Abstract
Since the outbreak of 2019 novel coronavirus (SARS‐CoV‐2) pneumonia, many patients with underlying disease, such as interstitial lung disease (ILD), were admitted to Tongji hospital in Wuhan, China. To date, no data have ever been reported to reflect the clinical features of Corona Virus Disease 2019 (COVID‐19) among these patients with preexisting ILD. We analyzed the incidence and severity of COVID‐19 patients with ILD among 3201 COVID‐19 inpatients, and compared two independent cohorts of COVID‐19 patients with pre‐existing ILD (n = 28) and non‐ILD COVID‐19 patients (n = 130). Among those 3201 COVID‐19 inpatients, 28 of whom were COVID‐19 with ILD (0.88%). Fever was the predominant symptom both in COVID‐19 with ILD (81.54%) and non‐ILD COVID‐19 patients (72.22%). However, COVID‐19 patients with ILD were more likely to have cough, sputum, fatigue, dyspnea, and diarrhea. A very significantly higher number of neutrophils, monocytes, interleukin (IL)‐8, IL‐10, IL‐1β, and D‐Dimer was characterized in COVID‐19 with ILD as compared to those of non‐ILD COVID‐19 patients. Furthermore, logistic regression models showed neutrophils counts, proinflammatory cytokines (tumor necrosis factor‐alpha, IL6, IL1β, IL2R), and coagulation dysfunction biomarkers (D‐Dimer, PT, Fbg) were significantly associated with the poor clinical outcomes of COVID‐19. ILD patients could be less vulnerable to SARS‐CoV‐2. However, ILD patients tend to severity condition after being infected with SARS‐CoV‐2. The prognosis of COVID‐19 patients with per‐existing ILD is significantly worse than that of non‐ILD patients. And more, aggravated inflammatory responses and coagulation dysfunction appear to be the critical mechanisms in the COVID‐19 patients with ILD.
Keywords: clinical features, ILD, incidence, SARS‐CoV‐2
Highlights
ILD patients could be less vulnerable to SARS‐CoV‐2. However, ILD patients tend to severity condition after being infected with SARS‐CoV‐2.
The prognosis of COVID‐19 patients with per‐existing ILD is significantly worse than that of non‐ILD patients. And more, aggravated inflammatory responses and coagulation dysfunction appear to be the critical mechanisms in the COVID‐19 patients with ILD.
1. INTRODUCTION
COVID‐19, full name is Coronavirus disease 2019, is an infectious disease caused by a coronavirus called severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), and first appeared in Wuhan, Hubei, and has rapidly spread throughout China and around the world. 1 , 2 , 3 Up to 31 March 2020, the total number of patients has risen to 82 547 in China, and 50 006 (60.58%) of whom were in Wuhan. 4 Previous studies have only described the general epidemiological findings, clinical presentation, and clinical outcomes from COVID patients with chronic underlying comorbidities. 1 , 5 , 6 To our knowledge, none of the fatal case reports has been associated with interstitial lung disease (ILD), which is featured by variable degrees of inflammation and fibrosis. Similarly, specific information characterizing COVID‐19 patients with or without preexisting ILD remains unknown.
As a leading hospital in Wuhan, Tongji Hospital serves as one of the main designated hospitals to receive and treat COVID‐19 patients. This study is designed to analyze the incidence and severity of COVID‐19 patients with preexisting ILD, and to compare the clinical features between COVID‐19 patients with preexisting ILD and COVID‐19 in patients without preexisting ILD based on the epidemiological, clinical, laboratory, and CT scan results.
2. METHODS
2.1. Patients
Tongji Hospital, as the center of COVID‐19 epidemic, received 3201 COVID‐19 inpatients from 7 February to 27 March 2020. Among those 3201 COVID‐19 inpatients, 28 of whom were COVID‐19 with pre‐existing ILD. In this retrospective study, we recruited all COVID‐19 patients with preexisting ILD, and subsequently, a total of 130 non‐ILD patients with COVID‐19 that were statistically matched with COVID‐19 patients with preexisting ILD at an approximate ratio of 4:1 based on age, sex, and illness severity were enrolled in this study. The clinical features, laboratory findings, computed tomography (CT) imaging, patient outcomes, and management data were obtained from each patient.
