Key Points
Male sex is an independent risk factor for presentation and outcomes in MPNs.
Men have a higher CD34+ cell mutational burden and higher risk of non–MPN-specific mutational burden compared with women.
Abstract
The factors underlying the variable presentation and clinical course of myeloproliferative neoplasms (MPNs) remain unclear. The aim of this study was to evaluate the independent effect of sex on MPN presentation and outcomes. A total of 815 patients with essential thrombocytosis, polycythemia vera, or primary myelofibrosis were evaluated between 2005 and 2019, and the association of sex with presenting phenotype, JAK2 V617F burden, progression, and survival was examined. Men presented more often with primary myelofibrosis vs essential thrombocytosis (relative risk, 3.2; P < .001) and polycythemia vera (relative risk, 2.1; P < .001), had higher rates of transformation to secondary myelofibrosis (hazard ratio [HR], 1.55; P = .013) and acute myeloid leukemia (HR, 3.67; P < .001), and worse survival (HR, 1.63; P = .001) independent of age, phenotype at diagnosis, and MPN-specific mutation. Men had higher JAK2 V617F allele burdens in their CD34+ cells (P = .001), acquired more somatic mutations (P = .012) apart from the MPN-specific mutations, and had an increased frequency of 1 (odds ratio, 2.35; P = .017) and 2 (odds ratio, 20.20; P = .011) high-risk mutations independent of age, phenotype, and driver mutation. Male sex is an independent predictor of poor outcomes in MPNs. This seems to be due to an increased risk of non–MPN-specific somatic mutations, particularly high-risk mutations, rather than MPN-specific mutation allele frequency. Conversely, disease progression in female subjects is more dependent on JAK2 mutation allele burden than on acquisition of other somatic mutations. Sex should be considered in prognostic models and when evaluating therapeutic strategies in MPNs.
Visual Abstract
Introduction
Chronic myeloproliferative neoplasms (MPNs), including essential thrombocytosis (ET), polycythemia vera (PV), and primary myelofibrosis (PMF), share driver mutations in the JAK2, MPL, and CALR genes but vary significantly in terms of natural history and outcomes.1 JAK2 V617F variant allele frequency (VAF) and the acquisition of additional non–MPN-specific somatic mutations influence MPN phenotype and outcomes2,3; however, these do not fully explain the variability in MPN presentation and progression.
Sex influences MPN presentation, symptom burden, and natural history. Women have a higher prevalence of MPNs, although women predominate in ET, and men predominate in PMF.4-10 Paradoxically, men with an MPN have milder symptoms4 but significantly worse clinical outcomes.5 Based on this, male sex was included in a recent prognostic tool based on a retrospective analysis of >2000 individuals with MPNs.3 Despite significant implications on risk stratification and therapeutic strategies, the independent impact of sex on MPN phenotype, complications, and survival, as well as the biologic mechanisms underlying sex differences, remain poorly understood.
Here, we prospectively followed up a patient cohort of 815 patients with MPNs to test the hypothesis that sex is an independent factor in MPN presentation and outcomes. We found that male sex independently associated with higher rates of progression to PMF, acute myeloid leukemia (AML), second cancer, and mortality. Poor outcomes in men were associated with higher rates of non–MPN-specific somatic mutations and higher proportions of high-risk mutations.
Design and methods
Patients, data collection, and study outcomes
We prospectively enrolled 815 individuals with MPN (ET, PV, and myelofibrosis, including PMF and post-ET/post-PV myelofibrosis [secondary myelofibrosis [sMF]) referred to the Johns Hopkins Center for chronic MPNs between May 2005 and September 2019. The diagnoses of PV and ET were based on the Polycythemia Vera Study Group criteria,6,7 and the diagnosis of PMF was based initially on the Italian Consensus criteria,8,9 and more recently on the 2016 World Health Organization criteria.10 Clinical and laboratory data were recorded and blood samples were collected for neutrophil and CD34+ cell isolation. Follow-up data and samples were collected at subsequent clinical encounters whenever possible. Patients were followed up from enrollment until death or last clinical contact. Outcomes of interest were overall survival, transformation to sMF (post-ET and post-PV), thrombosis, development of second cancer, and transformation to AML. Informed consent was obtained from all patients, and the study was approved by the Johns Hopkins University Institutional Review Board.
Mutation analysis
JAK2 V617F VAF was measured in peripheral blood neutrophils by using an allele-specific, quantitative real-time polymerase chain reaction assay sensitive to 5% of the wild-type or mutant JAK2 V617F allele. As previously described,11 intra-assay replicates did not vary by more than 5%, and the measurement was performed in all cohort patients; 226 had serial measurements. The peripheral blood CD34+ cell JAK2 V617F VAF was analyzed in 121 JAK2 V617F–positive individuals. JAK2 V617F–negative individuals underwent genotyping for other JAK2 mutations, CALR exon 9, and MPL 515K/L mutations using Sanger sequencing or next-generation sequencing (NGS). Karyotyping was performed in 389 patients. NGS to identify non–MPN-specific somatic mutations was performed in 227 patients by using an established panel of 63 genes at the Johns Hopkins Molecular Pathology Laboratory (supplemental Table 1).12
Statistical analysis
Data were summarized by using counts (and proportions) and means (and standard deviations) for categorical and continuous variables, respectively. The Fisher's exact test was used to compare the presenting phenotype between men and women in the entire cohort and then stratified according to age and initial driver mutation. Because the presenting phenotype had 3 possibilities, multinomial logistic regression was also used to evaluate associations of sex, age, and driver mutations with presenting phenotype (with ET as the reference category). Given that the JAK2 V617F VAF in neutrophil and CD34+ cells was available in a subgroup of patients, the associations including those variables were evaluated by performing subgroup analyses. Covariates of interest were selected a priori based on biological plausibility.
Separate Kaplan-Meier analyses were used to compare overall survival and progression-free survival between male and female subjects. We evaluated the independent effect of sex on these outcomes using Cox regression models adjusted for age, initial MPN-specific mutation, presenting phenotype, and treatment with hydroxyurea. The specific causes of death in both sexes were analyzed with the Nelson-Aalen cumulative hazard estimator. Multivariable logistic regression was used to evaluate the association of age and other clinical and genetic risk factors with venous thromboembolism (VTE), and Cox regression analysis was used to evaluate the association of age and other clinical and genetic risk factors with the progression to sMF and AML. We evaluated the association of sex with JAK2 V617F VAF in neutrophils and CD34+ cells using linear regression models adjusted for age and disease phenotype at the time of the measurement. The association between sex and number of non–MPN-specific somatic mutations was evaluated in a linear regression model adjusted for age and phenotype. All analyses were performed by using STATA version 13.1 software. P < .05 was considered significant. The number of patients included in the different analyses is shown in supplemental Figure 1.
