Key Points
Pembrolizumab provided durable responses over long-term follow-up in a subset of heavily pretreated patients with classical Hodgkin lymphoma.
Pembrolizumab had a favorable safety profile in heavily pretreated patients with classical Hodgkin lymphoma over long-term follow-up.
Abstract
The KEYNOTE-013 study was conducted to evaluate pembrolizumab monotherapy in hematologic malignancies; classical Hodgkin lymphoma (cHL) was an independent expansion cohort. We present long-term results based on >4 years of median follow-up for the cHL cohort. The trial enrolled cHL patients who experienced relapse after, were ineligible for, or declined autologous stem cell transplantation and experienced progression with or did not respond to brentuximab vedotin. Patients received IV pembrolizumab 10 mg/kg every 2 weeks for up to 2 years or until confirmed progression or unacceptable toxicity. Primary end points were safety and complete response (CR) rate by central review. Enrolled patients (N = 31) had received a median of 5 therapies (range, 2 to 15). After a median follow-up of 52.8 months (range, 7.0 to 57.6 months), CR rate was 19%, and median duration of response (DOR) was not reached; 24-month and 36-month DOR rates were both 50% by the Kaplan-Meier method. Median overall survival was not reached; 36-month overall survival was 81%. Six patients (19%) experienced grade 3 treatment-related adverse events (AEs); there were no grade 4 or 5 treatment-related AEs. With long-term follow-up among a heavily pretreated cohort, pembrolizumab had a favorable safety profile; some patients maintained long-term response with pembrolizumab years after end of treatment. This trial was registered at www.clinicaltrials.gov as #NCT01953692.
Visual Abstract
Introduction
Most patients with classical Hodgkin lymphoma (cHL) are cured after combination chemotherapy with or without radiation. Patients who experience relapse or are refractory to first-line treatment are treated with salvage chemotherapy, and, if eligible, those with chemosensitive disease undergo autologous stem cell transplantation (ASCT).1 Patients who are refractory to salvage chemotherapy or experience relapse after ASCT may benefit from additional chemotherapy or use of brentuximab vedotin (BV), but outcomes are historically poor.2 Results of the phase 1b KEYNOTE-013 study (#NCT01953692) and the phase 2 KEYNOTE-087 study (#NCT02453594) demonstrated strong antitumor activity with pembrolizumab, a programmed death 1 (PD-1) inhibitor, in patients with relapsed/refractory cHL.3-5 Based on KEYNOTE-087 results, pembrolizumab was approved by the US Food and Drug Administration for adult and pediatric patients with relapsed/refractory cHL.6 Other PD-1 inhibitors have also shown activity in cHL7-9; however, durability of responses with PD-1 inhibitor monotherapy remains to be established, and it is unknown whether benefit is maintained after treatment discontinuation. To better understand durability of responses, outcomes after >4 years of median follow-up for the KEYNOTE-013 cHL cohort are reported.
Methods
Detailed methods have been published previously.4 The protocol was approved by an independent institutional review board or the ethics committees of the participating institutions. The study was conducted according to the principles of the Declaration of Helsinki, and all patients provided written informed consent. Patients had confirmed cHL and experienced relapse after, were ineligible for, or declined to undergo ASCT, and either experienced progression with or did not respond to BV. Patients received 10 mg/kg of pembrolizumab IV every 2 weeks for up to 2 years or until confirmed progression or unacceptable toxicity. At the investigator’s discretion, patients with complete response (CR) could discontinue pembrolizumab after receiving ≥24 weeks of treatment, with ≥2 doses administered after confirmed CR. Weight-based pembrolizumab dosing provides equivalent efficacy to the fixed-dose regimen of 200 mg every 3 weeks approved for cHL.10,11
Primary end points were safety and CR rate. Secondary end points were objective response rate (ORR), duration of response (DOR), progression-free survival (PFS), and overall survival (OS). Responses (CR, partial response [PR], stable disease, progressive disease [PD]) were determined by central review using International Harmonization Project 2007 criteria.12 For CR rate and ORR, 95% confidence intervals (CIs) were calculated based on binomial distribution. DOR, PFS, and OS were estimated using the Kaplan-Meier method. Patients who underwent stem cell transplantation (SCT) or initiated another anticancer therapy without experiencing PD were censored on the date of their last assessment before SCT or new anticancer therapy for DOR and PFS analyses. Patients who experienced progression or died after >1 missed disease assessment were censored at their last assessment without PD for DOR. Adverse events (AEs) were graded per Common Terminology Criteria for Adverse Events, version 4.0.13 Immune-mediated AEs were based on a list of terms specified by the sponsor and reported regardless of attribution to study treatment or immune relatedness. The safety population included all patients who received ≥1 dose of pembrolizumab; the efficacy population included all patients who had ≥1 postbaseline assessment. Data cutoff was 28 September 2018.