All patients with ILD enrolled in this study were diagnosed on the basis of the ILD guideline. 7 According to the COVID‐19 Diagnosis and Treatment Protocol, 8 patients diagnosed as COVID‐19 were classified based on their clinical manifestations. The mild case was defined as mild clinical manifestations, with or without pneumonia changes of CT scans. Severe case was defined as: (a) respiratory distress, RR ≥30 times/min; (b) oxygen saturation ≤93% at rest; and (c) Pao2/Fio2 ≤300 mm Hg (1 mm Hg = 0.133 kPa). The critical case was defined as (a) respiratory failure requiring mechanical ventilation; (b) occurrence of shock; and (c) combined with the failure of other organs and intensive care unit care was required.
The study was approved by the Human Assurance Committee (HAC) of Tongji Hospital, and oral informed consent was obtained from each participant. In case some of the data were missed from the records or specific clarification was necessary, we obtained those data by directly communicating with the attending doctors and healthcare providers.
2.2. Procedures
The COVID‐19 nucleic acid assays were conducted in Tongji Hospital. Throatswab specimens from the upper respiratory tract were collected from all outpatients twice with a 24 hours interval. The throat swab was placed into a collection tube with a virus preservation solution, and total RNA was extracted using two different respiratory sample RNA isolation kits approved by the Food and Drug Administration (FDA) of China (Huirui and Bojie, Shanghai, China). Two target genes, including the open reading frame 1ab (ORF1ab) and the nucleocapsid protein (N), were simultaneously amplified by real‐time polymerase chain reaction (RT‐PCR). The reaction mixture consisted of 7.5 μL reaction buffer, 1.5 μL enzyme solution, 5 μL ORF1ab/N gene reaction solution, 5 to 11 μL RNA template and 25 μl RNase free pure water. The RT‐PCR reactions were subjected to 50°C for 15 minutes, incubation at 95°C for 5 minutes, denaturation at 95°C for 10 seconds with 45 cycles, and then subjected to the acquisition of fluorescence signal at 55°C for 45 seconds. A cycle threshold (Ct‐value) less than 35 is defined as positive for the tests, while ≧39 as negative. These diagnostic criteria are based on the recommendations of the National Institute for Viral Disease Control and Prevention (China). A medium load (Ct‐value >35 but <39.2) was required for a confirmed diagnosis.
2.3. CT scanning
All patients had undergone non‐contrast CT scanning using the standard‐dose chest CT protocols (GE Healthcare, Philips, or Toshiba Medical Systems) of the thorax in the supine position during end‐inspiration (80‐120 kVp, automated tube current modulation, mA ranges from 60 to 300, rotate time 0.5 seconds, pitch 0.984:1, a slice thickness of 1.25 mm. All patients did not conduct enhanced CT scanning.
2.4. Data collection
Epidemiological data including patients' age and sex information, clinical symptoms, blood routine results, and CT scans were collected through the standardized data collection tables from the electronic medical records.
2.5. Statistical analysis
Continuous variables were presented as the median and interquartile range (IQR) for skewed distributed data or mean and standard deviation (SD) for normally distributed data. Categorical variables were expressed as number (%). Differences between ILD and non‐ILD patients with COVID‐19. For continuous variables, Student t‐test was used for normally distributed data, whereas the Mann‐Whitney U non‐parameter test was used for skewed distributed data. Pearson's χ² test or Fisher's exact test were applied for categorical variables. Unconditional logistic regression was applied to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for the association between factors and the clinical outcomes of COVID‐19. A two‐sided P value < .05 was considered statistically significant. All statistical analyses were performed using SPSS (22.0).
2.6. Role of the funding source
The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. RESULTS
Since Tongji Hospital is almost located in the center of COVID‐19 epidemic, it received 3201 COVID‐19 inpatients between 7 February to 27 March 2020, and 28 of whom were COVID‐19 patients with preexisting ILD, and thus the incidence of COVID‐19 with preexisting ILD was 0.88%. Specifically, nine of 28 cases are idiopathic interstitial cases of pneumonia (IIPs), 10 cases are connective tissue disease‐interstitial lung disease (CTD‐ILD), three cases are antineutrophil cytoplasmic antibodies‐associated vasculitis combined with pulmonary fibrosis, two cases are chronic hypersensitivity pneumonitis, two cases are sarcoidosis, one case is pneumoconiosis, and the last one case is radiation pneumonitis. We subsequently recruited a total of 130 non‐ILD patients with COVID‐19 that were statistically matched with COVID‐19 patients with preexisting ILD at an approximate ratio of 4:1 based on age, sex, and illness severity in this study. In terms of age, gender, and severity distribution, no significant difference was noted between the COVID‐19 with preexisting ILD and COVID‐19 without preexisting ILD patients (Table 1).
Table 1.
