Abstract
Objective: The study evaluated the nurse-led intervention “Community Health Consultation Offices for Seniors (CHCO)” on health-related and care needs–related outcomes in community-dwelling older people (⩾60 years). Method: With a quasi-experimental design, the CHCO intervention was evaluated on health-related and care needs–related outcomes after 1-year follow-up. Older people who received the intervention were frail, overweight, or were smoking. The comparison group received care as usual. In both groups, similar data were collected on health status, falls and fractures, and care needs. In the intervention group, additional data were collected on biometric measures and health-related behavior. Results: The intervention group and the care-as-usual group included 403 seniors and 984 seniors, respectively. Health-related outcomes, behaviors, and biometric measures, remained stable. After 1 year, care needs increased for both groups, but at a lower rate for the care-as-usual group. Discussion: The CHCO intervention showed no significant improvement on health-related outcomes or stability in care needs–related outcomes.
Keywords: frailty, geriatric assessment, nursing, health behaviors, interventions
Background
The global increase in the number of older people and the accompanying increase of chronic conditions underline the necessity of health promotion and preventive intervention in high-risk populations (Anstey, von Sanden, Salim, & O’Kearney, 2007; Booth, Roberts, & Laye, 2012; Hagger-Johnson et al., 2013; van Baak & Visscher, 2006). Research has shown that unhealthy behaviors such as lack of exercise, smoking, excessive alcohol consumption, and poor nutrition are the main factors contributing to the development of chronic diseases, functional decline, and frailty (Hubbard, Lang, Llewellyn, & Rockwood, 2010; Hubbard, Searle, Mitnitski, & Rockwood, 2009; Kojima, Iliffe, & Walters, 2015; Peters, Boter, Burgerhof, Slaets, & Buskens, 2015; Whitlock, Orleans, Pender, & Allan, 2002). For example, physical inactivity is an important cause of most chronic diseases (Booth et al., 2012) and a poor diet increases the risks of cancers and cardiovascular diseases (Reedy et al., 2014). Also, unhealthy behaviors such as smoking combined with alcohol abuse led to a 36% faster cognitive decline in those individuals compared with nonsmokers who were moderate drinkers, over a 10-year follow-up (Hagger-Johnson et al., 2013). Even so, frail, community-dwelling older people are more likely to experience future falls, and falls are associated with negative consequences such as disabilities and even mortality (Kojima, 2015).
Interventions can be divided into population-based interventions targeting either the general community or specific high-risk groups (i.e., frail older people) (Boult, Pualwan, Fox, Pacala, & Management, 1998; Rose, 1992). The reason for targeting high-risk groups is the high level of motivation of these older people to change their behavior, because they are likely to be aware of their increased risks of adverse health outcomes (Rose, 1992). Concepts of frailty and case complexity have been introduced to identify individuals at risk of becoming dependent, and those in need of better care (Bergman et al., 2007; Peters, Boter, Buskens, & Slaets, 2012; Peters, Boter, Slaets, & Buskens, 2013). Frailty reflects dependence in daily activities due to the losses and interplay between physical, psychological, social, cognitive, and environmental factors (Bergman et al., 2007; Peters et al., 2012; Peters et al., 2013; Peters, Burgerhof, et al., 2015; Schuurmans, Steverink, Lindenberg, Frieswijk, & Slaets, 2004; Walston et al., 2006). Thus, combining interventions that detect frailty in an early stage and promote health-related behavior seem promising to prevent falls, decrease morbidity, and improve quality of life in community-dwelling older people (Ng et al., 2015; Slaets, 2006; Stegeman, Kraaijenhagen, & Bossuyt, 2009; Vina, Rodriguez-Manas, Salvador-Pascual, Tarazona-Santabalbina, & Gomez-Cabrera, 2016). Case complexity in older people highlights the progression in care needs based on the past, the present, and the future needs in biopsychosocial domains of older people themselves (Eissens van der Laan, van Offenbeek, Broekhuis, & Slaets, 2014; Peters et al., 2013). A higher risk of care needs indicates a need of more intensive interdisciplinary care (Peters, Boter, et al., 2015). The combination of both frailty and case complexity to segment older people’s health profiles (i.e., vital, psychosocial coping, physical and mobility, and multidomain) has been shown to facilitate preventive interventions tailored to the needs of individuals with a different health profile (Eissens van der Laan et al., 2014).
Nurses have a prominent role in delivering health promotion and preventive interventions to older people (Goodman, Davies, Dinan, See Tai, & Iliffe, 2011; Kemppainen, Tossavainen, & Turunen, 2013). This role is evident because (a) nurses use systematic assessments to facilitate early recognition of complaints experienced by older people (Borglin, Jakobsson, Edberg, & Hallberg, 2005; Kemppainen et al., 2013), (b) nurses use a comprehensive approach matching the multiple complaints experienced by older people (Borglin et al., 2005), (c) nurses often work in multidisciplinary teams and refer to other health professionals if needed (Iliffe, 2016; Kemppainen et al., 2013), and (d) nurses are able to build personal relationships with clients based on trust (Hupcey & Miller, 2006). Thus, nurses are the preferred care professionals to offer health promotion and preventive interventions tailored to the needs of community-living older people (Kemppainen et al., 2013). However, little is known about the effectivity of nurse-led health promotion and preventive interventions in frail community-dwelling older people that also contribute to health-related behavior in the long term (Walters, Reijneveld, van der Meulen, Dijkstra, & de Winter, 2017).
Therefore, this study will evaluate a nurse-led health promotion and preventive intervention called “Community Health Consultation Offices for Seniors (CHCO)” (in Dutch Consultatiebureau voor Ouderen). This intervention targeted community-dwelling older people with increased risk of frailty and/or unhealthy behavior patterns. The aim of the present study was to evaluate the nurse-led intervention on the following outcomes:
health-related outcomes: (a) self-reported health status, (b) falls and fractures, (c) biometric measures (blood pressure, blood glucose, height, weight, and waist circumference), and (c) health-related behavior (smoking behavior, alcohol consumption, balanced diet, and physical activity); and
care needs–related outcomes: levels of dependency and progression of care needs as measured by transitions in the segmentation of the health profiles (vital, psychosocial coping, physical and mobility, and multidomain).
Method
Study Design
Using a quasi-experimental design, we evaluated the CHCO intervention in community-dwelling older people of 60 years and older in the northern regions of the Netherlands in the period 2011 to 2013. We included a care-as-usual group of community-dwelling older people who participated in an observational study, the National Program Elderly Care (NPEC, number 60-61,900-98-218) conducted in similar Dutch regions (Eissens van der Laan et al., 2014; Peters, Burgerhof, et al., 2015).
Study data were obtained at baseline and at a 1-year follow-up in the intervention and care-as-usual group. The ethics review board of the University Medical Center Groningen provided a waiver for the CHCO intervention study. Ethical committee approval for the longitudinal observational nonintrusive study is not required under Dutch legislation. Written informed consent was obtained from all older people who participated either in the intervention group or in the care-as-usual group.