Results
Among 31 patients enrolled in the cHL cohort, median follow-up was 52.8 months (range, 7.0 to 57.6 months). Among 18 responders, median follow-up was 52.7 months (range, 50.0 to 56.6 months). Baseline characteristics have been published elsewhere.4 The median number of previous therapies was 5 (range, 2 to 15). At data cutoff, 6 patients (19%) had completed 2 years of treatment and 25 (81%) had discontinued study medication because of PD (15 patients; 48%), AEs (3 patients; 10%), physician decision (3 patients; 10%), CR (2 patients; 7%), protocol violation (1 patient; 3%), and patient decision (1 patient; 3%).
Treatment-related AEs occurred in 22 patients (71%), and grade 3 to grade 5 treatment-related AEs occurred in 6 (19%). The most common treatment-related AEs were diarrhea (7 patients; 23%), hypothyroidism, nausea, and pneumonitis (4 patients each; 13%). Six patients (19%) experienced grade 3 treatment-related AEs (no grade 4 or 5 treatment-related AEs; Table 1). Three patients (10%) discontinued because of treatment-related AEs. Overall, 14 patients (45%) experienced immune-mediated AEs and infusion reactions, most commonly hypothyroidism (5 patients; 16%) and pneumonitis (4 patients; 13%; supplemental Table 1). Three patients (10%) experienced grade 3 immune-related AEs (colitis in 2 patients; nephrotic syndrome in 1 patient).
Table 1.
Treatment-related AEs (N = 31)
| AEs | n (%) |
|---|---|
| Any grade (≥2 patients) | |
| Any | 22 (71) |
| Diarrhea | 7 (23) |
| Hypothyroidism | 4 (13) |
| Nausea | 4 (13) |
| Pneumonitis | 4 (13) |
| Dyspnea | 3 (10) |
| Fatigue | 3 (10) |
| Abdominal distension | 2 (6) |
| Arthralgia | 2 (6) |
| Increased aspartate aminotransferase level | 2 (6) |
| Asthenia | 2 (6) |
| Increased blood creatine phosphokinase level | 2 (6) |
| Chills | 2 (6) |
| Decreased appetite | 2 (6) |
| Hypertriglyceridemia | 2 (6) |
| Thyroiditis | 2 (6) |
| Vomiting | 2 (6) |
| Increased weight | 2 (6) |
| Xerosis | 2 (6) |
| Grade 3 | |
| Any | 6 (19) |
| Colitis | 1 (3) |
| Axillary pain | 1 (3) |
| Increased aspartate aminotransferase level | 1 (3) |
| Back pain | 1 (3) |
| Joint swelling | 1 (3) |
| Nephrotic syndrome | 1 (3) |
| Dyspnea | 1 (3) |
There were no grade 4 or 5 treatment-related AEs.
ORR was 58% (95% CI, 39% to 76%); 6 patients (19%) achieved CR and 12 (39%) achieved PR (Table 1). Response rates were assessed in key subgroups (Table 2). ORR was 69% (95% CI, 41% to 89%) among patients who received BV after ASCT and subsequent PD and 57% (95% CI, 18% to 90%) among patients who received BV before ASCT and subsequent PD. Among patients who received BV after being deemed ineligible for ASCT, ORR was 38% (95% CI, 9% to 76%). When evaluated by response to first therapy, ORR was 55% (95% CI, 23% to 83%) and 60% (95% CI, 36% to 81%) among patients who were and those who were not refractory to their first therapy, respectively (Table 2).