Demographic, clinical, laboratory, and radiographic findings of patients
| Indicators | Total | Alive | Dead | P value | |||
|---|---|---|---|---|---|---|---|
| Characteristics | |||||||
| Age | N = 50 | 36.50(30.25‐44.00) | N = 40 | 36.00(30.75‐44.00) | N = 10 | 41.00(30.75‐44.00 | 0.980* |
| Sex | |||||||
| Male | N = 50 | 23(46.0%) | N = 40 | 18(45.0%) | N = 10 | 5(50.0%) | 1.000** |
| Female | N = 50 | 27(54.0%) | N = 40 | 5(55.0%) | N = 10 | 5(50.0%) | |
| BMI | N = 40 | 24.38 ± 4.00 | N = 31 | 23.38 ± 3.02 | N = 9 | 27.79 ± 5.18 | 0.036*** |
| N = 40 | 23.39(21.62‐26.34) | N = 31 | 23.18(21.62‐24.59) | N = 9 | 29.32(28.91‐29.40) | 0.021* | |
| Initial symptoms | |||||||
| Fever | N = 49 | 40(81.6%) | N = 40 | 33(82.5%) | N = 9 | 7(77.8%) | 0.663** |
| Chill | N = 49 | 1(2.0%) | N = 40 | 0(0%) | N = 9 | 1(11.1%) | 0.184** |
| Dry cough | N = 49 | 13(26.5%) | N = 40 | 8(20%) | N = 9 | 5(55.6%) | 0.043** |
| Expectoration | N = 49 | 2(4.1%) | N = 40 | 0(0%) | N = 9 | 2(22.2%) | 0.031** |
| Fatigue | N = 49 | 2(4.1%) | N = 40 | 1(2.5%) | N = 9 | 1(11.1%) | 0.337** |
| Dyspnea | N = 49 | 4(8.2%) | N = 40 | 0(0%) | N = 9 | 4(44.4%) | 5.94 × 10−4 ** |
| Diarrhea | N = 49 | 3(6.1%) | N = 40 | 2(5%) | N = 9 | 1(11.1%) | 0.464** |
| Muscle ache | N = 49 | 2(4.1%) | N = 40 | 1(2.5%) | N = 9 | 1(11.1%) | 0.337** |
| Chest pain | N = 48 | 0(0) | N = 40 | 0(0%) | N = 8 | 0(0%) | 1.000** |
| Sore throat | N = 48 | 1(2.1%) | N = 40 | 1(2.5%) | N = 8 | 0(0%) | 1.000** |
| Vomiting | N = 49 | 1(2.0%) | N = 40 | 0(0%) | N = 9 | 1(11.1%) | 0.184** |
| Headache | N = 48 | 0(0) | N = 40 | 0(0%) | N = 8 | 0(0%) | 1.000** |
| Dizziness | N = 49 | 0(0) | N = 40 | 0(0%) | N = 9 | 0(0%) | 1.000** |
| Others | N = 49 | 3(6.1%) | N = 40 | 1(2.5%) | N = 9 | 2(22.2%) | 0.083** |
| CT findings | N = 49 | N = 39 | N = 10 | ||||
| Ground‐glass opacity | 31(64.6%) | 24(61.5%) | 7(77.8%) | 0.460** | |||
| Patchy shadows | 28(58.3%) | 19(48.7%) | 9(100%) | 0.006** | |||
| Fibrous stripes | 10(20.8%) | 10(25.6%) | 0(0%) | 0.172** | |||
| Pericardial effusion | 7(14.6%) | 0(0%) | 7(77.8%) | 0.000** | |||
| Pleural thickening | 10(20.8%) | 5(12.8%) | 5(55.6%) | 0.012** | |||
| Lymphadenia | 10(20.8%) | 3(7.7%) | 7(77.8%) | 0.000** | |||
| Bilateral pulmonary | 37(77.1%) | 29(74.4%) | 8(88.9%) | 0.662** | |||
| Right lung | 17(35.4%) | 11(28.2%) | 6(66.7%) | 0.051** | |||
| Left lung | 13(27.1%) | 7(17.9%) | 6(66.7%) | 0.007** | |||
| Laboratory examination | |||||||
| Cytokines | |||||||
| IL6, pg/mL | N = 31 | 11.27(2.11‐20.91) | N = 27 | 9.50(1.79‐18.09) | N = 4 | 22.88(18.90‐27.76) | 0.117* |
| IL10, pg/mL | N = 31 | 5.20(5.00‐13.05) | N = 27 | 5.00(5.00‐7.90) | N = 4 | 22.00(14.73‐60.00) | 0.008* |
| IL8, pg/mL | N = 31 | 9.50(6.55‐17.35) | N = 27 | 9.40(6.55‐15.85) | N = 4 | 29.05(14.35‐56.75) | 0.118* |
| TNF‐α, pg/mL | N = 31 | 7.70(6.10‐10.10) | N = 27 | 7.60(5.65‐9.00) | N = 4 | 23.00(9.65‐44.23) | 0.042* |
| IL1β, pg/mL | N = 31 | 5.00(5.00‐5.00) | N = 27 | 5.00(5.00‐5.00) | N = 4 | 5.00(5.00‐25.88) | 0.390* |
| IL2R, U/mL | N = 29 | 536.00(426.00‐825.00) | N = 27 | 529.00(385.00‐754.50) | N = 2 | 1729.50(1277.25‐2181.75) | 0.078* |
| Inflammatory factors | |||||||
| CRP, mg/L | N = 50 | 25.80(7.23‐57.73) | N = 40 | 13.05(4.70‐47.68) | N = 10 | 58.40(51.45‐141.25) | 0.002* |