Results
Clinical, molecular, and cytogenetic features at MPN diagnosis
We investigated a cohort comprising 815 individuals (469 women and 346 men) with MPNs (Table 1). The median duration from initial diagnosis until enrollment was 5 ± 8 years, and median follow-up was 8 ± 8.7 years. Mean age at diagnosis was 51.28 ± 16.26 years, and men were older at presentation (53.55 ± 15.58 years vs 49.6 ± 16.5 years; P = .001). Women predominated in the entire cohort and was most pronounced in African-American subjects compared with white subjects (female/male ratio, 2.4 vs 1.27, respectively; P = .01). Treatment exposures varied as more men than women received hydroxyurea (41.3% vs 32%; P = .006) and ruxolitinib (19.4% vs 11.5%; P = .020) and more female subjects than male subjects received anagrelide (11.3% vs 6.1%; P = .013) and interferon (11.9% vs 7.5%; P = .045). Finally, more men underwent allogeneic stem cell transplantation (7.5% vs 2.8%; P = .002).
Table 1.
Cohort characteristics
| Variable | All patients (N = 815) | Female patients (n = 469) | Male patients (n = 346) | P |
|---|---|---|---|---|
| Age at diagnosis, mean ± SD, y | 51.28 ± 16.26 | 49.6 ± 16.56 | 53.55 ± 15.58 | .001 |
| Race | ||||
| White | 689 (84.5) | 386 (82.3) | 303 (87.6) | .040 |
| African American | 85 (10.4) | 60 (12.8) | 25 (7.2) | .010 |
| Hispanic | 5 (0.6) | 3 (0.6) | 2 (0.6) | 1.000 |
| Asian | 36 (4.4) | 20 (4.3) | 16 (4.6) | .864 |
| Phenotype at diagnosis | ||||
| ET | 381 (46.7) | 252 (53.7) | 129 (37.3) | <.001 |
| PV | 289 (35.5) | 163 (34.8) | 126 (36.4) | .656 |
| PMF | 145 (17.8) | 54 (11.5) | 91 (26.3) | <.001 |
| MPN-specific mutation | ||||
| JAK2 + | 606 (74.4) | 343 (73.1) | 263 (76) | .372 |
| JAK2 – | 209 (25.6) | 126 (26.9) | 83 (24) | .372 |
| CALR + | 129 (15.8) | 68 (14.5) | 61 (17.6) | .442 |
| MPL + | 21 (2.6) | 15 (3.2) | 6 (1.7) | .179 |
| Triple-negative | 45 (5.5) | 35 (7.5) | 10 (2.9) | .005 |
| JAK2– others not done | 14 (1.7) | 8 (1.7) | 6 (1.7) | .957 |
| Karyotype (n = 389) | ||||
| Normal | 226 (58.1) | 125 (62.2) | 101 (53.7) | .100 |
| 1 lesion | 81 (20.8) | 36 (17.9) | 45 (23.9) | .169 |
| ≥2 lesions | 82 (21.1) | 40 (19.9) | 42 (22.3) | .619 |
| Treatments | ||||
| None | 388 (47.6) | 230 (49) | 158 (45.7) | .356 |
| Hydroxyurea | 293 (36) | 150 (32) | 143 (41.3) | .006 |
| Anagrelide | 74 (9.1) | 53 (11.3) | 21 (6.1) | .013 |
| Interferon | 82 (10.1) | 56 (11.9) | 26 (7.5) | .045 |
| Ruxolitinib | 122 (15) | 54 (11.5) | 67 (19.4) | .002 |
| Allogeneic stem cell transplantation | 39 (4.8) | 13 (2.8) | 26 (7.5) | .002 |
| Enrolled within 1 y from diagnosis | 229 (28) | 116 (24.7) | 113 (32.7) | .015 |
Data are expressed as n (%) unless otherwise indicated.
More men presented with PMF independent of age and MPN-specific driver mutation
A presenting phenotype of PMF was more common in men (26.3% vs 11.2%; P < .001), whereas a presenting phenotype of ET was more common in women (53.7% vs 37.3%; P < .001) (Figure 1A), even when stratified according to MPN-specific mutation and by age at presentation (supplemental Figure 2A). Among patients positive for the JAK2 V617F mutation, women were more likely to present with ET (44.9% vs 28.1%; P < .001), whereas men were more likely to present with PMF (8.2% vs 24%; P < .001). In the JAK2 V617F–negative group (n = 209), men were also more likely than women to present with PMF (33.7% vs 20.6%; P = .042). In the CALR-mutated subgroup (n = 129), the overall proportion of PMF at presentation was relatively similar between men and women (22.1% vs 27.9%). Among patients with an MPL mutation (n = 21) or triple-negative patients (n = 28), more men presented with PMF (50% vs 13.3% [P = .290] and 50% vs 25.7% [P = .243]), but these differences did not reach statistical significance.
Figure 1.
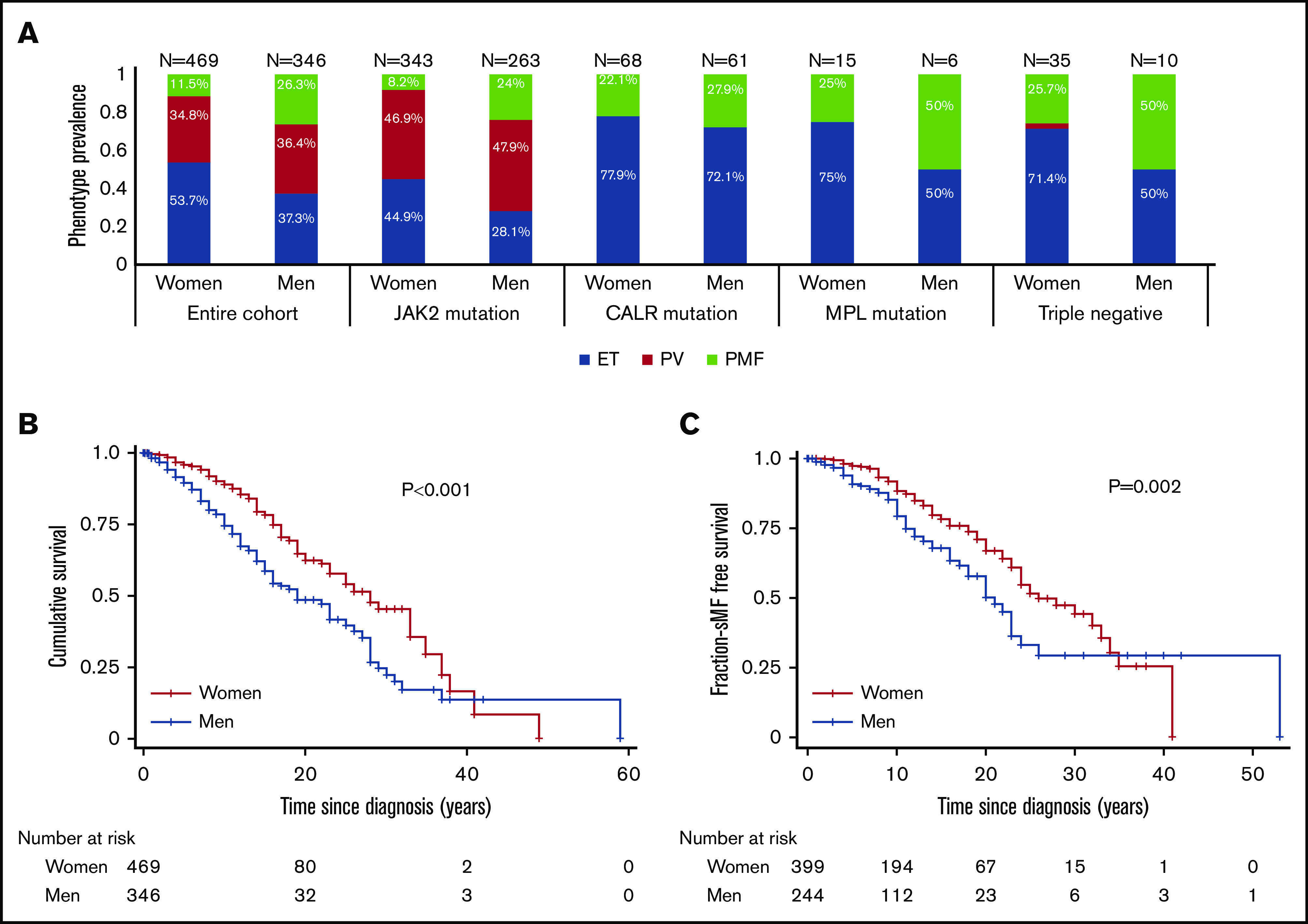
Men have a higher prevalence of PMF and a lower prevalence of ET at presentation, worse survival, and shorter time to MF transformation. (A) Men were more likely to present with PMF compared with women (P < .001), and women were more likely to present with ET (P < .001) in the entire cohort; male subjects with a JAK2 mutation have a higher prevalence of PMF (P < .001) and a lower prevalence of ET (P = .001) at presentation. Similarly, JAK2-negative men have a higher prevalence of PMF (P = .04) at presentation. The prevalence of PMF was similar among women and men only in the CALR mutation subgroup. The frequency of PMF in men in the MPL and triple-negative subgroups was higher compared with women (50% vs 13.3% [P = .290] and 50% vs 25.7% [P = .243], respectively), but the differences did not reach significance. (B) Kaplan-Meier curves showing that the survival of men from the whole cohort was significantly worse compared with women (P < .001). (C) Kaplan-Meier analysis showing that men had shorter sMF-free survival (P = .002).