Intervention Group
The CHCO intervention used the active aging model (World Health Organization [WHO], 2002), the life course perspective (Braveman & Barclay, 2009; Mayer, 2009), and the transtheoretical model (Prochaska & DiClemente, 1983) as a frame of reference targeting community-dwelling older people to promote healthy aging (Bakker, Jaspers, Kraakman, & Visser, 2008).
Older people received an informational letter about the intervention if they were members of the participating home care association and insured by the largest health insurer company in the region, enabling them to participate in the intervention without a financial contribution. To select frail older people, a postal questionnaire, including the Groningen Frailty Indicator (GFI), items about smoking, and anthropometric measures (i.e., weight and height to calculate body mass index [BMI]), was added to the information letter. After completion of the questionnaire, the older people returned it by regular post to the home care organization. Older people were considered at risk and, therefore, eligible to receive the CHCO intervention if they complied with at least one of the following inclusion criteria (Kojima et al., 2015; Ng et al., 2015; Peters, Boter, et al., 2015; Stegeman et al., 2009): (a) frailty (GFI > 3), (b) overweight (<70 years, BMI > 25 kg/m² and/or >70 years, BMI > 30 kg/m²), and (c) smoking.
Those eligible for participation received an invitation for a nurse-led consult at a consultation office in the area where they lived, or the community health nurse visited their homes if necessary. The community health nurse performed a comprehensive assessment of the health and well-being of the participating senior, offered tailored advice, and referred to other health professionals if needed. Also, nurses informed the local general practitioners (GPs) about the consultations. See Figure 1 for detailed information on the intervention.
Figure 1.
Phases of the intervention.
All involved community health nurses (N = 48, registered nurses) were offered training specifically to implement the intervention. This training consisted of the following elements: (a) general outline of the intervention (including knowledge about the theoretical framework, instructions on measurements, and protocols, (b) a one-time practice observation exchange, (c) 1-hr consultation with a dietician, and (d) a 2-hr workshop in motivational interviewing. All nurses attended an annual meeting to receive a 4-hr educational and intervision session.
Motivational interviewing is a conversation style aimed at partnership to search for the individuals’ own conviction for the need to change and the development of confidence to make a change (Miller & Rollnick, 2002). Using motivational interviewing skills and applying the transtheoretical model provides a useful strategy for guiding the change of unhealthy lifestyles (Noordman, de Vet, van der Weijden, & van Dulmen, 2013).
Care-as-Usual Group
The care-as-usual group was identified with the assistance of 25 health care organizations (e.g., hospitals, welfare organizations, homes for the elderly) and associations for the elderly (Peters, Burgerhof, et al., 2015). Older people were excluded if they were severely cognitively impaired or terminally ill. Older people were included if they were living independently. In the Netherlands, care as usual is mainly provided by the GP, who is a gatekeeper to hospital and specialist care. Also, referrals are registered by the GP. For specific details about the data collection in the observational study, we refer to previously published work (Eissens van der Laan et al., 2014; Peters, Burgerhof, et al., 2015).
Data Collection and Measures
Persons of the intervention and care-as-usual group completed similar postal questionnaires at baseline and at 1-year follow-up. Data of demographics (i.e., age, sex, and education level), physical health (i.e., presence of chronic diseases), self-reported health status (i.e., a five-level Likert-type item varied from excellent to poor), and falls and fractures in the previous year were collected. Frailty and case complexity were assessed with the GFI and INTERMED (a biopsychosocial assessment and classification system for case complexity) for the Elderly Self-Assessment (IM-E-SA), respectively.
The GFI consists of 15 items and assesses frailty from a multidimensional perspective because physical, cognitive, psychological, and social domains are included (Peters et al., 2012; Steverink, Westerhof, Bode, & Dittmann-Kohli, 2001). The theoretical range of this instrument is 0 to 15; a higher score indicated a higher level of frailty (Peters et al., 2012; Schuurmans et al., 2004; Steverink et al., 2001).
The current level of case complexity was assessed with items of the IM-E-SA in the physical, psychological, social, and health care domain (Peters et al., 2013). For the four items, a score between 0 and 3 can be filled out, and the items summed up to a theoretical range 0 to 12. Higher scores of the IM-E-SA indicated higher levels of case complexity (Peters et al., 2013). Both the GFI and IM-E-SA were evaluated with good psychometric properties in community-dwelling older people (Peters et al., 2012; Peters et al., 2013; Peters, Boter, et al., 2015).
In the intervention group, additional data were collected on self-reported lifestyle behaviors. Hazardous drinking was measured by the Alcohol Use Disorders Identification Test (AUDIT) alcohol consumption questions (AUDIT-C), a three-item alcohol screening instrument to identify persons who are hazardous drinkers or have active alcohol use disorders. It uses a scale of 0 to 12, with each question containing five answer options. In men, a score of 4 or higher, and in women, a score of 3 or higher indicate hazardous drinkers or having active alcohol use disorders (Bush et al., 1998). Balanced diet was measured by showing older people “the Wheel of Five,” a practical and well-known tool used by The Netherlands Nutrition Center. In 2011, “the Wheel of Five” consisted of the following elements: (a) fruits and vegetables, (b) grains/wheat/rice/legumes, (c) meat/fish/nuts/eggs/milk/dairy products, (d) healthy fats/oils, and (e) adequate liquids such as water/tea/coffee. A balanced diet consists of daily intake of each of these elements and provides the body with all the nutrients that it needs (Voedingscentrum, 2011). Older people were asked how many days per week they used all presented elements of “the Wheel of Five,” accompanied by a picture presenting all five elements. Physical activity included all sorts of activities that increase heart rate, such as working in the garden, cycling, walking, and other sports. Older people were asked how many days per week they performed such activities and, subsequently, the duration of these activities, resulting in a score of physical activity in minutes per week. Moreover, the nurse performed biometric measurements during the nurse-led consultations at baseline and 1-year follow-up. These included weight and height to calculate BMI as body weight divided by height squared, waist circumference, blood pressure, and blood glucose. The cutoff values for these biometric measures were as follows: (a) underweight (BMI < 19), (b) overweight (<70 years, BMI ⩾ 25, and/or waist circumference [women] = 88 cm, waist circumference [men] = 102 cm; or ⩾70 years, BMI ⩾ 30, and/or waist circumference [women] = 88 cm, waist circumference [men] = 102 cm), (c) hypertension (<75 years, systolic blood pressure ⩾ 140 mmHg; 75-80 years, systolic blood pressure ⩾ 160 mmHg; ⩾80 years, systolic blood pressure ⩾ 160 mmHg), and (d) hyperglycemia (fasting or nonfasting ⩾6.1 mmol/L).
