Editor
The pandemic of infection with the severe acute respiratory syndrome coronavirus (SARS‐CoV‐2) causing the atypical pneumonia coronavirus disease 19 (COVID‐2019) has become a global health emergency. In parallel with the spread of the infection, there is new information on cutaneous involvement reminiscent to autoimmune diseases and concerns about the risk of disease and management of patients with cutaneous autoimmunity under immunosuppression.
COVID‐2019 mainly presents with fever, cough, loss of smell and taste, myalgia and fatigue. 1 Main complication of the infection is progression to acute respiratory distress syndrome (ARDS), coagulopathy, vasculopathy, cardiovascular failure and a cytokine storm syndrome requiring intensive care for days or even weeks. 1 , 2
The risk factors for more severe COVID‐19 infection are cardiovascular illnesses, diabetics, renal failure, respiratory failure, morbid obesity and older age (>65). 3 , 4
Cutaneous symptoms of COVID‐19 include petechial skin rash 5 , 6 or digitate scaly thin plaques 7 associated with severe respiratory disease (Table 1). A maculopapular urticarial rash may present as early sign of disease 8 , 9 , 10 or during disease 11 without a yet known association to severity. 12 , 13 There was also a case of pityriasis rosea like rash in one patient with mild febrile COVID‐19. 14 In young children, infection with SARS‐CoV‐2 can be associated with Kawasaki syndrome including the maculopapular oedematous rash and conjunctival injection. 15 , 16
Table 1.
Cutaneous lesions associated with COVID‐19
| Cutaneous findings | Histopathology | COVID‐19 | Reference |
|---|---|---|---|
| Acral chilblain lesions | Vacuolar interface dermatitis and superficial and deep perivascular and periadnexal lymphohistiocytic infiltrates | Mild or none, late symptom | 17, 18, 19, 21, 27 |
| Violaceous papules and digital swelling | Diffuse perivascular involvement of the dermis and hypodermis by a dense lymphoid infiltrate | Mild or none, late symptom | 20 |
| Symmetrical petechial skin rash on buttocks, thighs that might be similar to dengue virus exanthema | Superficial perivascular infiltrate with erythrocyte extravasation, dermal papillary oedema, and scattered dyskeratotic keratinocytes | Associated with severe acute respiratory syndrome | 5, 6 |
| Digitate scaly thin plaques | Spongiosis in the epidermis and mild papillary oedema with lymphohistiocytic infiltrate in the dermis | Associated with severe acute respiratory syndrome | 7 |
| Erythematous and oedematous non‐pruritic annular fixed plaques involving the upper limbs, chest, neck, abdomen and palms | Superficial perivascular lymphocytic infiltrate, papillary dermal oedema, mild spongiosis, lichenoid and vacuolar interface dermatitis, dyskeratotic basilar keratinocytes | Mild | 9 |
| Maculopapular and urticarial rash | Early symptom | 8, 10 | |
| Maculopapular symmetrical rash | Superficial perivascular lymphocytic infiltrate, papillary dermal oedema, ectatic vessels, vacuolar interface dermatitis 14 | Mild, associated mild lung disease | 11, 13 |
| Maculopapular rash in young children | Kawasaki syndrome associated with COVID‐19 | 15 | |
| Pityriasis rosea | Mild | 14 |
Mild forms of disease in younger individuals seem to present with chilblain‐like lesions on acral locations especially the toes 17 (Table 1). The skin appears shiny red and is painful (Fig. 1). The lesions resolve spontaneously after weeks and indicate a rather favourable outcome. 17 The symptoms may represent a form of inflammation induced by small vessel endotheliitis. 17 In histological section, these lesions may have a slight vacuolar interface dermatitis and a superficial and deep perivascular and periadnexal lymphohistiocytic infiltrate. 18 Reports from Italy and France documented an outbreak of chilblain‐like lesions contemporarily to COVID‐19 epidemic. 19 , 20 , 21 Despite the low rate of positive mRNA or antibody testing, the coincidence of both events is highly suggestive for direct correlation.
Figure 1.