Table 2.
Best objective tumor response in the total population and by key patient subgroups
| Total population, N = 31 | Response by baseline transplantation status and BV status | Response by refractory status to first therapy | ||||
|---|---|---|---|---|---|---|
| Received BV after unsuccessful ASCT, n = 16 | Received BV and ASCT ineligible, n = 8 | Received BV before unsuccessful ASCT, n = 7 | Refractory to first therapy, n = 11 | Not refractory to first therapy, n = 20 | ||
| ORR (CR + PR), % (95% CI) | 58.1 (39.1-75.5) | 68.8 (41.3-89.0) | 37.5 (8.5-75.5) | 57.1(18.4-90.1) | 54.5 (23.4-83.3) | 60.0 (36.1-80.9) |
| Response, n (%) | ||||||
| CR | 6 (19.4) | 3 (18.8) | 2 (25.0) | 1 (14.3) | 3 (27.3) | 3 (15.0) |
| PR | 12 (38.7) | 8 (50.0) | 1 (12.5) | 3 (42.9) | 3 (27.3) | 9 (45.0) |
| SD | 7 (22.6) | 3 (18.8) | 3 (37.5) | 1 (14.3) | 4 (36.4) | 3 (15.0) |
| PD | 6 (19.4) | 2 (12.5) | 2 (25.0) | 2 (28.6) | 1 (9.1) | 5 (25.0) |