| Organ damage index | |||||||
| ALT, U/L | N = 50 | 19.50(11.00‐36.50) | N = 40 | 19.50(11.00‐32.00) | N = 10 | 19.50(12.50‐44.50) | 0.855* |
| AST, U/L | N = 50 | 27.00(20.00‐41.00) | N = 40 | 25.00(20.00‐35.00) | N = 10 | 38.00(28.75‐64.50) | 0.069* |
| Urea, mmol/L | N = 50 | 3.85(2.80‐5.00) | N = 40 | 3.75(2.80‐4.50) | N = 10 | 5.50(2.75‐7.48) | 0.104* |
| Estimated glomerular filtration rate | N = 49 | 114.10(103.80‐120.00) | N = 40 | 114.80(102.83‐118.85) | N = 9 | 112.70(107.90‐129.50) | 0.308* |
| Total cholesterol, mmol/L | N = 50 | 3.51(3.01‐4.08) | N = 40 | 3.66(3.26‐4.15) | N = 10 | 2.97(2.90‐3.34) | 0.016* |
| Triglyceride, mmol/L | N = 29 | 1.56(0.95‐2.08) | N = 23 | 1.42(0.89‐1.93) | N = 6 | 1.88(1.57‐3.52) | 0.118* |
| Creatinine, μmol/L | N = 50 | 62.50(55.25‐76.00) | N = 40 | 63.00(56.75‐77.50) | N = 10 | 61.50(51.25‐67.50) | 0.331* |
| NT‐proBNP, pg/mL | N = 32 | 41.50(11.50‐333.50) | N = 23 | 29.00(8.00‐47.50) | N = 9 | 639.00(504.00‐1602.00) | 1.55 × 10−5 * |
| hs‐cTnI, pg/mL | N = 35 | 2.10(1.90‐4.45) | N = 27 | 1.90(1.90‐2.30) | N = 8 | 19.45(12.55‐98.67) | 3.26 × 10−5 * |
| Creatine kinase, U/L | N = 30 | 78.50(47.25‐180.25) | N = 25 | 83.00(52.00‐181.00) | N = 5 | 27.00(19.00‐120.00) | 0.278* |
| Immune globulin and complement | |||||||
| Albumin, g/L | N = 50 | 36.91 ± 5.67 | N = 40 | 38.63 ± 4.59 | N = 10 | 30.02 ± 4.22 | 4.66 × 10−5 *** |
| IgA | N = 23 | 2.43(2.09‐2.85) | N = 22 | 2.39(2.05‐2.80) | N = 1 | 3.74(3.74‐3.74) | 0.152* |
| IgG | N = 23 | 11.20(10.30‐12.60) | N = 22 | 11.10(10.25‐12.55) | N = 1 | 21.60(21.60‐21.60) | 0.152* |
| IgM | N = 23 | 1.38(1.04‐1.66) | N = 22 | 1.36(0.97‐1.68) | N = 1 | 1.42(1.42‐1.42) | 0.940* |
| Blood routine | |||||||
| Lymphocytes count, /μL | N = 50 | 1.11 ± 0.53 | N = 40 | 1.25 ± 0.49 | N = 10 | 0.58 ± 0.35 | 9.63 × 10−5 *** |
| Monocytes count, ×109/L | N = 50 | 0.41(0.32‐0.53) | N = 40 | 0.39(0.31‐0.51) | N = 10 | 0.51(0.37‐0.60) | 0.403* |
| Neutrophils count, ×109/L | N = 50 | 2.98(1.95‐5.58) | N = 40 | 2.63(1.98‐4.19) | N = 10 | 5.58(1.76‐5.87) | 0.291* |
| Eosinophils count, ×109/L | N = 50 | 0.01(0‐0.03) | N = 40 | 0.01(0‐0.05) | N = 10 | 0 | 0.022* |
| N = 50 | 0.035 ± 0.092 | N = 40 | 0.042 ± 0.102 | N = 10 | 0.004 ± 0.010 | 0.024*** | |
| Platelets count, ×109/L | N = 50 | 165.50(138.00‐213.00) | N = 40 | 170.00(144.75‐215.50) | N = 10 | 126(33.50‐168.75) | 0.055* |
| Coagulation | |||||||
| APTT, s | N = 39 | 40.21 ± 5.06 | N = 32 | 40.25 ± 4.65 | N = 7 | 39.99 ± 7.12 | 0.927*** |
| PT, s | N = 50 | 13.75(13.20‐14.68) | N = 40 | 13.60(13.08‐14.43) | N = 10 | 14.50(14.15‐16.20) | 0.006* |
| D‐dimer, ug/mL | N = 48 | 0.47(0.36‐1.09) | N = 40 | 0.44(0.31‐0.57) | N = 8 | 2.42(1.55‐5.59) | 1.89 × 10−5 * |
| Fibrinogen, g/L | N = 39 | 4.15 ± 1.42 | N = 32 | 4.68 ± 1.19 | N = 7 | 3.77 ± 2.15 | 0.316*** |
Notes: Continuous variables were described as the median and interquartile range (IQR) or mean and standard deviation (SD). Categorical variables were described as number (%).
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; APTT, activated partial thromboplastin time; BMI, body mass index; BNP, brain natriuretic peptide; CRP, C‐reactive protein; hs‐cTnI, hypersensitive cardiac troponin; IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M; IL‐6, Interleukin 6; IL‐10, interleukin 10; IL‐8, interleukin 8; IL‐1β, interleukin 1β; IL‐2R, interleukin 2 receptor; PT, plasma prothrombin time; TNF, tumor necrosis factor.