Power Calculation
One goal of the proposed study is to test the hypothesis that the proportion of getting a worse health profile in the intervention group is lower than in the care-as-usual group: 0.10 versus 0.20. The criterion for significance (alpha) has been set at .05. Therefore, we calculated for 400 older people in the intervention group and 800 older people in the care-as-usual group, resulting in a power of >.80.
Statistical Analyses
Statistical differences on baseline characteristics between the older people who received the CHCO intervention at baseline and those who were lost to follow-up were calculated with Mann–Whitney tests and chi-square tests, and were appropriate. Baseline and follow-up characteristics of the older people in the intervention group and the care-as-usual group were reported. Statistical differences between both groups with respect to demographic characteristics, physical morbidity, GFI score, IM-E-SA score, health profiles, and self-reported health status were assessed with chi-square and Mann–Whitney tests where appropriate.
Next, based on scores of the GFI and IM-E-SA, a confirmatory factor analysis and latent class analysis were performed, resulting in a segmentation of the older people in four health profiles: vital, difficulties with psychosocial domains, physical and mobility complaints, and difficulties experienced in multiple domains (Eissens van der Laan et al., 2014). We calculated whether older people started transition toward a worse health profile between baseline and follow-up. We hypothesized fewer transitions toward a lower health profile, or stability in health profiles in the intervention group than in the care-as-usual group. Next, a multivariate logistic regression analysis was performed to assess the effect of the intervention on transitioning toward a worse health profile between baseline and 1-year follow-up. Results were adjusted for gender, age, physical morbidity, and psychological morbidity at baseline, and associations were expressed with odds ratios (ORs) and 95% confidence intervals (95% CIs). Statistical analyses were performed using SPSS Statistics 22.0 (Corp, 2013) and Latent Gold 4.5 (Vermunt & Magidson, 2008).
Results
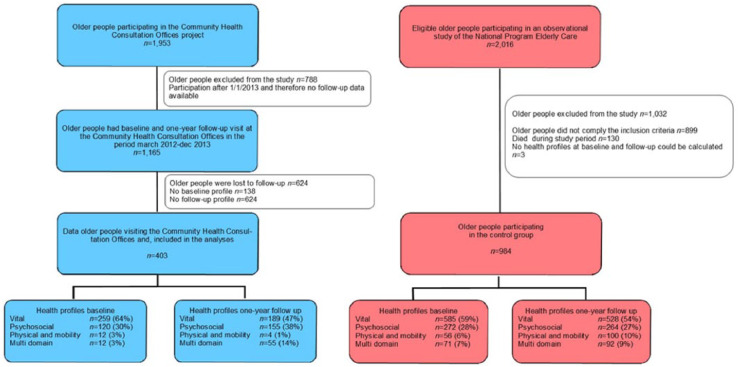
A total of 1,953 older people returned the postal questionnaire and, based on the inclusion criteria, they were invited for a first CHCO consultation. Whereas 2,016 older people were eligible to participate in the care-as-usual group (see Figure 2). In the intervention group, 403 older people were included, and in the care-as-usual group, 984 older people were included.
Figure 2.
Flowchart of the intervention group and care-as-usual group.
In the intervention group, 788 older people were excluded when they had a first CHCO consultation visit after January 2013. About 1,165 older people participated in the CHCO intervention in the period March 2012 until December 2013. However, from 138 older people, no baseline health profiles could be calculated, and from 624 older people, no data were collected at 1-year follow-up and, therefore, those older people were also excluded from the analyses. Eventually, data of 403 older people were included in the statistical analyses. In the care-as-usual group, 1,032 older people were excluded from the study for the following reasons: (a) older people did not comply with the inclusion criteria (n = 899), (b) no health profiles at baseline and follow-up could be calculated (n = 3), and (c) older people died during study period (n = 130).
Baseline Characteristics
At baseline, 1,165 older people attended a CHCO visit. Between the older people with no health profiles at follow-up (n = 624) and those who had calculated health profiles at 1-year follow-up (n = 403) showed no statistical differences on age, gender, education, and morbidity. However, older people who were lost to follow-up had at baseline a statistically significantly higher GFI score than the older people who had a follow-up CHCO visit, the median scores were 4 (interquartile range [IQR] = 2-6) versus 3 (IQR = 2-5), p = .03 respectively.
The intervention group and care-as-usual group had similar characteristics on gender and physical morbidity. The median age for the intervention and care-as-usual group was 73 (IQR = 67-78) and 75 (IQR = 71-80), respectively (Table 1). Compared with the care-as-usual group, the intervention group age was statistically significantly lower, whereas education was statistically significantly higher (p ⩽ .001). In the intervention group, 62% (n = 251) were considered frail, 68% (n = 275) were considered overweighed, and 12% (n = 49) were currently smoking. Older people of the intervention group had significantly higher scores on the GFI (median 3, IQR = 2-5), compared with the care-as-usual group (median 2, IQR = 1-4). In the intervention group as well as in the care-as-usual group, the majority of the older people were segmented into the vital health profile, 64% and 59%, respectively.
Table 1.
Baseline Characteristics of the Intervention Group and Care-as-Usual Group.
| Intervention group CHCO (N = 403) |
Care-as-usual group NPEC (N = 984) |
Statistical differences between CHCO and NPEC | |
|---|---|---|---|
| N (%) | N (%) | p | |
| Gender (% female) | 244 (61) | 541 (55) | .07 |
| Median age in years (IQR) | 73 (67-78) | 75 (71-80) | ⩽.001 |
| Education level (%) | ⩽.001 | ||
| None/primary school | 89 (22) | 339 (35) | |
| Secondary school | 263 (65) | 543 (55) | |
| Higher education | 34 (9) | 95 (10) | |
| Unknown | 17 (4) | ||
| Physical morbidity | .24 | ||
| No chronic disease | 143 (36) | 360 (37) | |
| One chronic disease | 154 (38) | 330 (33) | |
| ⩾ 2 chronic diseases | 106 (26) | 294 (30) | |
| Measures (median, IQR) | |||
| Frailty (GFI) | 3 (2-5) | 2 (1-4) | ⩽.01 |
| Case complexity current (IM-E-SA) | 2 (1-3) | 2 (1-4) | .004 |
Note. IM-E-SA = a case complexity measure. CHCO = Community Health Consultation Offices for Seniors; NPEC = National Program Elderly Care; IQR = interquartile range; GFI = Groningen Frailty Indicator; IM-E-SA = INTERMED for the Elderly Self-Assessment.
