcal urethritis. Int J STD AIDS. 2016;27(11):928. doi: 10.1177/0956462416648585. [DOI] [PubMed] [Google Scholar]
- 62.Munoz JL, Goje OJ. Mycoplasma genitalium: an emerging sexually transmitted infection. Scientifica (Cairo). 2012;2016(December):942–955. doi: 10.1155/2016/7537318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oakeshott P, Aghaizu A, Hay P, Reid F, Kerry S, Atherton H, et al. Is Mycoplasma genitalium in women the “new chlamydia?” a community-based prospective cohort study. Clin Infect Dis. 2010;51(10):1160–1166. doi: 10.1086/656739. [DOI] [PubMed] [Google Scholar]
- 64.Lee MY, Kim MH, Lee WI, Kang SY, La JY. Prevalence and antibiotic susceptibility of Mycoplasma hominis and Ureaplasma urealyticum in pregnant women. Int J Infect Dis. 2010;57(14):e90–e95. doi: 10.1016/j.ijid.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 65.Taylor-Robinson D. Infections due to species of Mycoplasma and Ureaplasma: an update. Clin Infect Dis. 1996;23(4):671–684. doi: 10.1093/clinids/23.4.671. [DOI] [PubMed] [Google Scholar]
- 66.Embree JE, Embil JA. Mycoplasmas in diseases of humans. Can Med Assoc J. 1980;123(2):105–111. [PMC free article] [PubMed] [Google Scholar]
- 67.Ghorbanalinezhad E, Amirmozafari N, Khavari-nejad R, Sepahi AA. International journal of molecular and clinical microbiology survey on the genital mycoplasmosis by multiplex PCR. 2014. pp. 451–456. [Google Scholar]
- 68.Ghadiri A, Ahmadi K, Rashno M, Moosavian M, Afzali M, Amirzadeh S. Investigating Chlamydia trachomatis and genital Mycoplasma prevalence and apoptosis markers in infertile and fertile couples. Jundishapur J Microbiol. 2019;12(1):1–7. [Google Scholar]
- 69.Leli C, Mencacci A, Latino MA, Clerici P, Rassu M, Perito S, et al. Prevalence of cervical colonization by Ureaplasma parvum, Ureaplasma urealyticum, Mycoplasma hominis and Mycoplasma genitalium in childbearing age women by a commercially available multiplex real-time PCR: an Italian observational multicentre study. J Microbiol Immunol Infect. 2018;51(2):220–225. doi: 10.1016/j.jmii.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 70.Zhu X, Li M, Cao H, Yang X, Zhang C. Epidemiology of Ureaplasma urealyticum and Mycoplasma hominis in the semen of male outpatients with reproductive disorders. Exp Ther Med. 2016;12(2):1165–1170. doi: 10.3892/etm.2016.3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mahlangu MP, Müller EE, Venter JME, Maseko DV, Kularatne RS. The prevalence of Mycoplasma genitalium and association with human immunodeficiency virus infection in symptomatic patients, Johannesburg, South Africa, 2007–2014. Sex Transm Dis. 2019;46(6):395–399. doi: 10.1097/OLQ.0000000000000984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baumann L, Cina M, Egli-Gany D, Goutaki M, Halbeisen FS, Lohrer GR, et al. Prevalence of Mycoplasma genitalium in different population groups: systematic review andmeta-analysis. Sex Transm Infect. 2018;94(4):255–262. doi: 10.1136/sextrans-2017-053384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang C, Zhu HL, Xu KR, Wang SY, Fan LQ, Zhu WB. Mycoplasma and ureaplasma infection and male infertility: a systematic review and meta-analysis. Andrology. 2015;3(5):809–816. doi: 10.1111/andr.12078. [DOI] [PubMed] [Google Scholar]
- 74.Kasprzykowska U, Sobieszczańska B, Duda-Madej A, Secewicz A, Nowicka J, Gościniak G. A twelve–year retrospective analysis of prevalence and antimicrobial susceptibility patterns of Ureaplasma spp. and Mycoplasma hominis in the province of lower Silesia in Poland. Eur J Obstet Gynecol Reprod Biol. 2018;220:44–49. doi: 10.1016/j.ejogrb.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 75.Cassell GH, Waites KB, Watson HL, Crouse DT, Harasawa R. Ureaplasma urealyticum intrauterine infection: role in prematurity and disease in newborns. Clin Microbiol Rev. 1993;6(1):69–87. doi: 10.1128/cmr.6.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zinzendorf NY, Kouassi-Agbessi BT, Lathro JS, Don C, Kouadio L, Loukou YG. Ureaplasma urealyticum or Mycoplasma hominis infections and semen quality of infertile men in Abidjan. J Reprod Contracept. 2008;19(2):65–72. [Google Scholar]
- 77.Taken K. Prevalence of Ureaplasma and Mycoplasma in infertile men in Van region and effects to semen parameters. J Clin Anal Med [Internet]. 2016;7(3):4–7. [Google Scholar]
- 78.Abusarah EA, Awwad ZM, Charvalos E, Shehabi AA. Molecular detection of potential sexually transmitted pathogens in semen and urine specimens of infertile and fertile males. Diagn Microbiol Infect Dis. 2013;77(4):283–286. doi: 10.1016/j.diagmicrobio.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 79.Jensen JS, Hansen HT, Seruminstitut S. S D-C. isolation of Mycoplasma genitalium strains from the male urethra. Microbiology. 1996;34(2):286–291. doi: 10.1128/jcm.34.2.286-291.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Al-Sweih NA, Al-Fadli AH, Omu AE, Rotimi VO. Prevalence of Chlamydia trachomatis, Mycoplasma hominis, Mycoplasma genitalium, and Ureaplasma urealyticum infections and seminal quality in infertile and fertile men in Kuwait. J Androl. 2012;33:1323–1329. doi: 10.2164/jandrol.111.013821. [DOI] [PubMed] [Google Scholar]
- 81.Lee JS, Kim KT, Lee HS, Yang KM, Seo JT, Choe JH. Concordance of Ureaplasma urealyticum and Mycoplasma hominis in infertile couples: impact on semen parameters. Urology. 2013;81(6):1219–1224. doi: 10.1016/j.urology.2013.02.044. [DOI] [PubMed] [Google Scholar]
- 82.Andersen B, Sokolowski I, Østergaard L, Kjølseth Møller J, Olesen F, Jensen JS. Mycoplasma genitalium: prevalence and behavioural risk factors in the general population. Sex Transm Infect. 2007;83(3):237–241. doi: 10.1136/sti.2006.022970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grześko J, Elias M, Maczyńska B, Kasprzykowska U, Tłaczała M, Goluda M. Occurrence of Mycoplasma genitalium in fertile and infertile women. Fertil Steril. 2009;91(6):2376–2380. doi: 10.1016/j.fertnstert.2008.03.060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.