P values were calculated by Wilcoxon sum‐rank test.
P values were calculated by Fisher's exact test.
P values were calculated by Student t test.
Fever was the predominant symptom both in COVID‐19 with pre‐existing ILD (81.54%) and COVID‐19 without preexisting ILD patients (72.22%). Similarly, the comparison of other symptoms between COVID‐19 with preexisting ILD and COVID‐19 without preexisting ILD patients also failed to detect a significant difference such as chill (5.56% vs 0.77%), muscle ache (11.11% vs 2.31%), nausea and vomiting (5.56% vs 0.00%), dizziness (5.56% vs 0.00%). However, a higher proportion of COVID‐19 with preexisting ILD patients displayed cough (66.67% vs 29.23%, P = .004), expectoration (38.89% vs 2.31%, P < .001), fatigue (22.22% vs 6.15%, P = .041), difficulty breathing (50.00% vs 8.46%, P < .001), and diarrhea (33.33% vs 6.15%, P = .002) as compared to those of COVID‐19 without preexisting ILD patients (Table 1).
All the COVID‐19 with preexisting ILD patients performed chest CT‐scans at the time of admission, and 114 out of 130 COVID‐19 patients without preexisting ILD performed chest CT scans. It was noted that the typical CT images derived either from COVID‐19 with preexisting ILD or COVID‐19 without preexisting ILD patients were all characterized by the ground glass‐like shadows (42.86% vs 49.12%), patchy shadow (78.57% vs 63.16%), and pleural thickening (50.00% vs 33.33%). Remarkably, COVID‐19 with preexisting ILD was featured by the higher severity of honeycomb shadow (7.14% vs 0.00%, P = .038) and interlobular septum and intralobular interstitial thickening (78.57% vs 0.88%, P < .001). Furthermore, analysis of the lesion sites in CT‐scans revealed that no significant difference was noted between the COVID‐19 with preexisting ILD and COVID‐19 without preexisting ILD patients (Table 1).
To understand the clinical risk of COVID‐19 with preexisting ILD patients, we compared laboratory findings between two groups. The average number of lymphocytes in the COVID‐19 with pre‐existing ILD patients was 0.82 × 109/L, while it was 0.85 × 109/L for the COVID‐19 without preexisting ILD patients, and no significant difference was observed between the two groups (P = .972). Furthermore, we have compared the differences between the two groups based on the counts of eosinophils, NK cell, T + B + Nk cell, B cell, T cell, CD3 + CD4 + T cell and CD3 + CD8 + T cell, but failed to detect a significant difference. Similarly, no significant difference was noted in terms of renal function, liver function and heart function. In sharp contrast, COVID‐19 with preexisting ILD patients displayed a significantly higher level of neutrophils counts (5.78 × 109/L vs 4.16 × 109/L; P = .017), monocytes counts (0.59 × 109/L vs 0.41 × 109/L; P = .005), IL‐8 (31.90 pg/L vs 12.70 pg/L; P = .015), IL‐10 (71.55 pg/mL vs 5.00 pg/mL; P < .001), IL‐1β (20.85 pg/mL vs 5.00 pg/mL; P < .001), D‐Dimer (2.81ug/mL vs 1.07ug/mL; P = .001), as compared to those of COVID‐19 without preexisting ILD patients (Table 1).