Health-Related Outcomes
Within the intervention group, an increase of 6% of older people, who rated their health status as “good,” was found between baseline and 1-year follow-up. Whereas in the care-as-usual group, a decrease of 4% of older people who rated their health as “good” was found between baseline and 1-year follow-up. However, these changes were not statistically significant (Table 2). No significant changes in falls were recorded in the intervention group and care-as-usual group between baseline and 1-year follow-up. A decrease in fractures in the previous year was recorded in the care-as-usual group (4% fewer fractures), not in the intervention group. At baseline, the characteristics of the older people in the intervention group showed relatively high rates on unhealthy behaviors and risk factors. Significant changes in health-related behaviors (smoking, alcohol consumption, balanced diet, and physical activity) between baseline and follow-up were not observed in the intervention group. The prevalence of hypertension, hyperglycemia, and overweight remained stable in the intervention group during the 1-year follow-up period.
Table 2.
Characteristics of the Intervention Group and Care-as-Usual Group Regarding Health Profiles, Health Status, Falls and Fractures, Biometric Measures, and Health-Related Behavior at Baseline and 1-Year Follow-Up.
| Intervention group Community Health Consultation Offices N = 403 |
p | Care-as-usual group National Program Elderly Care N = 984 |
p | |||
|---|---|---|---|---|---|---|
| Baseline N (%) |
1-year follow-up N (%) |
Baseline N (%) |
1-year follow-up N (%) |
|||
| Health profiles | ⩽.001 | ⩽.001 | ||||
| Vital | 259 (64) | 189 (47) | 585 (59) | 528 (54) | ||
| Psychosocial coping | 120 (30) | 155 (38) | 272 (28) | 264 (27) | ||
| Physical and mobility | 12 (3) | 4 (1) | 56 (6) | 100 (10) | ||
| Multidomain | 12 (3) | 55 (14) | 71 (7) | 92 (9) | ||
| Self-reported health status (N, %) | .19 | .14 | ||||
| Excellent | 9 (2) | 4 (1) | 63 (6) | 42 (4) | ||
| Very good | 32 (8) | 33 (8) | 107 (11) | 106 (11) | ||
| Good | 240 (60) | 266 (66) | 381 (39) | 346 (35) | ||
| Fair | 115 (28) | 93 (23) | 382 (39) | 388 (39) | ||
| Poor | 3 (1) | 5 (1) | 47 (5) | 60 (6) | ||
| Unknown | 4 (1) | 2 (1) | 7 (1) | 45 (5) | ||
| Falls and fractures | ||||||
| Fall previous year | 102 (25) | 103 (26) | .89 | 93 (10) | 84 (9) | .42 |
| Fracture previous year | 69 (17) | 76 (19) | .53 | 128 (13) | 92 (9) | .01 |
| Biometric measures | ||||||
| Hypertensiona | 169 (42) | 157 (39) | .72 | —b | — | — |
| Hyperglycemiac | 193 (48) | 201 (50) | ||||
| Weightd | ||||||
| Underweight | 5 (1) | 4 (1) | — | — | ||
| Normal weight | 123 (31) | 130 (32) | — | — | ||
| Overweight | 275 (68) | 269 (67) | ||||
| Health-related behavior | ||||||
| Hazardous drinkinge | 327 (81) | 322 (80) | .38 | — | — | |
| Current smoking | 49 (12) | 44 (11) | .81 | — | — | |
| Balanced diet | .78 | |||||
| 1-4 days/week | 22 (5) | 16 (4) | — | — | ||
| ⩾5 days/week | 378 (94) | 385 (95) | — | — | ||
| Unknown | 3 (1) | 2 (1) | — | — | ||
| Physical activity | .91 | |||||
| ⩽70 min/week | 247 (61) | 248 (61) | — | — | ||
| 71-149 min/week | 84 (21) | 91 (23) | — | — | ||
| 150 min/week | 53 (13) | 54 (13) | — | — | ||
| Unknown | 19 (5) | 10 (3) | — | — | ||
Note. BMI = body mass index. AUDIT = Alcohol Use Disorders Identification Test.
Hypertension: <75 years, systolic blood pressure ⩾ 140 mmHg; 75-80 years, systolic blood pressure ⩾ 160 mmHg; ⩾80 years, systolic blood pressure ⩾ 160 mmHg.
Not applicable because these data were not collected for the care-as-usual group.
Hyperglycemia: fasting or nonfasting ⩾ 6.1 mmol/L.
Underweight: BMI < 19. Overweight <70 years: BMI ⩾ 25 and/or waist circumference (women) = 88 cm or waist circumference (men) = 102 cm; or Overweight ⩾70 years: BMI ⩾ 30 and/or waist circumference (women) = 88 cm or waist circumference (men) = 102 cm.
Hazardous drinking if the total score on the AUDIT alcohol consumption questions was for females ⩾4 and for males ⩾5.
Care Needs–Related Outcomes
Within the intervention group, 17% of the older people started transition to a worse health profile between baseline and 1-year follow-up, whereas in the care-as-usual group, 5% older people started transition to a worse health profile. The multivariate logistic regression model showed that, from all predictors, physical morbidity was the strongest predictor of starting a transition toward a worse health profile, adjusted OR = 1.73, 95% CI = [1.30, 2.30]. The population predictor showed that, compared with the care-as-usual group, the intervention group had a 1.36 higher odds of a transition to a worse health segment in the 1-year follow-up (Table 3).
Table 3.
Multivariate Logistic Regression Analyses to Assess Whether the Intervention Group Differs From the Care-as-Usual Group Regarding Worse Segment Based at 1-Year Follow-Up.
| Predictors | Worse segmenta | |
|---|---|---|
| OR [95%CI] | p | |
| Populationb | 1.36 [1.01, 1.83] | .04 |
| Genderc | 1.14 [0.87, 1.49] | .34 |
| Age (in years) | 1.04 [1.02, 1.06] | ⩽.001 |
| Education leveld | 0.86 [0.69, 1.08] | .20 |
| Physical morbiditye | 1.73 [1.30, 2.30] | ⩽.001 |
| Psychological morbidityf | 1.06 [0.97, 1.16] | .22 |
Note. CHCO = Community Health Consultation Offices for Seniors; NPEC = National Program Elderly Care; CI = confidence interval.
Segment of follow-up period minus segment of baseline, afterward dichotomized into 0 = better or stable segment, 1 = worsen segment.
Population: 0 is NPEC care-as-usual group, 1 is CHCO-intervention group.
Gender: 0 is male, 1 is female.
Education level: 0 is none or primary school, 1 is secondary or higher education.
Physical morbidity 0 is no physical morbidity, 1 is at least one chronic morbidity.
Psychological morbidity: 0 is no psychological morbidity, 1 is at least one experienced psychosocial problem as measured with items of the Groningen Frailty Indicator.
Discussion
The present study did not show any significant increase in health-related outcomes (self-reported health status, falls and fractures, biometric measures, and health-related behavior) or stability in care needs–related outcomes (transitions in health profiles) in community-dwelling older people, who participated in the CHCO intervention. Another important finding was that the selection procedure of this study showed to be highly functional in terms of selecting high-risk frail community-dwelling older people who could benefit from health promotion programs.