Male sex was associated with an increased risk of presenting with PMF compared with ET or PMF compared with PV, independent of age at presentation and MPN-specific mutation (Table 2). Moreover, male sex was associated with increased risk of presenting with PV compared with ET independent of age at presentation. In a subgroup analysis of 389 patients with available karyotyping data, male sex was associated with PMF as opposed to ET (relative risk [RR], 2.92; 95% CI, 1.57-5.42; P = .001) independent of karyotype abnormality and age (supplemental Table 2).
Table 2.
Multinomial logistic regression analysis of association of sex, age at diagnosis, and MPN-specific mutation with presenting phenotype ET vs PMF
| Variable | ET vs PMF | ||
|---|---|---|---|
| RR | 95% CI | P | |
| Male sex | 3.2 | 2.12-4.93 | <.001 |
| Age at diagnosis, y | 1.05 | 1.04-1.07 | <.001 |
| MPN-specific mutation | |||
| JAK2 + | Ref | ||
| CALR + | 1.05 | 0.63-1.76 | .855 |
| MPL + | 0.79 | 0.26-2.43 | .686 |
| Triple-negative | 2.16 | 0.99-4.76 | .051 |
| JAK2– others not done | 1.09 | 0.27-4.39 | .760 |
Male sex was an independent risk factor for decreased survival and disease progression
Male sex was an independent predictor of worse survival (hazard ratio [HR], 1.63; 95% CI, 1.22-2.17; P = .001) after adjusting for age and phenotype at diagnosis, MPN-specific mutation, and hydroxyurea treatment (supplemental Table 3). Kaplan-Meier analysis showed that male sex was associated with worse survival in the entire cohort as well as the JAK2 V617F–positive and JAK2 V617F–negative subgroups (Figure 1B; supplemental Figure 2B-C). Nelson-Aalen cumulative hazard curves revealed that men had higher mortality related to MPN progression (P = .002), other cancers (P = .004), and aging or death due to unknown cause (P = .002) (supplemental Figure 3). Male sex was independently associated with a higher risk of progression to sMF (HR, 1.55; 95% CI, 1.1-2.18; P = .013) after adjusting for age, phenotype at diagnosis, and MPN-specific mutation. Kaplan-Meier analysis confirmed that men have a higher risk of sMF transformation (Figure 1C). Similarly, male sex was associated with a higher risk of transformation to AML (HR, 3.67; 95% CI, 1.95-6.99; P < .001) independent of age and phenotype at diagnosis and MPN-specific mutation. Kaplan-Meier analysis confirmed that men from the entire cohort and the ET and PV subgroups had a higher risk of AML transformation (supplemental Figure 4).
Male subjects had a higher CD34+ cell JAK2 V617F VAF and disease progression was less dependent on the neutrophil JAK2 V617F VAF
MPN-specific driver mutation allele burden is known to correlate with disease phenotype.13 We tested the hypothesis that JAK2 V617F VAF was associated with sex, potentially explaining the poorer clinical outcomes in male subjects. Thus, we analyzed the neutrophil JAK2 V617F VAF in 524 individuals and the peripheral blood CD34+ JAK2 V617F VAF in 121 individuals. Of note, this 121-patient subgroup included fewer patients with ET (P = .004) and more patients with PV (P = .003) compared with the subgroup that did not have a CD34+ JAK2 V617F VAF measurement (supplemental Table 4).
Across the entire JAK2 V617F–positive cohort, the median neutrophil JAK2 V617F VAF was similar between men and women (53.3% vs 57.9%; P = .528) (Figure 2A), and when adjusted for phenotype and age in a multivariable linear regression model, there were no significant differences (supplemental Table 5). Next, the rate of change of the neutrophil JAK2 V617F VAF was evaluated in 143 female subjects and 83 male subjects who had ≥2 measurements during the study period. The VAF increased in female subjects by 0.52% ± 5.89% per year and in male subjects by 0.33% ± 6.8% per year. Because hydroxyurea may constrain the JAK2 V617F VAF,14 analysis was also performed after controlling for hydroxyurea therapy. Despite a higher percentage of male subjects having received hydroxyurea (Table 1), there was no sex difference in the rate of change in JAK2 V617F VAF after controlling for hydroxyurea therapy (P = .11).
Figure 2.