All patients received the same protocol of antivirus treatments during hospitalization. It is worth reminding that COVID‐19 patients with preexisting ILD were more likely to have a poor outcome (39.29%), a percentage much higher than COVID‐19 without preexisting ILD patients (15.38%), P = .004 (Figure 1).
Figure 1.
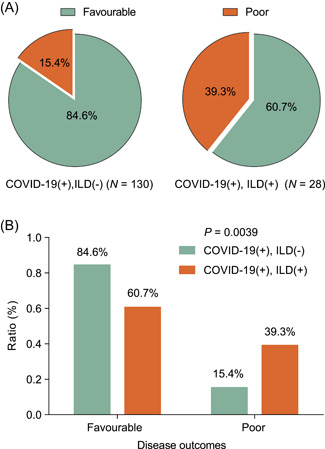
COVID‐19 patients with preexisting ILD were more likely to have a poor outcome (39.29%), a percentage much higher than COVID‐19 without preexisting ILD patients (15.38%), P = .004. COVID‐19, Coronavirus disease 2019; ILD, interstitial lung disease
Furthermore, Logistic regression models showed several factors related to the clinical outcomes of COVID‐19 (Figure 2). Among these factors, neutrophils counts (OR = 1.285; 95% CI = 1.116‐1.478; P < .0001), proinflammatory cytokines (tumor necrosis factor‐alpha [TNF‐α] (OR = 1.134; 95% CI = 1.030‐1.248; P = .010), interleukin (IL)6 (OR = 1.022; 95% CI = 1.011‐1.033; P < .0001), IL1β (OR = 1.160; 95% CI = 1.044‐1.289; P = .006), and IL2R (OR = 1.001; 95% CI = 1.000‐1.002; P = .024) were significantly associated with the poor clinical outcomes of COVID‐19. Moreover, we noted that organ damage indexes, such as liver damage biomarker TBIL (OR = 1.074; 95% CI = 1.005‐1.148; P = .035), Urea damage biomarker (OR = 1.231; 95% CI = 1.092‐1.388; P = .001), especially various coagulation dysfunction biomarkers (D‐Dimer, PT and Fbg; OR = 1.098~1.405; 95% CI = 1.034~1.912; P = .035~.001), were observed to be significantly associated with the poor clinical outcomes of COVID‐19. Strikingly, these proinflammatory and coagulation dysfunction factors were substantially elevated in ILD patients as compared to non‐ILD patients with COVID‐19 (Table 1), suggesting upregulation of those factors might result in poor outcomes of COVID‐19 in ILD patients.
Figure 2.
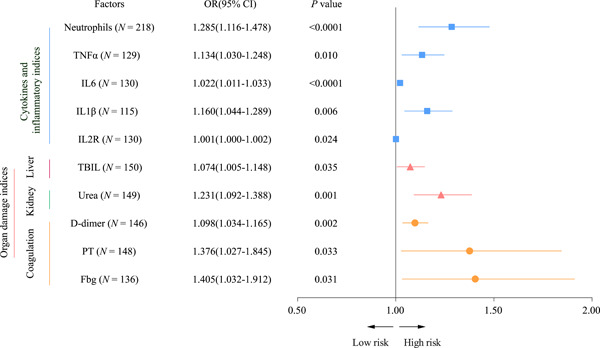
Logistic regression models showed several factors related to the clinical outcomes of COVID‐19. COVID‐19, Coronavirus disease 2019
4. DISCUSSION
This is the first retrospective study with a comparison between COVID‐19 patients with preexisting ILD and COVID‐19 in patients without preexisting ILD. ILD, a group of diseases with impaired interstitial lungs, the incidence of which is increasing by years and it has increasingly captured attention from both clinicians and patients. 9 , 10 Nevertheless, the incidence and severity of COVID‐19 among ILD patients remain unknown. Given such facts, the clinical features of COVID‐19 patients with preexisting ILD are worth exploring. Therefore, this study is designed to analyze the clinical features between COVID‐19 and COVID‐19 patients with preexisting ILD based on the incidence, severity, clinical features, laboratory findings, CT imaging, and patient outcomes.