It was unexpected that an increased self-reported health status by participants in the intervention, although not statistically significant, was not accompanied by increases in health-related outcomes, falls and fractures, biometric measures, and health-related behavior. A possible explanation for this might be that this increase is partly explained by the effect of screening, in which it is assumed that people seek reassurance that they have no unusual problems (Rose, 1992). Interestingly, even at 1-year follow-up, the majority of the CHCO intervention group scored their self-reported health status as “good.” This seems contradicting to other outcomes that showed that a substantial part of these older people are living with high risk factors such as hypertension or suffering from at least one chronic condition. Moreover, 17% of the intervention group started transition toward a worse health profile between baseline and 1-year follow-up. In accordance with the present results, a prospective cohort study, on the comparison of the Rowe–Kahn model of successful aging with self-rated health, demonstrated that many older people reported that they were content with their health while living with disease or disability (Whitley, Popham, & Benzeval, 2016). Another explanation of this rather contradictory result may be that the older people in the CHCO intervention group did not experience inconvenience or substantial distresses related to their daily functioning, yet. Even though they were selected because of certain risk factors, they might not have experienced these risks themselves. This could mean that many older people in the CHCO intervention group were in the precontemplation stage (i.e., no intention of changing health-related behavior and the person is unaware that a problem exists) when they participated in the first consultation with the nurse (Norcross, Krebs, & Prochaska, 2011). In the many cases that participants did not experience a need to change their behavior, nurses were highly challenged to use motivational interviewing skills to facilitate changes in older people from precontemplation to the contemplation stage (raising awareness), or even other stages of change in one consultation (Noordman et al., 2013; Norcross et al., 2011; Prochaska & DiClemente, 1983). Nurses received only a 2-hr workshop to develop motivational interviewing skills, previous studies have noted that it is quite challenging for nurses to apply motivational interviewing in daily practice for behavior change (Noordman et al., 2012). Also, it is challenging to come to active modification of behavior in the participating older people, even more, because behavior has long been entrenched in a majority of older people (Noordman et al., 2013; Norcross et al., 2011).
As the results of the current study did not show improvements in health-related outcomes or stability in care needs–related outcomes, we might consider that the CHCO intervention was too brief. The CHCO intervention consisted of only two/three consultation moments between the older person and the community nurse a year. For example, the Diabetes Prevention Study shows that future diabetes incidence was reduced among persons at increased risk, and receiving a comprehensive intervention, which included seven consultations a year with a health nutritionist (Tuomilehto et al., 2001). Another preventive intervention for older people, where they combined one preventive home visit by a health professional with four weekly senior group meetings, postponed progression of morbidity up to 2 years (Behm et al., 2014). The group meetings also included peer education (focusing on the aging process and approaches to solve problems in the home environment), giving older people the opportunity to learn from each other, as they are seen as reliable sources of information to the older people (Behm et al., 2014; Shiner, 1999). In addition, peer coaching groups (a face-to-face intervention given by an older person and sharing similarities with other older people in the group) are promising to positively influence health behavior (Ginis, Nigg, & Smith, 2013; Joseph, Griffin, Hall, & Sullivan, 2001). For example, a peer coaching initiative with older people in the Netherlands is the Free Wheel club, which shows an increase in daily physical activity in older people (van de Vijver, Wielens, Slaets, & van Bodegom, 2018).
Another explanation for not finding improvements or stability in health- and care-related outcomes might be that the Netherlands has a strong primary health care system (Looman, Fabbricotti, de Kuyper, & Huijsman, 2016). Because basic health insurance is obligatory in the Netherlands, all inhabitants have free access to almost all primary and secondary care, providing support to older people (Schafer et al., 2010). Possibly, the CHCO intervention did not provide extra care benefits compared with older people living in the community who receive care as usual, in which care is mainly provided by the GP who acts as a gatekeeper for specialized medical care (Erler et al., 2011). Moreover, because health profiles reflect a broad range of physical, psychological, social, cognitive, environmental, and care needs (Eissens van der Laan et al., 2014; Peters, Boter, et al., 2015; Steverink, Slaets, Schuurmans, & Van Lis, 2001), and to establish stability or improvements in health profiles, a more integrated approach and intensive interdisciplinary care is needed (Peters, Boter, et al., 2015; Peters, Burgerhof, et al., 2015).
Finally, other studies on supporting frail community-dwelling older people to age healthily did not show major effects, and evidence for effective interventions with significant health outcomes for frail community-dwelling older people is scarce (Beswick, Gooberman-Hill, Smith, Wylde, & Ebrahim, 2010; Smith, Wallace, O’Dowd, & Fortin, 2016). Therefore, it could be argued that other evaluation measurements or study designs are desirable to understand the complexity of these interventions, and of the context in which these frail older people function (Hopman et al., 2016).
Strengths
A strength of the present study is that it included a comprehensive (biopsychosocial) approach using validated instruments such as the GFI and IM-E-SA. Also, the present study included measurements at baseline and follow-up in a real-life setting of a large group of community-dwelling older people in the Netherlands, and relate many of these findings to those of a comparison group. Finally, the results of this study have the potential to improve care provision in daily practice because the involved home care organizations and health care professionals are able to adapt their policy and care protocols.
Limitations
The quasi-experimental design of our study has some limitations. First and foremost, our study lacks random assignment. Also, the care-as-usual group was compiled after the start of the study and, therefore, no data on health-related behavior (alcohol consumption, smoking, diet, and physical activity) and biometric measurements were collected. Second, we did not blind the study population. Nurses have not been blinded, indeed, as they were aware that they were trained for the implementation of the intervention. Not blinding the nurses could have caused detection bias and, this could have led to too positive estimates on our outcome measures. Also, we used mostly self-reported questionnaires, which could generate socially desirable responding in relation to certain behaviors (Paulhus, 2002). For example, older people scored very high on keeping a balanced diet in the present study, and these data must be interpreted with caution. We did add biometric measurements to the intervention population to control for detection bias. Third, we were not able to collect data on reasons for loss to follow-up in the study. It is clear that this kind of selection can lead to biased results. If so, the expected direction of bias due to the loss of follow-up could be in favor of a positive effect of the intervention. However, due to the lack of an effect of the CHCO intervention, it seems unlikely that the results were affected by selection bias. Taking all these circumstances into account, it is unlikely that the conclusions of this study are biased. In the fourth place, the intervention included a comprehensive health assessment, but there are some important health issues that were not included, such as an oral health assessment. Recent studies show that older people with remaining teeth scored significantly better on frailty, quality of life, physical functioning, and general health (Hoeksema et al., 2017; Hoeksema et al., 2018). Therefore, dentists as well as nurses should support older people in maintaining a good oral health status (Hoeksema et al., 2018).
Recommendations
In clinical practice, using the health profiles as a starting point in the nurse consultations could support nurses to (a) identify in which domain older people need more support based on their experienced needs and contextual information, (b) discuss personal preferences during the consultation and check present priorities of the older people, and (c) refer to the best suitable intervention or (health) professional, in cooperation with the GP (Eissens van der Laan et al., 2014; Stuck et al., 2015).