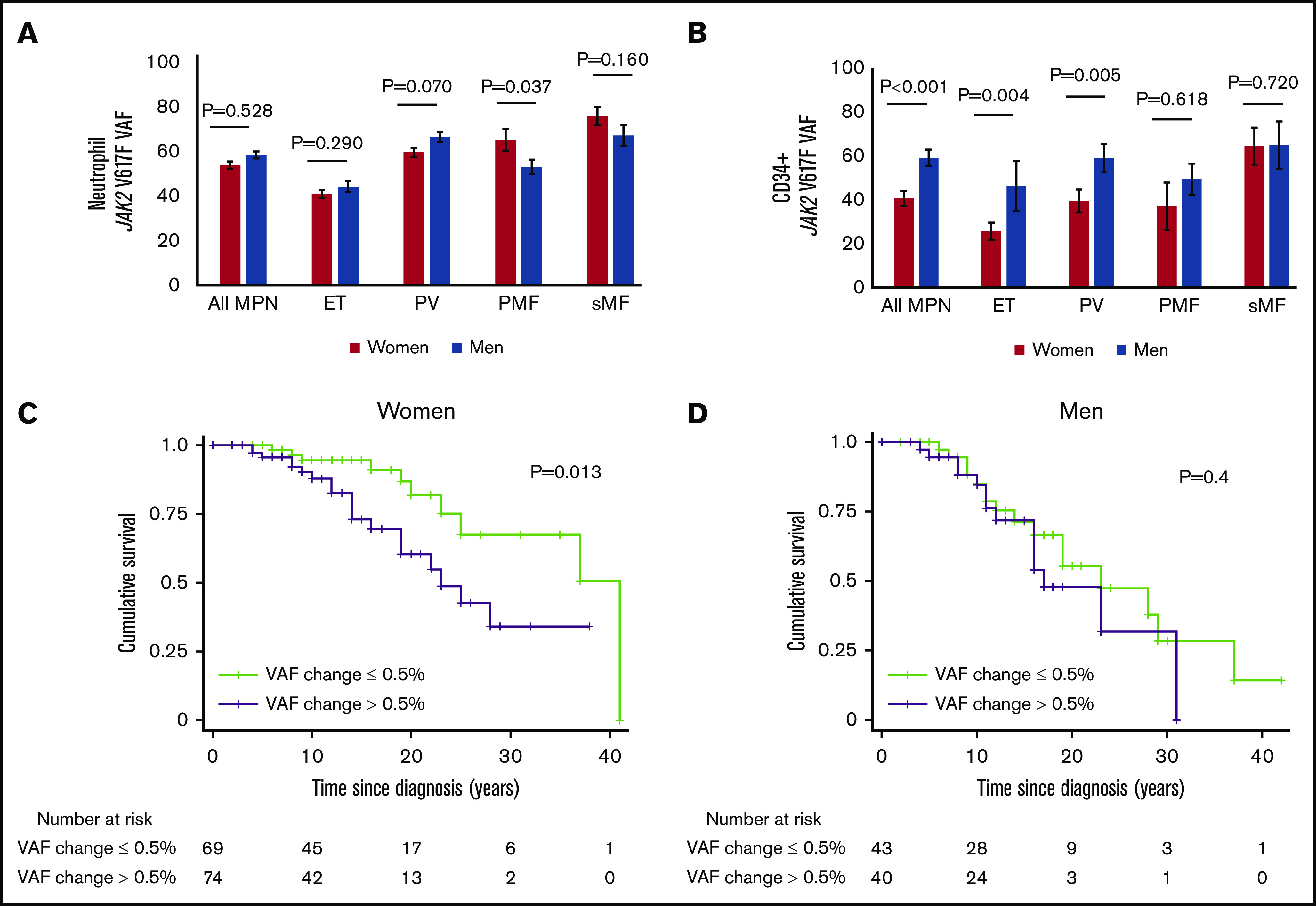
Men have higher JAK2 V617F VAF in CD34+ cells across different phenotypes but not in neutrophils, and their survival outcomes are less dependent on JAK2 V617F VAF change per year. (A) The neutrophil JAK2 V617F VAF in men was not higher compared with women across the whole cohort (P = .528), and among patients with ET (P = .290), whereas among patients with PV, men exhibited a trend of increased JAK2 V617F VAF, but the difference did not reach significance (P = .07). Among patients with PMF, women had higher JAK2 V617F VAF compared with men (P = .037), whereas differences did not reach significance among patients with sMF (P = .160). (B) The CD34+ cell JAK2 V617F VAF in men was higher compared with women across the whole cohort (P < .001), among patients with ET (P = .004), and among patients with PV (P = .005); there was no difference among PMF (P = .618) and sMF (P = .720) patients. (C) Kaplan-Meier analysis showed that women with JAK2 V617F VAF change per year >0.5% have worse survival compared with women with ≤0.5% change per year (P = .013). (D) Kaplan-Meier analysis of men showed no difference in the survival of those with JAK2 V617F VAF change >0.5% per year (P = .4). All MPN = cohort including all the MPN phenotypes.
We hypothesized that MPN-specific driver mutation VAF in CD34+ cells might explain the finding that male subjects have more aggressive disease. Men had higher JAK2 V617F allele burden in their CD34+ cells across the entire cohort (P < .001) and among patients with ET (P = .004) and PV (P = .005) (Figure 2B). In a linear regression model, the JAK2 V617F VAF in CD34+ cells was significantly higher in male subjects than in female subjects (P = .001), independent of age and phenotype (supplemental Table 5). However, male sex remained significantly associated with both presentation with PMF vs ET and overall worse survival, even when we controlled for the CD34+ cell JAK2 V617F VAF (supplemental Table 6).
We then queried if the difference in survival was associated with differences in the dependence of disease progression on the MPN-specific mutation VAF. Neutrophil JAK2 V617F VAF and its change per year were not significantly associated with survival in the entire cohort (HR of 1 [95% CI, 0.99-1.01; P = .120] and HR of 1.05 [95% CI, 0.97-1.12; P = .220], respectively). Similarly, these 2 variables were not associated with survival in men (HR of 1 [95% CI, 0.99-1; P = .320] and HR of 0.97 [95% CI, 0.85-1.06; P = .530]). On the contrary, both JAK2 V617F VAF (HR, 1.01; 95% CI, 1-1.02; P = .024) and its change per year (HR, 1.12; 95% CI, 1.01-1.25; P = .040) were associated with overall survival in women. To explore this finding, a Kaplan-Meier analysis was performed to compare the survival of patients based on the median rate of change of the neutrophil JAK2 V617F VAF (0.53% per year and 0.50% per year in women and men). In the entire cohort, patients with a JAK2 V617F VAF increase >0.5% per year had worse overall survival (supplemental Figure 5). This scenario remained significant for women but not for men (Figure 2C-D).
Men had an increased number of additional non–MPN-specific somatic mutations independent of age and phenotype
Although men presented with more aggressive disease and had a worse overall survival, this finding was not dependent on the MPN-specific mutation VAF. To examine whether increased acquisition of non–MPN-specific somatic mutations in men was associated with inferior outcomes, we analyzed the mutational burden in a subset of patients (n = 227) who underwent clinical evaluation using NGS for myeloid malignancy–associated genes (Table 3). Overall, men were more likely to have additional non–MPN-specific (non-JAK2, CALR, or MPL) mutations compared with women, even when controlled for age at the time of sequencing (Figure 3A). Multivariable linear regression analysis supported the observation that men acquire more non–MPN-specific somatic mutations (P = .012) independently of age, phenotype, and MPN-specific mutation (supplemental Figure 6; supplemental Table 7).