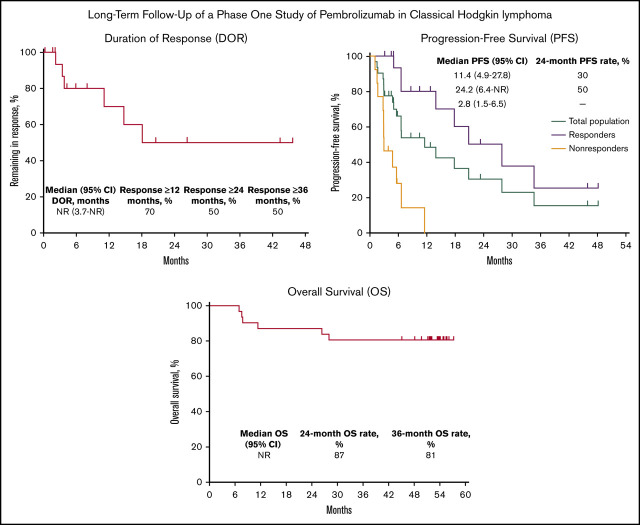
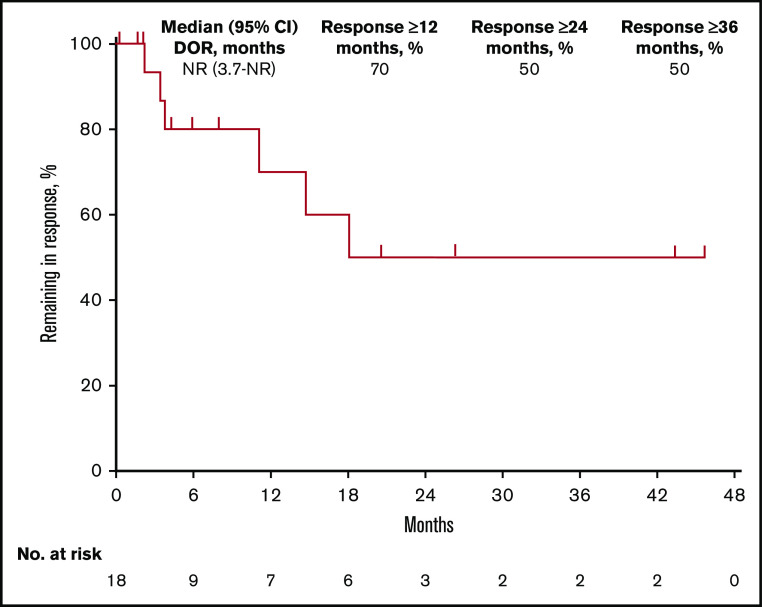
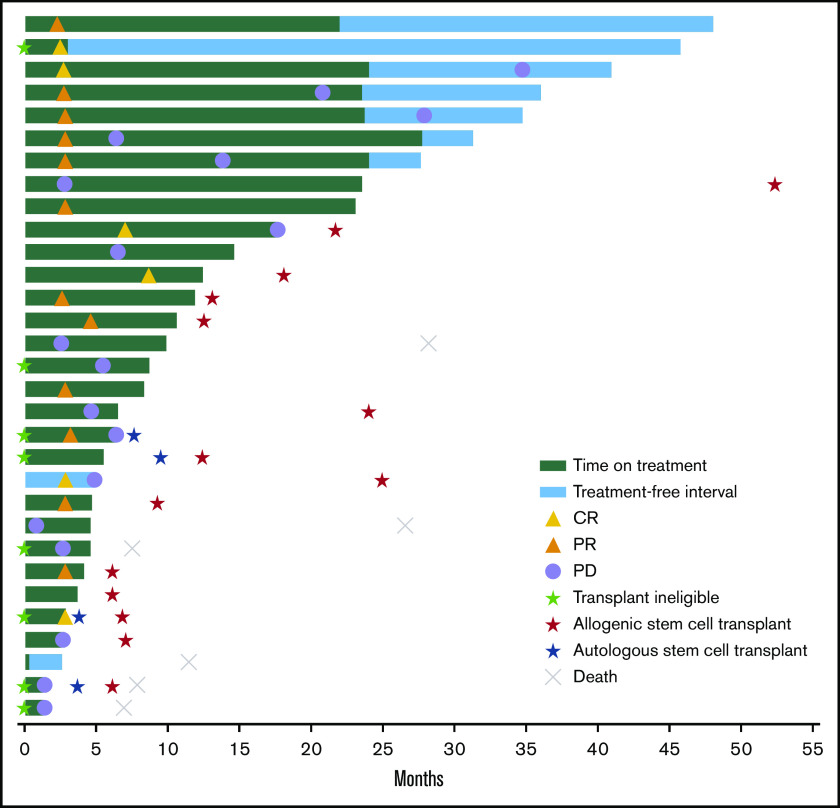
Among 18 responders, median DOR was not reached (95% CI, 3.7 months to not reached) (Figure 1). Estimated DOR ≥12 months, ≥24 months, and ≥36 months was 70%, 50%, and 50%, respectively. Among responders, 8 experienced disease progression (Figure 2). Of 6 patients who achieved CR then discontinued pembrolizumab, 3 subsequently underwent allogeneic stem cell transplant and 1 underwent ASCT followed by allogeneic stem cell transplant. Of 12 patients who achieved PR then discontinued pembrolizumab, 4 subsequently underwent allogeneic SCT and 1 underwent ASCT.
Figure 1.
Kaplan-Meier analysis of DOR in the total population. NR, not reached.
Figure 2.
Time to response and DOR in the total population. Six patients died after the study because of malignant neoplasm progression (n = 4), veno-occlusive liver disease (n = 1), and an unknown cause (n = 1).