The present study looked at health- and care-related outcomes to evaluate the CHCO intervention; however, several questions remain unanswered at present. To develop a full picture of the CHCO intervention, additional studies will be needed to enhance our understanding of the “black box” (Smit et al., 2018) such as (a) understanding the experiences and perceptions of older people participating in the CHCO intervention, (b) evaluating the motivational interviewing skills of community health nurses, and (c) understanding the organizational culture in which the CHCO intervention was implemented (Kemppainen et al., 2013).
Conclusion
The CHCO intervention did not show a significant improvement on health-related outcomes or stability in care needs–related outcomes in older people measured after a 1-year follow-up. With the CHCO intervention, we managed to reach community-dwelling older people with increased risk of frailty and/or unhealthy behavior patterns on a large scale. Further research is recommended to understand and evaluate nurse-led health promotion and preventive interventions targeting frail older populations, using diverse research designs.
Acknowledgments
We would like to express our gratitude to Jan van het Hof, who coordinated the data collection for the CHCO intervention. Also, we would like to thank Ronald Uittenbroek (manager of the bachelor of nursing at Windesheim University of Applied Sciences), for critically reviewing a draft version of our article. Writing assistance by Laura Allen, U.S. Fulbright researcher at Research Group Innovating With Older Adults, is greatly appreciated.
Footnotes
Authors’ Note: Anne Esther Marcus-Varwijk and Lilian L. Peters are first joint authorship.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: As funding organization, the Dutch Organization for Health Research and Development (ZonMw) played no role in the interpretation of the data or the approval of the article. The implementation of the CHCO intervention was financed by the health insurer Zilveren Kruis/Achmea in corporation with care organization Espria (Icare en Evean). The care-as-usual group was part of the National Care for the Elderly Program (NPO), which is funded by the Dutch Organization for Health Research and Development (ZonMW—number 60-61900-98-218).
ORCID iD: Anne Esther Marcus-Varwijk  https://orcid.org/0000-0003-3650-3964
https://orcid.org/0000-0003-3650-3964
References
- Anstey K. J., von Sanden C., Salim A., O’Kearney R. (2007). Smoking as a risk factor for dementia and cognitive decline: A meta-analysis of prospective studies. American Journal of Epidemiology, 166, 367-378. doi: 10.1093/aje/kwm116 [DOI] [PubMed] [Google Scholar]
- Bakker E., Jaspers N., Kraakman M., Visser G. (2008). Consultatiebureau voor Ouderen (Community Health Consultation Offices for Seniors). Visiedocument (Vision Document). Utrecht, The Netherlands: Vilans. [Google Scholar]
- Behm L., Wilhelmson K., Falk K., Eklund K., Zidén L., Dahlin-Ivanoff S. (2014). Positive health outcomes following health-promoting and disease-preventive interventions for independent very old persons: Long-term results of the three-armed RCT Elderly Persons in the Risk Zone. Archives of Gerontology and Geriatrics, 58, 376-383. doi: 10.1016/j.archger.2013.12.010 [DOI] [PubMed] [Google Scholar]
- Bergman H., Ferrucci L., Guralnik J., Hogan D. B., Hummel S., Karunananthan S., Wolfson C. (2007). Frailty: An emerging research and clinical paradigm: Issues and controversies. Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 62, 731-737. doi: 10.1093/gerona/62.7.731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beswick A. D., Gooberman-Hill R., Smith A., Wylde V., Ebrahim S. (2010). Maintaining independence in older people. Reviews in Clinical Gerontology, 20, 128-153. doi: 10.1017/S0959259810000079 [DOI] [Google Scholar]
- Booth F. W., Roberts C. K., Laye M. J. (2012). Lack of exercise is a major cause of chronic diseases. Comprehensive Physiology, 2, 1143-1211. doi: 10.1002/cphy.c110025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borglin G., Jakobsson U., Edberg A.-K., Hallberg I. R. (2005). Self-reported health complaints and their prediction of overall and health-related quality of life among elderly people. International Journal of Nursing Studies, 42, 147-158. doi: 10.1016/j.ijnurstu.2004.06.003 [DOI] [PubMed] [Google Scholar]
- Boult C., Pualwan T. F., Fox P. D., Pacala J. T., Management H. W. C. (1998). Identification and assessment of high-risk seniors. American Journal of Managed Care, 4, 1137-1146. [PubMed] [Google Scholar]
- Braveman P., Barclay C. (2009). Health disparities beginning in childhood: A life-course perspective. Pediatrics, 124(Suppl. 3), S163-175. doi: 10.1542/peds.2009-1100D [DOI] [PubMed] [Google Scholar]
- Bush K., Kivlahan D. R., McDonell M. B., Fihn S. D., Bradley K. A., P. (1998). The AUDIT alcohol consumption questions (AUDIT-C): An effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Archives of Internal Medicine, 158, 1789-1795. doi: 10.1001/archinte.158.16.1789 [DOI] [PubMed] [Google Scholar]
- Butler C. C., Simpson S. A., Hood K., Cohen D., Pickles T., Spanou C., . . . Rollnick S. (2013). Training practitioners to deliver opportunistic multiple behaviour change counselling in primary care: A cluster randomised trial. British Medical Journal, 346, Article f1191. doi: 10.1136/bmj.f1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corp I. (2013). IBM SPSS statistics for windows (Version 22.0). Armonk, NY: IBM Corp. [Google Scholar]
- Eissens van der Laan M. R., van Offenbeek M. A., Broekhuis H., Slaets J. P. (2014). A person-centred segmentation study in elderly care: Towards efficient demand-driven care. Social Science & Medicine, 113, 68-76. doi: 10.1016/j.socscimed.2014.05.012 [DOI] [PubMed] [Google Scholar]
- Erler A., Bodenheimer T., Baker R., Goodwin N., Spreeuwenberg C., Vrijhoef H. J. M., . . . Gerlach F. M. (2011). Preparing primary care for the future: Perspectives from the Netherlands, England, and USA. Zeitschrift für Evidenz, Fortbildung und Qualität im Gesundheitswesen, 105, 571-580. doi: 10.1016/j.zefq.2011.09.029 [DOI] [PubMed] [Google Scholar]
- Ginis K. A. M., Nigg C. R., Smith A. L. (2013). Peer-delivered physical activity interventions: An overlooked opportunity for physical activity promotion. Translational Behavioral Medicine, 3, 434-443. doi: 10.1007/s13142-013-0215-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman C., Davies S. L., Dinan S., See Tai S., Iliffe S. (2011). Activity promotion for community-dwelling older people: A survey of the contribution of primary care nurses. British Journal of Psychiatry, 16, 12-17. doi: 10.12968/bjcn.2011.16.1.12 [DOI] [PubMed] [Google Scholar]