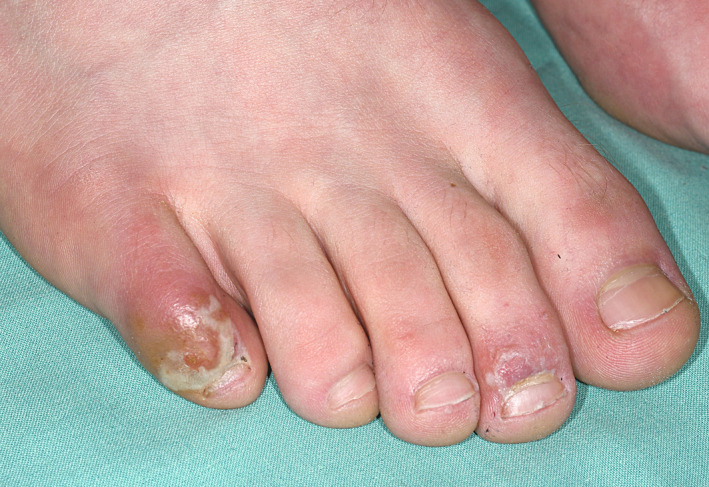
Chilblain lesions on acral locations occurring in a young man six weeks after visiting an area with high SARS‐CoV‐2 infection rate. He did not develop respiratory symptoms. Late swab testing at onset of chilblain lesions was negative. Lesions resolved with topical immunosuppressive treatment. There were no systemic or laboratory signs of autoimmunity.
The induction of the lesions might be related to a direct viral effect, pathogenic priming 22 or mediated by upregulation of the antiviral cytokine type I interferon. Type I interferons cause a stimulation and activation of the immune response and a thrombotic microangiopathy by a dose‐dependent toxic effect on the microvasculature. 23 Patients with high levels of type I interferon‐mediated autoimmune diseases or type I interferonopathies frequently develop chilblain lesions. 21 , 24 , 25 , 26
Chilblain or erythema multiforme like acral ischaemic lesions were also observed contemporarily to COVID‐19 epidemic in Spain. 27 None of the 132 patients reported developed COVID‐19 pneumonia or any other complication. The authors hypothesize that the latency time of up to 30 days between mild COVID‐19 symptoms and skin manifestations, and the low positive rate for nasopharyngeal swabs (only 2 positive results) suggest that these chilblain‐like ischaemic lesions represent a late manifestation of SARS‐CoV‐2 infection. 27
There are no large reports on the frequency of infection or the risk of severe COVID‐19 in patients with autoimmune diseases involving the skin such as lupus erythematosus, dermatomyositis, systemic sclerosis and autoimmune bullous diseases (Table 2). Currently, limited data suggest that patients with autoimmune disorders, especially rheumatoid arthritis, and immunosuppression do not appear to be more severely infected by COVID‐19. 28 , 29 , 30
Table 2.
Outcome of patients with autoimmune diseases and COVID‐19
| Autoimmune disease (number of patients) | Relevant comorbidities † | Ongoing treatment | Outcome 19 | Reference |
|---|---|---|---|---|
| Systemic sclerosis (1) | Yes | Tocilizumab | Mild | 38 |
| Granulomatosis with polyangiitis (1) | Yes | Rituximab | ARDS, survived | 39 |
| Systemic lupus erythematosus (17) | Yes | Hydroxychloroquine, other immunosuppressives interrupted |
14 admitted to hospital 2 died |
40 |
| Rheumatoid arthritis, spondyloarthritis (4) | Yes | DMARDs, temporarily withdrawn |
3 mild 1 hospitalized, survived |
41 |
| Systemic sclerosis (1 with positive swab of 123 total patients with connective tissue diseases), | Yes | Hydroxychloroquine, rituximab | severe pneumonia, died | 42 |
| Psoriasis, psoriatic arthritis, rheumatoid arthritis, ulcerative colitis, Crohn’s disease and ankylosing spondylitis under (86) | Yes | Methotrexate, hydroxychloroquine, JAK inhibitors, TNF inhibitor or IL‐17‐, IL‐23‐, IL‐12/23 blocker |
mild 14 admitted to hospital, 1 died |
43 |
| Pemphigus vulgaris (1) | None | Mycophenolate mofetil | Nausea and fever, mild | 44 |
| Psoriasis (1) | None | IL‐17 inhibitor | No symptoms | 45 |
| Psoriasis, arthritis, Crohn (1) | None | IL‐23 inhibitor | Mild | 46 |
DMARDs, methotrexate, etanercept, tofacitinib, leflunomide, abatacept; IL, interleukin.