Table 3.
Clinical and molecular characteristics of MPN cohorts with and without NGS
| Variable | Cohort with NGS (n = 227) | Cohort without NGS (n = 588) | P |
|---|---|---|---|
| Age at diagnosis, mean ± SD, y | 53.45 ± 15.8 | 50.56 ± 16.32 | .020 |
| Male sex | 117 (51.5) | 229 (38.8) | .001 |
| Phenotype at diagnosis | |||
| ET | 106 (46.7) | 275 (46.8) | 1.000 |
| PV | 57 (25.1) | 232 (39.4) | <.001 |
| PMF | 64 (28.2) | 81 (13.8) | <.001 |
| MPN-specific mutation | |||
| JAK2 + | 147 (64.8) | 459 (78.1) | <.001 |
| CALR + | 56 (24.7) | 73 (12.4) | <.001 |
| MPL + | 11 (4.8) | 10 (1.7) | .023 |
| Triple negative | 13 (5.7) | 32 (5.4) | .865 |
| JAK2– others not done | 0 (0) | 14 (2.4) | .014 |
| Karyotype | |||
| No lesion | 41 (34.7) | 184 (68.1) | <.001 |
| 1 lesion | 42 (35.6) | 39 (14.4) | <.001 |
| ≥2 lesions | 35 (29.7) | 47 (17.4) | .010 |
| Treatment with hydroxyurea | 129 (56.8) | 164 (27.9) | <.001 |
Data are expressed as n (%) unless otherwise indicated.
Figure 3.
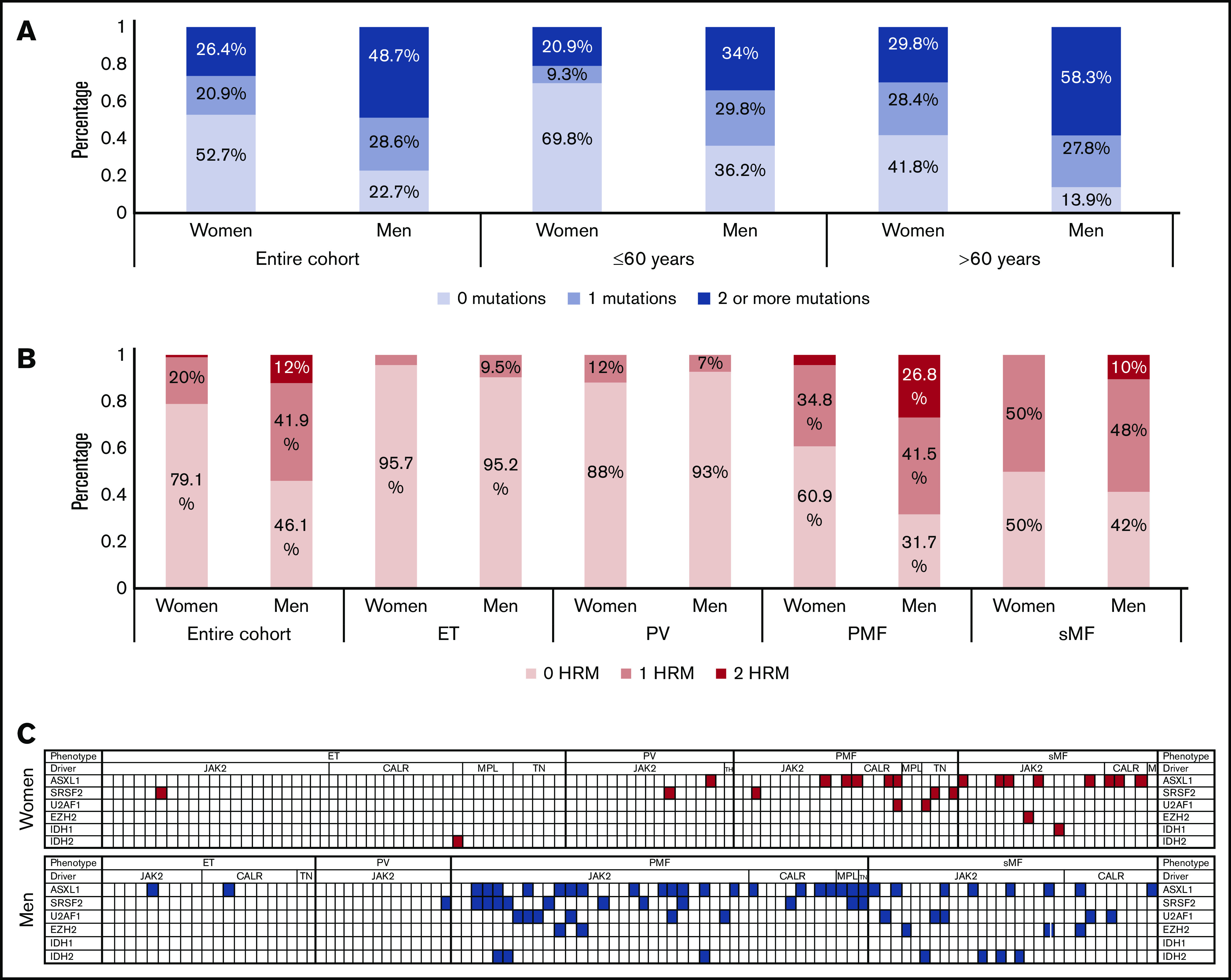
Men have a higher prevalence of non–MPN-specific somatic mutations and particularly 1 or 2 HRMs independent of age and phenotype at the time of NGS. (A) Higher percentage of women have no non–MPN-specific somatic mutations, whereas a higher percentage of men have ≥1 non–MPN-specific somatic mutations if stratified per age (≤60 years and >60 years). (B) Men have a higher prevalence of mutations in the high-risk genes ASXL1, SRSF2, U2AF1, EZH2, IDH1, and IDH2. (C) Men have a higher incidence of at least 1 HRM (P = .017) and 2 HRMs (P = .011) compared with women, independent of age and phenotype at the NGS and MPN-specific mutation.
Mutations in genes associated with myelodysplastic syndrome (MDS)/MPN phenotype (KRAS, NRAS, EZH2, and CBL) were more common among men (15.4% vs 6.4%; P = .035) (supplemental Figure 6). Interestingly, 7 men (5.9%) but only 2 women (1.8%) had coexisting JAK2 V617F and CALR or MPL mutations. Mutation of ASXL1, EZH2, SRSF2, U2AF1, or IDH1/2 has been associated with particularly poor prognosis in MF, and they are therefore defined as high-risk mutations (HRMs)15,16; the presence of at least 2 HRMs is associated with even worse outcomes.17 Men had a higher prevalence of 1 HRM (53.8% vs 20.9%; P < .001) and 2 HRMs (17.1% vs 0.9%; P < .001) (Figure 3B-C). In a regression model, the presence of 1 and 2 HRMs was associated with male sex (P = .017 and P = .011, respectively) independent of the age and phenotype at the time of NGS and MPN-specific mutation (supplemental Table 8). Apart from IDH1, all the other high-risk genes were more frequently mutated in men compared with women with PMF and sMF. Finally, women had a slightly higher incidence of SF3B1 mutations (6.4% vs 5.1%). Interestingly, across the entire cohort, the majority of women (5 of 7) had substitutions in the K700 residue, whereas the majority of men (4 of 6) had substitutions in the K666 residue. Focusing on the patients with MF, all the women (4 of 4) had K700 substitutions and all the men (4 of 4) had K666 substitutions.