- Hagger-Johnson G., Sabia S., Brunner E. J., Shipley M., Bobak M., Marmot M., . . . Singh-Manoux A. (2013). Combined impact of smoking and heavy alcohol use on cognitive decline in early old age: Whitehall II prospective cohort study. British Journal of Psychiatry, 203, 120-125. doi: 10.1192/bjp.bp.112.122960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeksema A. R., Peters L. L., Raghoebar G. M., Meijer H. J. A., Vissink A., Visser A. (2017). Oral health status and need for oral care of care-dependent indwelling elderly: From admission to death. Clinical Oral Investigations, 21, 2189-2196. doi: 10.1007/s00784-016-2011-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeksema A. R., Peters L. L., Raghoebar G. M., Meijer H. J. A., Vissink A., Visser A. (2018). Health and quality of life differ between community living older people with and without remaining teeth who recently received formal home care: A cross sectional study. Clinical Oral Investigations, 22, 2615-2622. doi: 10.1007/s00784-018-2360-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopman P., de Bruin S. R., Forjaz M. J., Rodriguez-Blazquez C., Tonnara G., Lemmens L. C., . . . Rijken M. (2016). Effectiveness of comprehensive care programs for patients with multiple chronic conditions or frailty: A systematic literature review. Health Policy, 120, 818-832. doi: 10.1016/j.healthpol.2016.04.002 [DOI] [PubMed] [Google Scholar]
- Hubbard R. E., Lang I. A., Llewellyn D. J., Rockwood K. (2010). Frailty, body mass index, and abdominal obesity in older people. The Journals of Gerontology, Series A: Psychological Sciences and Social Sciences, 65, 377-381. doi: 10.1093/gerona/glp186 [DOI] [PubMed] [Google Scholar]
- Hubbard R. E., Searle S. D., Mitnitski A., Rockwood K. (2009). Effect of smoking on the accumulation of deficits, frailty and survival in older adults: A secondary analysis from the Canadian study of health and aging. Journal of Nutrition Health & Aging, 13, 468-472. doi: 10.1007/s12603-009-0085-y [DOI] [PubMed] [Google Scholar]
- Hupcey J. E., Miller J. (2006). Community dwelling adults’ perception of interpersonal trust vs. trust in health care providers. Journal of Clinical Nursing, 15, 1132-1139. [DOI] [PubMed] [Google Scholar]
- Iliffe S. (2016). Community-based interventions for older people with complex needs: Time to think again? Age Ageing, 45, 2-3. doi: 10.1093/ageing/afv185 [DOI] [PubMed] [Google Scholar]
- Joseph D. H., Griffin M., Hall R. F., Sullivan E. D. (2001). Peer coaching: An intervention for individuals struggling with diabetes. The Diabetes Educator, 27, 703-710. doi: 10.1177/014572170102700511 [DOI] [PubMed] [Google Scholar]
- Kemppainen V., Tossavainen K., Turunen H. (2013). Nurses’ roles in health promotion practice: An integrative review. Health Promotion International, 28, 490-501. doi: 10.1093/heapro/das034 [DOI] [PubMed] [Google Scholar]
- Kojima G. (2015). Frailty as a predictor of future falls among community-dwelling older people: A systematic review and meta-analysis. Journal of the American Medical Directors Association, 16, 1027-1033. doi: 10.1016/j.jamda.2015.06.018 [DOI] [PubMed] [Google Scholar]
- Kojima G., Iliffe S., Walters K. (2015). Smoking as a predictor of frailty: A systematic review. BMC Geriatrics, 15, Article 131. doi: 10.1186/s12877-015-0134-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looman W. M., Fabbricotti I. N., de Kuyper R., Huijsman R. (2016). The effects of a pro-active integrated care intervention for frail community-dwelling older people: A quasi-experimental study with the GP-practice as single entry point. BMC Geriatrics, 16, Article 43. doi: 10.1186/s12877-016-0214-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer K. U. (2009). New directions in life course research. Annual Review of Sociology, 35, 413-433. doi: 10.1146/annurev.soc.34.040507.134619 [DOI] [Google Scholar]
- Miller W. R., Rollnick S. (2002). Motivational interviewing: Preparing people for change (2nd ed.). New York, NY: Guilford Press. [Google Scholar]
- Ng T. P., Feng L., Nyunt M. S. Z., Feng L., Niti M., Tan B. Y., . . . Yap K. B. (2015). Nutritional, physical, cognitive, and combination interventions and frailty reversal among older adults: A randomized controlled trial. The American Journal of Medicine, 128, 1225-1236.e1. doi: 10.1016/j.amjmed.2015.06.017 [DOI] [PubMed] [Google Scholar]
- Noordman J., de Vet E., van der Weijden T., van Dulmen S. (2013). Motivational interviewing within the different stages of change: An analysis of practice nurse-patient consultations aimed at promoting a healthier lifestyle. Social Science & Medicine, 87, 60-67. doi: 10.1016/j.socscimed.2013.03.019 [DOI] [PubMed] [Google Scholar]
- Noordman J., van Lee I., Nielen M., Vlek H., van Weijden T., van Dulmen S. (2012). Do trained practice nurses apply motivational interviewing techniques in primary care consultations? Journal of Clinical Medicine Research, 4, 393-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norcross J. C., Krebs P. M., Prochaska J. O. (2011). Stages of change. Journal of Clinical Psychology, 67, 143-154. doi: 10.1002/jclp.20758 [DOI] [PubMed] [Google Scholar]
- Paulhus D. L. (2002). Socially desirable responding: The evolution of a construct. In Braun H. I., Jackson D. N., Wiley D. E. (Eds.), The role of constructs in psychological and educational measurement (pp. 49-69). Mahwah, NJ: Lawrence Erlbaum. [Google Scholar]
- Peters L. L., Boter H., Burgerhof J. G., Slaets J. P., Buskens E. (2015). Construct validity of the Groningen Frailty Indicator established in a large sample of home-dwelling elderly persons: Evidence of stability across age and gender. Experimental Gerontology, 69, 129-141. doi: 10.1016/j.exger.2015.05.006 [DOI] [PubMed] [Google Scholar]
- Peters L. L., Boter H., Buskens E., Slaets J. P. (2012). Measurement properties of the Groningen Frailty Indicator in home-dwelling and institutionalized elderly people. Journal of the American Medical Directors Association, 13, 546-551. doi: 10.1016/j.jamda.2012.04.007 [DOI] [PubMed] [Google Scholar]
- Peters L. L., Boter H., Slaets J. P., Buskens E. (2013). Development and measurement properties of the self assessment version of the INTERMED for the elderly to assess case complexity. Journal of Psychosomatic Research, 74, 518-522. doi: 10.1016/j.jpsychores.2013.02.003 [DOI] [PubMed] [Google Scholar]