Older age > 65, obesity, cardiovascular disease, diabetes, kidney disease, lung disease, smoker
However, immunosuppression and especially long‐term hydroxychloroquine treatment did not prevent infection of lupus patients. 31 These patients may get infected and can also suffer from severe COVID‐19 if they have concomitant risk factors such as obesity or chronic kidney disease 31 , 32 (Table 2).
Immunosuppressive treatment is necessary for the management of autoimmune diseases, and it is known from patients with rheumatoid arthritis that the risk for viral infection is increased if the disease is not controlled. 33
Therefore, we should continue immunosuppressive treatment in patients with autoimmune diseases and not postpone diagnostic procedures if ever possible. 30 There are concerns especially regarding the long‐term immunosuppressive effect of rituximab used for treatment of pemphigus. 34 The initiation of rituximab in patients with autoimmune bullous disease must be weighed against the risks of conventional immunomodulatory regimens on an individual basis. 35
To reduce the risk of infection routine face‐to‐face appointments could be delayed if they are not urgently needed or substituted by teledermatology. 36 Patients should be encouraged to update appropriate flu and pneumococcal vaccination and maintain the hygiene and protection measures.
To improve current knowledge on the disease course of COVID‐19 in patients with autoimmune disease, their risk of infection and potential treatment options, all cases should be reported to the COVID‐19 registries set by organization such as the EULAR, EUSTAR, task force for autoimmune bullous diseases, the German network for systemic sclerosis and local epidemiology registries. 37
Conflicts of interest
The authors have declared no conflict of interest.
Funding source
This work was supported by the Deutsche Forschungsgemeinschaft (German Research Foundation), grant 369799452/404458960 to CG.
References
- 1. Huang C, Wang Y, Li X et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mehra MR, Desai SS, Kuy S, Henry TD, Patel AN. Cardiovascular disease, drug therapy, and mortality in Covid‐19. N Engl J Med 2020; 382: e102. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3. Zhou F, Yu T, Du R et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ackermann M, Verleden SE, Kuehnel M et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid‐19. N Engl J Med 2020. 10.1056/NEJMoa2015432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Diaz‐Guimaraens B, Dominguez‐Santas M, Suarez‐Valle A et al. Petechial skin rash associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol 2020. 10.1001/jamadermatol.2020.1741 [DOI] [PubMed] [Google Scholar]
- 6. Joob B, Wiwanitkit V. COVID‐19 can present with a rash and be mistaken for dengue. J Am Acad Dermatol 2020; 82: e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sanchez A, Sohier P, Benghanem S et al. Digitate papulosquamous eruption associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol 2020. 10.1001/jamadermatol.2020.1704 [DOI] [PubMed] [Google Scholar]
- 8. Henry D, Ackerman M, Sancelme E, Finon A, Esteve E. Urticarial eruption in COVID‐19 infection. J Eur Acad Dermatol Venereol 2020; 34: e244– e245. 10.1111/jdv.16472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Amatore F, Macagno N, Mailhe M et al. SARS‐CoV‐2 infection presenting as a febrile rash. J Eur Acad Dermatol Venereol 2020. 10.1111/jdv.16528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Damme C, Berlingin E, Saussez S, Accaputo O. Acute urticaria with pyrexia as the first manifestations of a COVID‐19 infection. J Eur Acad Dermatol Venereol 2020. 10.1111/jdv.16523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Avellana Moreno R, Villa E, Avellana Moreno V, Estela Villa C, Aparicio M, Fontanella A. Cutaneous manifestation of COVID‐19 in images: a case report. J Eur Acad Dermatol Venereol 2020. 10.1111/jdv.16531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Recalcati S. Cutaneous manifestations in COVID‐ 19: a first perspective. J Eur Acad Dermatol Venereol 2020; 34: e212– e213. 10.1111/jdv.16387 [DOI] [PubMed] [Google Scholar]