Venous thrombosis but not arterial thrombosis was less common in male subjects
VTE was less common in men (7.2% vs 14.7%; P < .001). Male sex was associated with a lower risk of VTE (odds ratio, 0.45; 95% CI, 0.27-0.74; P = .002) independent of age and phenotype at diagnosis and MPN-specific mutation (supplemental Table 9). There was no significant difference in the incidence of arterial ischemic events between men and women (11.3% vs 10.5%; P = .732).
Discussion
We found that men with an MPN present at an older age18,19 and with a striking difference in the frequency of PMF at diagnosis, consistent with findings from other large cohorts.20,21 In addition, male sex was associated with worse outcomes, regardless of age, presenting phenotype, or MPN-specific driver mutation. The MPN-specific mutation (JAK2, CALR, and MPL) VAF at diagnosis was not associated with worse outcomes; however, MPN-specific mutation allele burden change over time did predict overall survival in women. In contrast, men had a higher prevalence of non–MPN-specific somatic mutations, including high-risk mutations. Men had worse overall survival and higher risk of transformation to sMF and AML, and higher mortality related to second cancers. The more rapid progression to sMF in men confirmed similar findings from other groups.22 The only disease complication in which men fared better than women was venous thrombosis; venous thrombotic complications were more common in women, yet these did not account for increased mortality in women.23-25
Our group has previously shown that women with PV had a lower neutrophil JAK2 V617F allele burden compared with male subjects.26 The current study confirmed that in PV, men have a higher neutrophil JAK2 V617F allele burden compared with women; in PMF, men have a lower neutrophil JAK2 V617F allele burden compared with women. However, there was no significant difference in the neutrophil JAK2 V617F allele burden between sexes when adjusted for age and phenotype, suggesting that the neutrophil JAK2 V617F allele burden per se is not the cause of the more advanced disease presentation and progression in men. On the contrary, we showed for the first time that the CD34+ cell JAK2 V617F allele burden is significantly higher in men, even after controlling for age and phenotype. We questioned whether the higher progenitor compartment allele burden was the sole cause of the differences between men and women but found that male sex was associated with PMF at diagnosis and worse survival, independent of the progenitor cell VAF. Thus, it is likely that other biologic mechanisms in addition to progenitor JAK2 V617F allele burden are implicated in these differences.
We examined the association between the dynamic disease burden and outcomes. The neutrophil JAK2 V617F VAF and the rate of increase per year were associated with survival only in women. This outcome suggests that disease progression is more dependent on JAK2 mutation VAF in women, likely due to the fact that women with the JAK2 mutation commonly present with ET and PV, phenotypes in which increases in allele burden are associated with phenotype evolution. Large population studies examining the prevalence and outcomes associated with JAK2 V617F in the general population report sex differences in presentation and outcomes. The presence of JAK2 V617F mutation in the general population is associated with higher hemoglobin levels, a difference that was more prominent in women than in men.27 On the contrary and consistent with our results that disease progression and survival seem to be less dependent on the JAK2 mutation in men, men in the general population with a JAK2 V617F mutation have a higher risk of hematologic malignancies, cancer, and worse survival compared with women.28
Men also had a significantly higher burden of non–MPN-specific somatic mutations and higher burdens of mutations in high-risk genes, including ASXL1, EZH2, SRSF2, U2AF1, and IDH1/2. The presence of a mutation in one of those genes is reportedly associated with poor prognosis in patients with PMF.15,16 Moreover, the presence of mutations in at least 2 of these genes that is independently associated with particularly poor survival17 was more common among male subjects in our cohort. Similarly, mutations in genes such as KRAS, NRAS, EZH2, and CBL, which are associated with MDS/MPN phenotype, and coexistence of CALR and JAK2 mutations,29 were more common in men, further indicating that they have a distinct and higher risk molecular profile. In the context of SF3B1 mutation among patients with MF, higher risk SF3B1 lesions were associated with male sex: all the men with MF had K666 substitutions, lesions more frequently associated with AML,30 whereas all the women had K700 substitutions, with the most common SF3B1 substitutions in low-grade MDS.31 Finally, the increased number of non–MPN-specific somatic mutations in men provides a rationale for the decreased dependence of their disease progression on MPN-specific mutation.
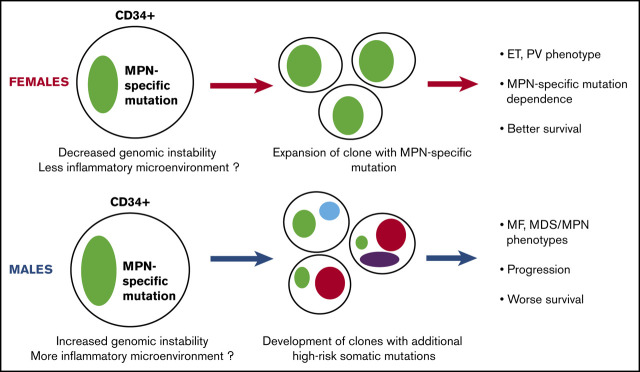
Our data provide an explanation for the different biology of MPNs in men and women. First, men had a higher MPN-specific mutation VAF in their CD34+ stem and progenitor cell compartment, which could reflect increased genomic instability of these cells and a potentially different cytokine profile in the hematopoietic niche in male subjects,32,33 leading to a more advanced disease at presentation. Second, men had increased acquisition of additional high-risk somatic mutations and decreased dependency on the MPN-specific mutation for disease progression. Importantly, in the population at large, higher clonal hematopoiesis rates not only associate with higher hematologic malignancy rates but also with higher cancer rates overall, whether MPN or non-MPN populations28,34,35 were ascertained, and higher all-cause mortality.36 These forces combine in men with MPN to increase both MPN-specific mortality due to disease progression and transformation but also all-cause mortality, including cancer mortality (Figure 4).
Figure 4.