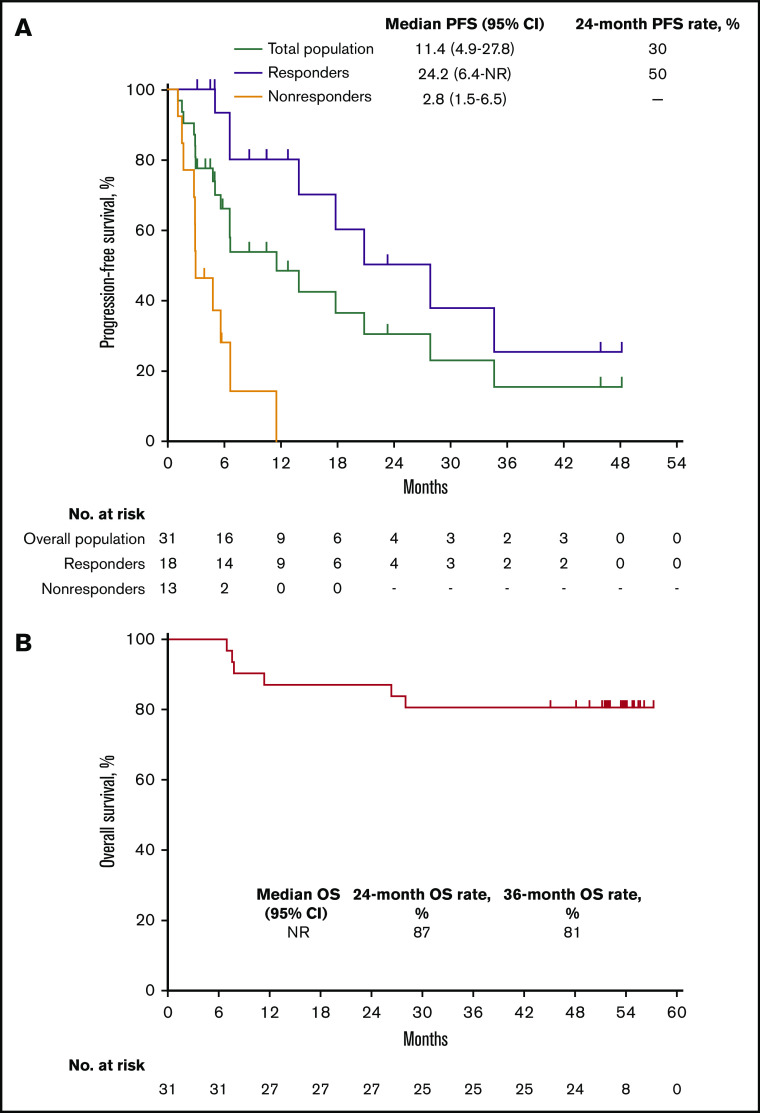
Median PFS in the total population was 11.4 months (95% CI, 4.9 to 27.8 months) and the 24-month PFS rate was 30% (Figure 3A). Median PFS was 24.2 months (95% CI, 6.4 months to not reached) among responders (24-month PFS rate 50%) and 2.8 months (95% CI, 1.5 to 6.5 months) among nonresponders. Median OS in the total population was not reached; 24- and 36-month OS rates were 87% and 81%, respectively (Figure 3B). Six deaths occurred, all in nonresponders (Figure 2).
Figure 3.
Kaplan-Meier analysis of PFS and OS in the total population. (A) PFS in the total population and by best response (responders vs nonresponders). (B) OS in the total population.
Discussion
Results of this long-term follow-up analysis of KEYNOTE-013 showed durable antitumor activity of pembrolizumab monotherapy in heavily pretreated patients with relapsed/refractory cHL. Phase 2 studies of PD-1 blockade that followed KEYNOTE-013 (for example, KEYNOTE-087 and CheckMate 205) had larger sample sizes and, therefore, more robust estimates of response rate and safety.5,7 Fortunately, those estimates are similar to those of KEYNOTE-013 (for example, ORR 58% in KEYNOTE-013 vs 72% in KEYNOTE-087, with 71% vs 73% incidence of treatment-related AEs).5 These similarities lend support to the hypothesis that the long-term results presented here reliably reflect the long-term benefit of pembrolizumab in this patient population, despite the smaller sample size of the phase 1 cohort.
One limitation of this study is that data on responses were censored at the date of last assessment prior to any stem-cell transplant (in the absence of PD). Subsequent data on continued duration of remission are not available. This limits our ability to estimate what the long-term durability of responses from pembrolizumab alone would have been in the entire population of responders. Because data on outcome after subsequent therapy were not collected in this study, we also cannot use this data set to examine the outcome of postpembrolizumab salvage therapy. Significant controversy remains regarding the optimal use and timing of allogeneic SCT after PD-1 blockade. Because transplantation in this study (as in all others) was performed at the discretion of treating clinicians, our data do not allow us to comment on this question or on the contribution of transplantation to long-term survival.