As a leading hospital in Hubei Province, Tongji Hospital serves as one of the main designated hospitals to carry outpatient admission. In particular, From 7 February to 27 March 2020, 3201 COVID‐19 cases were hospitalized, while 28 of which had ILD as the underlying disease, which accounted for a relatively low proportion (0.88%) of the total hospitalized cases. In general, however, based on the analysis of SARS‐CoV‐2 in previous studies, SARS‐CoV‐2 is highly transmissible in humans, especially in the elderly and people with underlying diseases. 1 , 6 , 11 The low incidence in our report could be caused by following reasons: First, the incidence of ILD is indeed not as high as other underlying diseases including hypertension, diabetes, cerebrovascular disease, etc. 9 , 12 , 13 , 14 ; Second, ILD has undergone significant evolution in recent years, with more complex, ever‐expanding disease classification. Despite notable advances, progress has been challenged by a poor understanding of pathological mechanisms and difficult diagnose, 15 meaning that some ILD patients may escape diagnosis in the clinical practice; Finally, and most important, it's known that SARS‐CoV‐2 infects cells with angiotensin‐converting enzyme 2 (ACE2) as a receptor, while previous studies suggested that decreased angiotensin II mRNA and its activity not only in the lung tissue of patients with idiopathic pulmonary fibrosis but also in a mouse model of bleomycin‐induced pulmonary fibrosis. 16 , 17 , 18 Therefore, we assume that the reduced infection rate of ILD could be caused by decreased angiotensin II mRNA and its biological activity. However, due to the lack of research data on this conclusion, more exploration is needed.
Among 28 cases included in this study, the median age was 68 years, this finding is different from the average age (55.5 years) of the entire hospitalized COVID‐19 patients previously published in Lancet, 6 but consistent with the fact that ILD patients are mostly elderly. 19 In terms of gender, 23 were males (82.1%), while five were females (17.9%), which is consistent with the epidemiology that ILD is always encountered primarily among males. Furthermore, nine cases were common cases (32.14%), while 19 cases were severe or critical (67.85%), suggesting that even though ILD patients could be less vulnerable to SARS‐CoV‐2, once SARS‐CoV‐2 infection occurs, COVID‐19 patients with ILD tend to serious condition.
Subsequently, 28 COVID‐19 patients with ILD were statistically matched with 130 patients without ILD at a ratio of 1: 4 based on age, gender, and disease severity. The main symptom of COVID‐19 patients with ILD is fever, which is similar to the clinical manifestations of patients with COVID‐19 without ILD. Note that patients with COVID‐19 who had preexisting ILD are more likely to have cough, sputum, fatigue, dyspnea, and diarrhea, which is consistent with the characteristic that ILD patients. Given the fact that the number of COVID‐19 patients with preexisting ILD is limited, further investigations with large‐scale of patients would be necessary.
Intriguingly, we did not found a significant difference in terms of lymphocytes and lymphocyte subsets between COVID‐19 patients with preexisting ILD and COVID‐19 patients without ILD, while the number of neutrophils, monocyte was significantly higher in COVID‐19 patients with preexisting ILD as compared to that of COVID‐19 patients without ILD. Also, the levels of IL‐8, IL‐10, IL‐1β, and D‐D dimer were significantly higher in COVID‐19 patients with preexisting ILD. Indeed, previous studies have confirmed that IL‐8, IL‐10, and IL‐1β are related to the occurrence and development of ILD. 20 , 21 , 22 Also, mostly studied has been done on IL‐8, which can be secreted by a variety of inflammatory cells. Then, in turn, IL‐8 could migrate neutrophils and macrophages from pulmonary capillaries to alveoli, intralobular interstitial and interlobular septum, and activate neutrophils and macrophages, causing lung inflammation and forming a vicious cycle. 23 , 24 In this process, if the alveolar inflammation is not controlled (such as the inability to clear SARS‐CoV‐2), the alveolar inflammation will further aggravate the original pulmonary interstitial fibrosis. Meanwhile, D‐D dimer has been confirmed by Cao et al 25 as an indicator of coagulation function and can be used as a high‐risk factor to reflect the prognosis of COVID patients.
CT imagings suggested no differences in characteristics that can reflect the severity of the disease, including ground glass shadow, patch shadow, and viral lung distribution area. However, COVID‐19 patients with per‐existing ILD had more intralobular interstitial and interlobular septum thickening and honeycomb shadows, which are the typical images derived from chest CT scan in ILD patients. It is worthy of note that even the two groups were statistically matched in age, gender, and disease severity, COVID‐19 patients with per‐existing ILD (11 cases, 39.29%) had a significantly worse prognosis than COVID‐19 patients without ILD (20 cases, 15.38%), P = .004.