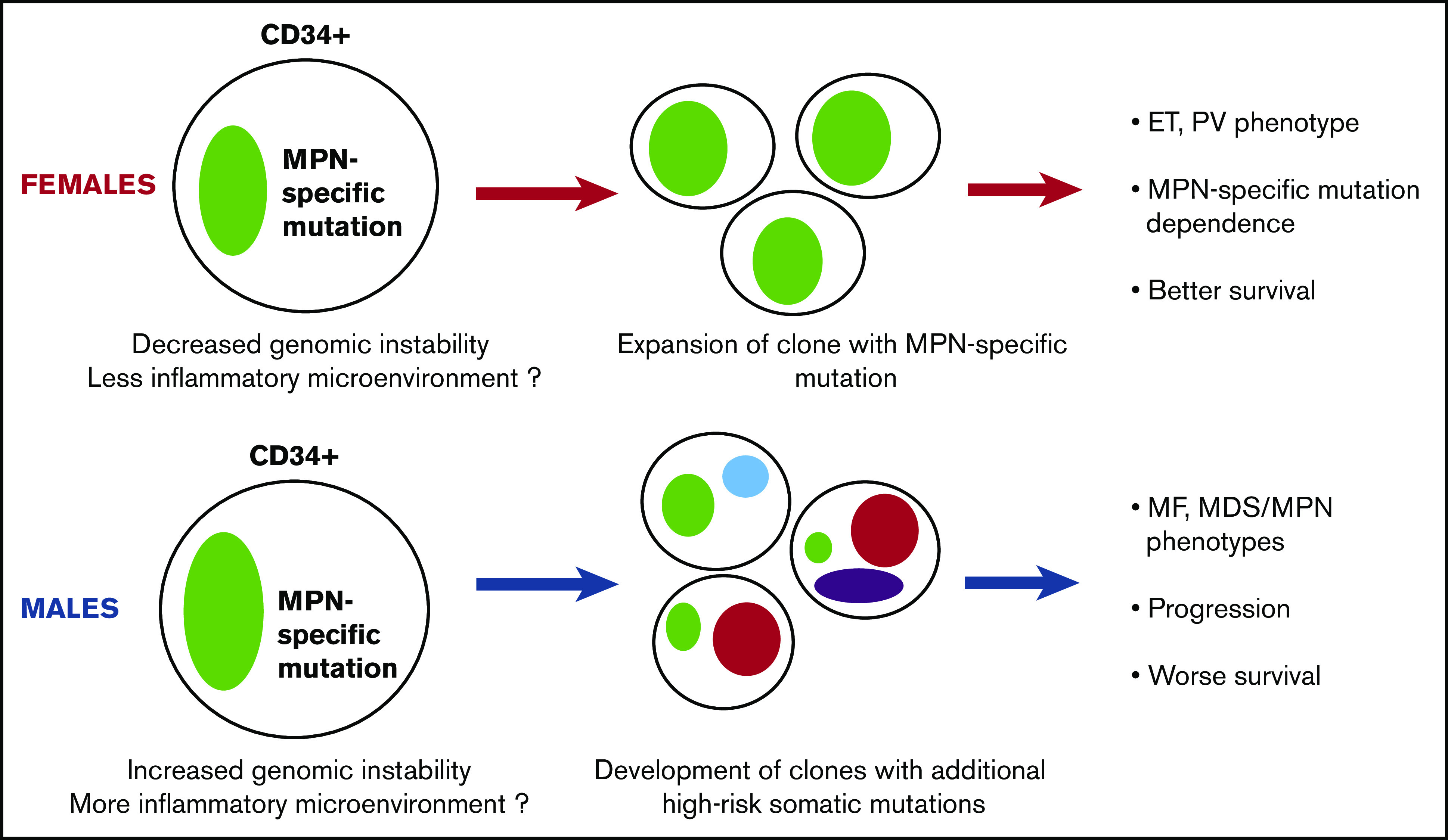
Hypothetical model of the different biology of MPN development and progression between women and men. Men may have higher genomic instability in their primitive cell compartment and a potentially more inflammatory microenvironment, causing the increased allele burden of their MPN-specific mutation. These characteristics can also induce the acquisition of additional non–MPN-specific somatic mutations, rendering the disease less dependent on the MPN-specific mutation and promoting the development of clonal hematopoiesis, MDS/MPN, or MF phenotype, accelerated disease progression, and worse survival.
Overall, these results suggest that risk stratification according to sex could be considered in everyday practice. Moreover, clinical trials should factor sex into their analysis given the radically different context that sex introduces in these diseases. However, deeper understanding of the underlying mechanisms, and particularly further study of the hypothesis that these differences are mediated by higher-risk mutational burden, is warranted. Finally, our findings introduce a novel biologic model that implicates progenitor compartment, MPN-specific, non–MPN-specific mutations, and high-risk mutation burden as the basis of sex differences in presentation and outcomes in MPNs.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
This study was supported by the National Institutes of Health, National Heart, Lung, and Blood Institute (T32HL007525, R01 HL145780, and K08HL138142), and The Leukemia & Lymphoma Society Quest for Cures (0864-15).
Footnotes
Requests for data sharing should be submitted to the corresponding author (Alison R. Moliterno; e-mail: amoliter@jhmi.edu).
Authorship
Contribution: T.K. and A.R.M. wrote the manuscript; E.M.B., S.C., J.S., and L.R. edited the manuscript; E.M.B., J.S., L.R., D.M.W., and O.R. enrolled patients and collected clinical data; D.M.W., O.R., J.S., and A.R.M. performed and interpreted genomic analyses; C.D.G. performed and analyzed the NGS; T.K., E.M.B., S.K., and S.C. performed the statistical analysis; and A.R.M. supervised the project.
Conflict-of-interest disclosure: The authors declare no competing financial interest.
Correspondence: Alison R. Moliterno, Division of Adult Hematology, Department of Medicine, Johns Hopkins University School of Medicine, 720 Rutland Ave, Ross Building #1025, Baltimore, MD 21205; e-mail: amoliter@jhmi.edu.