Another limitation is that the small patient numbers used for subgroup response evaluation and the resulting large CIs preclude robust conclusions; the differences noted here should be considered hypothesis generating only. Although this phase 1 study reports data on pembrolizumab monotherapy and thus cannot be directly compared with available data on BV efficacy and safety, the ongoing phase 3 KEYNOTE-204 study (NCT02684292) evaluates the safety and efficacy of pembrolizumab monotherapy compared with BV in relapsed/refractory cHL, and results from this study should shed light on the relative efficacy and safety of each drug.
Additionally, the dose used in this study is different from the currently used (and approved) dose. Although the optimal dose has been shown, after the design of this study, to be 200 mg every 3 weeks, studies have shown no significant exposure dependency for safety or efficacy with doses of 2 to 10 mg/kg, and weight-based pembrolizumab dosing provides equivalent exposure to the fixed-dose regimen of 200 mg every 3 weeks.10,11
Despite these limitations, the long-term results presented here may add important information about the durability of response of pembrolizumab monotherapy in patients with relapsed/refractory cHL. Among this population of heavily pretreated patients with cHL for whom BV therapy was ineffective, some maintained a long-term response with median DOR not reached after >4 years of follow-up. It will be important to understand the characteristics of patients who have such durable responses and who probably should not receive further therapy after remission with pembrolizumab or who would be good candidates to receive pembrolizumab earlier in the treatment course.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank the patients and their families, investigators, and site personnel. The authors also thank Mohammed Farooqui for study oversight (Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ). Medical writing and/or editorial assistance was provided by Cathy R. Winter and Matthew Grzywacz of the ApotheCom pembrolizumab team (Yardley, PA).
This work was supported by Merck Sharp & Dohme Corp. (a subsidiary of Merck & Co., Inc., Kenilworth, NJ). Writing assistance was funded by Merck Sharp & Dohme Corp. (a subsidiary of Merck & Co., Inc., Kenilworth, NJ). P.A. acknowledges the generous support of the Harold and Virginia Lash Foundation as well as the Leukemia and Lymphoma Society.
Footnotes
Merck & Co., Inc.'s data sharing policy, including restrictions, is available at http://engagezone.merck.com/ds_documentation.php. Requests for access to clinical study data can be submitted through the EngageZone site or via email to dataaccess@merck.com.
Authorship
Contribution: P.A., P.L.Z., and C.H.M. conceived, designed, and planned the study; P.A., J.K., J.-M.M., and V.R. acquired the data; Y.Z., A.N., P.M., and C.H.M. analyzed the data; P.A., J.K., J.-M.M., V.R., P.L.Z., A.N., P.M., and C.H.M. interpreted the results; P.A., J.K., J.-M.M., V.R., P.L.Z., Y.Z., A.N., P.M., and C.H.M. critically reviewed or revised the manuscript for important intellectual content; and V.R., A.N., P.M., and C.H.M. drafted the manuscript.