- 13. Mahé A, Birckel E, Krieger S, Merklen C, Bottlaender L. A distinctive skin rash associated with coronavirus disease 2019? J Eur Acad Dermatol Venereol 2020; 34: e246– e247. 10.1111/jdv.16471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ehsani AH, Nasimi M, Bigdelo Z. Pityriasis rosea as a cutaneous manifestation of COVID‐19 infection. J Eur Acad Dermatol Venereol 2020. 10.1111/jdv.16579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jones VG, Mills M, Suarez D et al. COVID‐19 and Kawasaki disease: novel virus and novel case. Hospital Pediatrics 2020; 10: 537–540. [DOI] [PubMed] [Google Scholar]
- 16. Viner RM, Whittaker E. Kawasaki‐like disease: emerging complication during the COVID‐19 pandemic. Lancet 2020; 395: 1741–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Landa N, Mendieta‐Eckert M, Fonda‐Pascual P, Aguirre T. Chilblain‐like lesions on feet and hands during the COVID‐19 Pandemic. Int J Dermatol 2020; 59: 739–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kolivras A, Dehavay F, Delplace D et al. Coronavirus (COVID‐19) infection‐induced chilblains: a case report with histopathologic findings. JAAD Case Rep 2020; 6: 489–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Piccolo V, Neri I, Filippeschi C et al. Chilblain‐like lesions during COVID‐19 epidemic: a preliminary study on 63 patients. J Eur Acad Dermatol Venereol 2020. 10.1111/jdv.16526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Recalcati S, Barbagallo T, Frasin LA et al. Acral cutaneous lesions in the time of COVID‐19. J Eur Acad Dermatol Venereol 2020. 10.1111/jdv.16533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bouaziz J, Duong T, Jachiet M et al. Vascular skin symptoms in COVID‐ 19: a French observational study. J Eur Acad Dermatol Venereol 2020. 10.1111/jdv.16544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lyons‐Weiler J. Pathogenic priming likely contributes to serious and critical illness and mortality in COVID‐19 via autoimmunity. J Transl Autoimmun 2020; 3: 100051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kavanagh D, McGlasson S, Jury A et al. Type I interferon causes thrombotic microangiopathy by a dose‐dependent toxic effect on the microvasculature. Blood 2016; 128: 2824–2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zimmermann N, Wolf C, Schwenke R et al. Assessment of clinical response to janus kinase inhibition in patients with familial chilblain lupus and TREX1 mutation. JAMA Dermatol 2019; 155(3): 342–346. 10.1001/jamadermatol.2018.5077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gunther C, Berndt N, Wolf C, Lee‐Kirsch MA. Familial chilblain lupus due to a novel mutation in the exonuclease III domain of 3' repair exonuclease 1 (TREX1). JAMA Dermatol 2015; 151: 426–431. [DOI] [PubMed] [Google Scholar]
- 26. Lee‐Kirsch MA. The type I interferonopathies. Annu Rev Med 2017; 68: 297–315. [DOI] [PubMed] [Google Scholar]
- 27. Fernandez‐Nieto D, Jimenez‐Cauhe J, Suarez‐Valle A et al. Characterization of acute acro‐ischemic lesions in non‐hospitalized patients: a case series of 132 patients during the COVID‐19 outbreak. J Am Acad Dermatol 2020; 83(1): e61– e63. 10.1016/j.jaad.2020.04.093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Monti S, Balduzzi S, Delvino P, Bellis E, Quadrelli VS, Montecucco C. Clinical course of COVID‐19 in a series of patients with chronic arthritis treated with immunosuppressive targeted therapies. Ann Rheum Dis 2020; 79: 667–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Favalli EG, Agape E, Caporali R. Incidence and clinical course of COVID‐19 in patients with connective tissue diseases: a descriptive observational analysis. J Rheumatol 2020; 47. 10.3899/jrheum.200507 [DOI] [PubMed] [Google Scholar]
- 30. D'Antiga L. Coronaviruses and immunosuppressed patients: the facts during the third epidemic. Liver Transplant 2020; 26: 832–834. [DOI] [PubMed] [Google Scholar]