References
- 1.Spivak JL. Myeloproliferative neoplasms. N Engl J Med. 2017;377(9):895-896. [DOI] [PubMed] [Google Scholar]
- 2.Vannucchi AM, Lasho TL, Guglielmelli P, et al. Mutations and prognosis in primary myelofibrosis. Leukemia. 2013;27(9):1861-1869. [DOI] [PubMed] [Google Scholar]
- 3.Grinfeld J, Nangalia J, Baxter EJ, et al. Classification and personalized prognosis in myeloproliferative neoplasms. N Engl J Med. 2018;379(15):1416-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geyer HL, Kosiorek H, Dueck AC, et al. Associations between gender, disease features and symptom burden in patients with myeloproliferative neoplasms: an analysis by the MPN QOL International Working Group. Haematologica. 2017;102(1):85-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tefferi A, Betti S, Barraco D, et al. Gender and survival in essential thrombocythemia: a two-center study of 1,494 patients. Am J Hematol. 2017;92(11):1193-1197. [DOI] [PubMed] [Google Scholar]
- 6.Berk PD, Goldberg JD, Donovan PB, Fruchtman SM, Berlin NI, Wasserman LR. Therapeutic recommendations in polycythemia vera based on Polycythemia Vera Study Group protocols. Semin Hematol. 1986;23(2):132-143. [PubMed] [Google Scholar]
- 7.Murphy S, Peterson P, Iland H, Laszlo J. Experience of the Polycythemia Vera Study Group with essential thrombocythemia: a final report on diagnostic criteria, survival, and leukemic transition by treatment. Semin Hematol. 1997;34(1):29-39. [PubMed] [Google Scholar]
- 8.Mesa RA, Verstovsek S, Cervantes F, et al. ; International Working Group for Myelofibrosis Research and Treatment (IWG-MRT) . Primary myelofibrosis (PMF), post polycythemia vera myelofibrosis (post-PV MF), post essential thrombocythemia myelofibrosis (post-ET MF), blast phase PMF (PMF-BP): consensus on terminology by the International Working Group for Myelofibrosis Research and Treatment (IWG-MRT). Leuk Res. 2007;31(6):737-740. [DOI] [PubMed] [Google Scholar]
- 9.Barosi G. Myelofibrosis with myeloid metaplasia: diagnostic definition and prognostic classification for clinical studies and treatment guidelines. J Clin Oncol. 1999;17(9):2954-2970. [DOI] [PubMed] [Google Scholar]
- 10.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391-2405. [DOI] [PubMed] [Google Scholar]
- 11.Moliterno AR, Williams DM, Rogers O, Spivak JL. Molecular mimicry in the chronic myeloproliferative disorders: reciprocity between quantitative JAK2 V617F and Mpl expression. Blood. 2006;108(12):3913-3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gondek LP, Zheng G, Ghiaur G, et al. Donor cell leukemia arising from clonal hematopoiesis after bone marrow transplantation. Leukemia. 2016;30(9):1916-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vannucchi AM, Antonioli E, Guglielmelli P, Pardanani A, Tefferi A. Clinical correlates of JAK2V617F presence or allele burden in myeloproliferative neoplasms: a critical reappraisal. Leukemia. 2008;22(7):1299-1307. [DOI] [PubMed] [Google Scholar]
- 14.Girodon F, Schaeffer C, Cleyrat C, et al. Frequent reduction or absence of detection of the JAK2-mutated clone in JAK2V617F-positive patients within the first years of hydroxyurea therapy [published correction appears in Haematologica. 2010;95(2):344 ]. Haematologica. 2008;93(11):1723-1727. [DOI] [PubMed] [Google Scholar]
- 15.Guglielmelli P, Lasho TL, Rotunno G, et al. The number of prognostically detrimental mutations and prognosis in primary myelofibrosis: an international study of 797 patients. Leukemia. 2014;28(9):1804-1810. [DOI] [PubMed] [Google Scholar]
- 16.Tamari R, Rapaport F, Zhang N, et al. Impact of high-molecular-risk mutations on transplantation outcomes in patients with myelofibrosis. Biol Blood Marrow Transplant. 2019;25(6):1142-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guglielmelli P, Lasho TL, Rotunno G, et al. MIPSS70: mutation-enhanced international prognostic score system for transplantation-age patients with primary myelofibrosis. J Clin Oncol. 2018;36(4):310-318. [DOI] [PubMed] [Google Scholar]
- 18.Resar L, Williams DM, Braunstein EM, Spivak JL, Moliterno AR. Myeloproliferative neoplasms in pediatrics and young adults: genetic lesions, diagnostic features, and clinical outcomes. Blood. 2017;130(suppl 1):4180. [Google Scholar]
- 19.Videbaek A. Polycythaemia vera. Course and prognosis. Acta Med Scand. 1950;138(3):179-187. [DOI] [PubMed] [Google Scholar]
- 20.Passamonti F, Rumi E, Pungolino E, et al. Life expectancy and prognostic factors for survival in patients with polycythemia vera and essential thrombocythemia. Am J Med. 2004;117(10):755-761. [DOI] [PubMed] [Google Scholar]
- 21.McNally RJ, Rowland D, Roman E, Cartwright RA. Age and sex distributions of hematological malignancies in the U.K. Hematol Oncol. 1997;15(4):173-189. [DOI] [PubMed] [Google Scholar]
- 22.Barraco D, Mora B, Guglielmelli P, et al. Gender effect on phenotype and genotype in patients with post-polycythemia vera and post-essential thrombocythemia myelofibrosis: results from the MYSEC project. Blood Cancer J. 2018;8(10):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stein BL, Rademaker A, Spivak JL, Moliterno AR. Gender and vascular complications in the JAK2 V617F-positive myeloproliferative neoplasms. Thrombosis. 2011;2011:874146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borowczyk M, Wojtaszewska M, Lewandowski K, et al. The JAK2 V617F mutational status and allele burden may be related with the risk of venous thromboembolic events in patients with Philadelphia-negative myeloproliferative neoplasms. Thromb Res. 2015;135(2):272-280. [DOI] [PubMed] [Google Scholar]
- 25.Hultcrantz M, Björkholm M, Dickman PW, et al. Risk for arterial and venous thrombosis in patients with myeloproliferative neoplasms: a population-based cohort study. Ann Intern Med. 2018;168(5):317-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stein BL, Williams DM, Wang NY, et al. Sex differences in the JAK2 V617F allele burden in chronic myeloproliferative disorders. Haematologica. 2010;95(7):1090-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cordua S, Kjaer L, Skov V, Pallisgaard N, Hasselbalch HC, Ellervik C. Prevalence and phenotypes of JAK2 V617F and calreticulin mutations in a Danish general population. Blood. 2019;134(5):469-479. [DOI] [PubMed] [Google Scholar]
- 28.Nielsen C, Birgens HS, Nordestgaard BG, Kjaer L, Bojesen SE. The JAK2 V617F somatic mutation, mortality and cancer risk in the general population. Haematologica. 2011;96(3):450-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang MG, Choi HW, Lee JH, et al. Coexistence of JAK2 and CALR mutations and their clinical implications in patients with essential thrombocythemia. Oncotarget. 2016;7(35):57036-57049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hou HA, Liu CY, Kuo YY, et al. Splicing factor mutations predict poor prognosis in patients with de novo acute myeloid leukemia. Oncotarget. 2016;7(8):9084-9101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Obeng EA, Chappell RJ, Seiler M, et al. Physiologic expression of Sf3b1(K700E) causes impaired erythropoiesis, aberrant splicing, and sensitivity to therapeutic spliceosome modulation. Cancer Cell. 2016;30(3):404-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pietras EM. Inflammation: a key regulator of hematopoietic stem cell fate in health and disease. Blood. 2017;130(15):1693-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pedersen KM, Çolak Y, Ellervik C, Hasselbach HC, Bojesen SE, Nordestgaard BG. Loss-of-function polymorphism in IL6R reduces risk of JAK2 V617F somatic mutation and myeloproliferative neoplasm: a Mendelian randomization study. EClinicalMedicine. 2020;;21(100280): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hong J, Lee JH, Byun JM, et al. Risk of disease transformation and second primary solid tumors in patients with myeloproliferative neoplasms. Blood Adv. 2019;3(22):3700-3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Genovese G, Kähler AK, Handsaker RE, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med. 2014;371(26):2477-2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jaiswal S, Natarajan P, Silver AJ, et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377(2):111-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.