Conflict-of-interest disclosure: P.A. has received honoraria from Merck & Co., Inc., Bristol-Myers Squibb, Pfizer, Affimed, Adaptive, Infinity, ADC Therapeutics, Celgene, Morphosys, Daiichi Sankyo, Miltenyi, Tessa, GenMab, C4, and Enterome and has received research funding from Merck & Co., Inc., Bristol-Myers Squibb, Affimed, Adaptive, Roche, Tensha, Otsuka, Sigma Tau, Genentech, and IGM. J.K. has been a consultant for Merck & Co., Inc., and has received payment for lectures and speakers’ bureaus from Merck & Co., Inc. V.R. has been a consultant/advisor for Gilead, Infinity, Bristol-Myers Squibb, NanoString, and Incyte; has received research funding from ArgenX; has provided expert testimony for Servier; and has received travel, accommodations, and expenses from Roche and Bristol-Myers Squibb. P.L.Z. has served on speakers’ bureaus for Verastem, Celltrion, Gilead, Janssen-Cilag, Bristol-Myers Squibb, Servier, Sandoz, Merck & Co., Inc., Immune Design, Celgene, Portola, Roche, Eusapharma, Kyowa Kirin, and Sanofi; has served on advisory boards for Verastem, Celltrion, Gilead, Janssen-Cilag, Bristol-Myers Squibb, Servier, Merck & Co., Inc., Immune Design, Celgene, Portola, Roche, Eusapharma, Kyowa Kirin, and Sanofi; and has been a consultant for Verastem, Merck & Co., Inc., Eusapharma, and Sanofi. Y.Z. and A.N. are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ. P.M. is an employee and stockholder of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, and has received travel expenses from Merck & Co., Inc. C.H.M. has held an employment/leadership position/advisory role for Celgene, Genentech, Merck & Co., Seattle Genetics Inc., participated in an advisory board for Molecular Templates, and has received research funding from Pharmacyclics, Genentech, Merck & Co., Inc., and Seattle Genetics Inc. J.-M.M. declares no competing financial interests.
Correspondence: Philippe Armand, Dana-Farber Cancer Institute, 459 Brookline Ave, Boston, MA 02215; e-mail: philippe_armand@dfci.harvard.edu or parmand@partners.org.
References
- 1.Shanbhag S, Ambinder R. Hodgkin lymphoma: a review and update on recent progress. CA Cancer J Clin. 2018;68(2):116-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elshenawy M, Shahzad Rauf M, Elhassan T, Maghfoor I, Akhtar S. Survival analysis of patients with Hodgkin lymphoma who failed high dose chemotherapy and autologous stem cell transplant. Ann Hematol. 2018;97(7):1229-1240. [DOI] [PubMed] [Google Scholar]
- 3.Chen R, Zinzani P, Fanale M, et al. ; KEYNOTE-087 . Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic Hodgkin lymphoma. J Clin Oncol. 2017;35(19):2125-2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armand P, Shipp M, Ribrag V, et al. Programmed death-1 blockade with pembrolizumab in patients with classical Hodgkin lymphoma after brentuximab vedotin failure. J Clin Oncol. 2016;34(31):3733-3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen R, Zinzani P, Lee H, et al. Pembrolizumab in relapsed or refractory Hodgkin lymphoma: 2-year follow-up of KEYNOTE-087. Blood. 2019;134(14):1144-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.KEYTRUDA (pembrolizumab) for injection, for intravenous use [package insert 1/2020]. Whitehouse Station, NJ: Merck Sharp & Dohme Corp.
- 7.Armand P, Engert A, Younes A, et al. Nivolumab for relapsed/refractory classic Hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: extended follow-up of the multicohort single-arm phase II CheckMate 205 trial. J Clin Oncol. 2018;36(14):1428-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song Y, Wu J, Chen X, et al. A single-arm, multicenter, phase II study of camrelizumab in relapsed or refractory classical Hodgkin lymphoma. Clin Cancer Res. 2019;25(24):7363-7369. [DOI] [PubMed] [Google Scholar]
- 9.Shi Y, Su H, Song Y, et al. Safety and activity of sintilimab in patients with relapsed or refractory classical Hodgkin lymphoma (ORIENT-1): a multicentre, single-arm, phase 2 trial. Lancet Haematol. 2019;6(1):e12-e19. [DOI] [PubMed] [Google Scholar]
- 10.Freshwater T, Kondic A, Ahamadi M, et al. Evaluation of dosing strategy for pembrolizumab for oncology indications. J Immunother Cancer. 2017;5(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chatterjee M, Turner D, Felip E, et al. Systematic evaluation of pembrolizumab dosing in patients with advanced non-small-cell lung cancer. Ann Oncol. 2016;27(7):1291-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheson B, Pfistner B, Juweid M, et al. ; International Harmonization Project on Lymphoma . Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579-586. [DOI] [PubMed] [Google Scholar]
- 13.National Institutes of Health . National Cancer Institute. Common terminology criteria for adverse events (CTCAE) version 4.0. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. Accessed 3 December 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.