- Peters L. L., Burgerhof J. G., Boter H., Wild B., Buskens E., Slaets J. P. (2015). Predictive validity of a frailty measure (GFI) and a case complexity measure (IM-E-SA) on healthcare costs in an elderly population. Journal of Psychosomatic Research, 79, 404-411. doi: 10.1016/j.jpsychores.2015.09.015 [DOI] [PubMed] [Google Scholar]
- Prochaska J. O., DiClemente C. C. (1983). Stages and processes of self-change of smoking: Toward an integrative model of change. Journal of Consulting and Clinical Psychology, 51, 390-395. [DOI] [PubMed] [Google Scholar]
- Reedy J., Krebs-Smith S. M., Miller P. E., Liese A. D., Kahle L. L., Park Y., Subar A. F. (2014). Higher diet quality is associated with decreased risk of all-cause, cardiovascular disease, and cancer mortality among older adults. Journal of Nutrition, 144, 881-889. doi: 10.3945/jn.113.189407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose G. A. (1992). The strategy of preventive medicine. Oxford, UK: Oxford University Press. [Google Scholar]
- Schafer W., Kroneman M., Boerma W., van den Berg M., Westert G., Deville W., van Ginneken E. (2010). The Netherlands: Health system review. Health systems in Transition, 12(1), v-xxvii, 1-228. [PubMed] [Google Scholar]
- Schuurmans H., Steverink N., Lindenberg S., Frieswijk N., Slaets J. P. (2004). Old or frail: What tells us more? The Journals of Gerontology, Series A: Psychological Sciences and Social Sciences, 59, M962-M965. [DOI] [PubMed] [Google Scholar]
- Shiner M. (1999). Defining peer education. Journal of Adolescence, 22, 555-566. doi: 10.1006/jado.1999.0248 [DOI] [PubMed] [Google Scholar]
- Slaets J. P. (2006). Vulnerability in the elderly: Frailty. Medical Clinics of North America, 90, 593-601. doi: 10.1016/j.mcna.2006.05.008 [DOI] [PubMed] [Google Scholar]
- Smit L. C., Schuurmans M. J., Blom J. W., Fabbricotti I. N., Jansen A. P. D., Kempen G. I. J. M., . . . Bleijenberg N. (2018). Unravelling complex primary-care programs to maintain independent living in older people: A systematic overview. Journal of Clinical Epidemiology, 96, 110-119. doi: 10.1016/j.jclinepi.2017.12.013 [DOI] [PubMed] [Google Scholar]
- Smith S. M., Wallace E., O’Dowd T., Fortin M. (2016). Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database of Systematic Reviews, 3, Article CD006560. doi: 10.1002/14651858.CD006560.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegeman I., Kraaijenhagen R., Bossuyt P. (2009). Integrated risk profiling allows prevention and early intervention. Nederlands Tijdschrift Voor Geneeskunde, 154, A1906. [PubMed] [Google Scholar]
- Steverink N., Slaets J., Schuurmans H., Van Lis M. (2001). Measuring frailty: Developing and testing the GFI (Groningen Frailty Indicator). Gerontologist, 41, 236-237. [Google Scholar]
- Steverink N., Westerhof G. J., Bode C., Dittmann-Kohli F. (2001). The personal experience of aging, individual resources, and subjective well-being. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 56, P364-P373. [DOI] [PubMed] [Google Scholar]
- Stuck A. E., Moser A., Morf U., Wirz U., Wyser J., Gillmann G., . . . Egger M. (2015). Effect of health risk assessment and counselling on health behaviour and survival in older people: A pragmatic randomised trial. PLoS Medicine, 12(10), e1001889. doi: 10.1371/journal.pmed.1001889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomilehto J., Lindström J., Eriksson J. G., Valle T. T., Hämäläinen H., Ilanne-Parikka P., . . . Uusitupa M. (2001). Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. New England Journal of Medicine, 344, 1343-1350. doi: 10.1056/nejm200105033441801 [DOI] [PubMed] [Google Scholar]
- van Baak M. A., Visscher T. L. S. (2006). Public health success in recent decades may be in danger if lifestyles of the elderly are neglected. American Journal of Clinical Nutrition, 84, 1257-1258. [DOI] [PubMed] [Google Scholar]
- van de Vijver P. L., Wielens H., Slaets J. P. J., van Bodegom D. (2018). Vitality club: A proof-of-principle of peer coaching for daily physical activity by older adults. Translational Behavioral Medicine, 8, 204-211. doi: 10.1093/tbm/ibx035 [DOI] [PubMed] [Google Scholar]
- Vermunt J. K., Magidson J. (2008). LG-Syntax user’s guide: Manual for Latent GOLD 4.5 syntax module. Belmont, MA: Statistical Innovations. [Google Scholar]
- Vina J., Rodriguez-Manas L., Salvador-Pascual A., Tarazona-Santabalbina F. J., Gomez-Cabrera M. C. (2016). Exercise: The lifelong supplement for healthy ageing and slowing down the onset of frailty. Journal of Physiology, 594, 1989-1999. doi: 10.1113/Jp270536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voedingscentrum. (2011, April 12). Richtlijnen Voedselkeuze (Guidelines on food choices). Retrieved from http://www.voedingscentrum.nl/Assets/Uploads/voedingscentrum/Documents/Professionals/Schijf%20van%20Vijf/20110412_Richtlijnen%20voedselkeuze%20VC.pdf
- Walston J., Hadley E. C., Ferrucci L., Guralnik J. M., Newman A. B., Studenski S. A., . . . Fried L. P. (2006). Research agenda for frailty in older adults: Toward a better understanding of physiology and etiology: Summary from the American Geriatrics Society/National Institute on Aging Research Conference on frailty in older adults. Journal of the American Geriatrics Society, 54, 991-1001. doi: 10.1111/j.1532-5415.2006.00745.x [DOI] [PubMed] [Google Scholar]
- Walters M. E., Reijneveld S. A., van der Meulen A., Dijkstra A., de Winter A. F. (2017). Effects of a training program for home health care workers on the provision of preventive activities and on the health-related behavior of their clients: A quasi-experimental study. International Journal of Nursing Studies, 74, 61-66. doi: 10.1016/j.ijnurstu.2017.05.012 [DOI] [PubMed] [Google Scholar]
- Whitley E., Popham F., Benzeval M. (2016). Comparison of the Rowe–Kahn model of successful aging with self-rated health and life satisfaction: The West of Scotland twenty-07 prospective cohort study. The Gerontologist, 56, 1082-1092. doi: 10.1093/geront/gnv054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock E. P., Orleans C. T., Pender N., Allan J. (2002). Evaluating primary care behavioral counseling interventions: An evidence-based approach. American Journal of Preventive Medicine, 22, 267-284. [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2002). Active ageing: A policy framework. Retrieved from https://extranet.who.int/agefriendlyworld/wp-content/uploads/2014/06/WHO-Active-Ageing-Framework.pdf