- 31. Mathian A, Mahevas M, Rohmer J et al. Clinical course of coronavirus disease 2019 (COVID‐19) in a series of 17 patients with systemic lupus erythematosus under long‐term treatment with hydroxychloroquine. Ann Rheum Dis 2019; 79: 837–839. [DOI] [PubMed] [Google Scholar]
- 32. Bozzalla Cassione E, Zanframundo G, Biglia A, Codullo V, Montecucco C, Cavagna L. COVID‐19 infection in a northern‐Italian cohort of systemic lupus erythematosus assessed by telemedicine. Ann Rheum Dis 2020. 10.1136/annrheumdis-2020-217717 [DOI] [PubMed] [Google Scholar]
- 33. Au K, Reed G, Curtis JR et al. High disease activity is associated with an increased risk of infection in patients with rheumatoid arthritis. Ann Rheum Dis 2011; 70: 785–791. [DOI] [PubMed] [Google Scholar]
- 34. Shakshouk H, Daneshpazhooh M, Murrell DF, Lehman JS. Treatment considerations for patients with pemphigus during the COVID‐19 pandemic. J Am Acad Dermatol 2020; 82: e235–e236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kasperkiewicz M, Schmidt E, Fairley JA et al. Expert recommendations for the management of autoimmune bullous diseases during the COVID‐19 pandemic. J Eur Acad Dermatol Venereol 2020. 10.1111/jdv.16525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Misra DP, Agarwal V, Gasparyan AY, Zimba O. Rheumatologists' perspective on coronavirus disease 19 (COVID‐19) and potential therapeutic targets. Clin Rheumatol 2020; 39: 2055–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gianfrancesco MA, Hyrich KL, Gossec L et al. Rheumatic disease and COVID‐19: initial data from the COVID‐19 Global Rheumatology Alliance provider registries. Lancet Rheumatol 2020; 2: e250– e253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mihai C, Dobrota R, Schröder M et al. COVID‐19 in a patient with systemic sclerosis treated with tocilizumab for SSc‐ILD. Ann Rheum Dis 2020; 79: 668–669. [DOI] [PubMed] [Google Scholar]
- 39. Guilpain P, Le Bihan C, Foulongne V et al. Rituximab for granulomatosis with polyangiitis in the pandemic of covid‐19: lessons from a case with severe pneumonia. Ann Rheum Dis 2020. 10.1136/annrheumdis-2020-217549 [DOI] [PubMed] [Google Scholar]
- 40. Mathian A, Mahevas M, Rohmer J et al. Clinical Course of Coronavirus Disease 2019 (COVID‐19) in a series of 17 patients with systemic lupus erythematosus under long‐term treatment with hydroxychloroquine. Ann Rheum Dis 2020; 79: 837–839. [DOI] [PubMed] [Google Scholar]
- 41. Monti S, Balduzzi S, Delvino P, Bellis E, Quadrelli VS, Montecucco C. Clinical Course of COVID‐19 in a series of patients with chronic arthritis treated with immunosuppressive targeted therapies. Ann Rheum Dis 2020; 79: 667–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Favalli EG, Agape E, Caporali R. Incidence and Clinical Course of COVID‐19 in patients with connective tissue diseases: a descriptive observational analysis. J Rheumatol 2020; 47. 10.3899/jrheum.200507 [DOI] [PubMed] [Google Scholar]
- 43. Haberman R, Axelrad J, Chen A et al. Covid‐19 in immune‐mediated inflammatory diseases – case series from New York. N Engl J Med 2020. 10.1056/NEJMc2009567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Balestri R, Rech G, Girardelli CR. Occurrence of SARS‐CoV‐2 during mycophenolate mofetil treatment for pemphigus. J Eur Acad Dermatol Venereol 2020. 10.1111/jdv.16578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Balestri R, Rech G, Girardelli CR. SARS‐CoV‐2 infection in a psoriatic patient treated with IL‐17 inhibitor. J Eur Acad Dermatol Venereol 2020. 10.1111/jdv.16571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Messina F, Piaserico S. SARS‐CoV‐2 infection in a psoriatic patient treated with IL‐23 inhibitor. J Eur Acad Dermatol Venereol 2020; 34: e254– e255. 10.1111/jdv.16468 [DOI] [PMC free article] [PubMed] [Google Scholar]
Acknowledgement
The patient in this manuscript has given written informed consent to the publication of his case details.