To further analyze which clinical indicators are independent risk factors affecting the clinical outcome of patients with COVID‐19 with per‐existing ILD, we proposed a logistic regression model to analyze several factors related to COVID‐19. Neutrophils counts, proinflammatory cytokines (IL6, IL1β, and IL2R) were significantly associated with the poor clinical outcomes of COVID‐19. And more, various coagulation dysfunction biomarkers (D‐Dimer, PT, and Fbg), were observed to be significantly associated with the poor clinical outcomes of COVID‐19. All of these findings demonstrate that aggravated inflammatory responses and coagulation dysfunction appear to be the cruicial mechanisms in COVID‐19 patients with ILD.
Our retrospective study included 28 COVID‐19 cases with per‐existing ILD in Tongji Hospital from 7 February to 27 March 2020. During the same period, a total of 3201 inpatients with COVID were admitted to Tongji Hospital, of which only 0.88% had COVID‐19 patients with preexisting ILD, which could be related to ILD low incidence, escaping diagnosis and the low expression of angiotensin II in ILD patients, while once COVID‐19 occurs in patients with ILD, the severity could reach up to 67.85%. Even with statistically matched age, gender, and disease severity, the prognosis of COVID‐19 patients with per‐existing ILD is still significantly worse than that of patients without ILD. And more, our findings demonstrate that aggravated inflammatory responses and coagulation dysfunction appear to be the key mechanisms in COVID‐19 patients with ILD.
4.1. Interpretation
ILD patients could be less vulnerable to SARS‐CoV‐2. However, ILD patients tend to severity condition after being infected with SARS‐CoV‐2. The prognosis of COVID‐19 patients with per‐existing ILD is significantly worse than that of non‐ILD patients. And more, aggravated inflammatory responses and coagulation dysfunction appear to be the critical mechanisms in the COVID‐19 patients with ILD.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
HZ, AD, YD, JS, and HD collected the epidemiological and clinical data. JT contributed to the statistical analysis. HH, MZ, CC, and YW drafted the original manuscript. All authors contributed to data acquisition, data analysis, or data interpretation, and reviewed and approved the final version.
Huang H, Zhang M, Chen C, et al. Clinical characteristics of COVID‐19 in patients with preexisting ILD: A retrospective study in a single center in Wuhan, China. J Med Virol. 2020;92:2742–2750. 10.1002/jmv.26174
Contributor Information
Jin Shang, Email: 15972949829@163.com.
Yan Deng, Email: dy102398@163.com.
Aihua Du, Email: ahdu@tjh.tjmu.edu.cn.
Huaping Dai, Email: daihuaping@ccmu.edu.cn.
REFERENCES
- 1. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hui DS, Azhar EI, Madani TA, et al. The continuing 2019nCoV epidemic threat of novel coronaviruses to global health the latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan China: the mystery and the miracle. J Med Virol. 2020;92:401‐402. 10.1002/jmv.25678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Update on the Epidemic Situation of New Coronavirus Pneumonia as of 24:00 on March 31. http://www.nhc.gov.cn/xcs/yqfkdt/202004/28668f987f3a4e58b1a2a75db60d8cf2.shtml. Accessed April 1, 2020.
- 5. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323:1061‐1069. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bradley B, Branley HM, Egan JJ, et al. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. Thorax. 2008;63(suppl 5):v1‐v58. [DOI] [PubMed] [Google Scholar]
- 8.Notice on Issuing a New Coronavirus Infected Pneumonia Diagnosis and Treatment Plan (Trial Version 5). http://www.gov.cn/zhengce/zhengceku/2020-02/05/content_5474791.htm. Accessed April 1, 2020.
- 9. Ozawa Y, Abe T, Omae M, et al. Impact of preexisting interstitial lung disease on acute, extensive radiation pneumonitis: retrospective analysis of patients with lung cancer. PLoS One. 2015;10(10):e0140437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jones Kirk D. Unclassifiable interstitial lung disease: a pathologist's perspective. Eur Respir Rev. 2018;27(147):170132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8:475‐481. 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hajjar I, Kotchen JM, Kotchen TA. Hypertension: trends in prevalence, incidence, and control. Annu Rev Public Health. 2006;27(1):465‐490. [DOI] [PubMed] [Google Scholar]
- 13. Ramsey SD, Newton K, Blough D, et al. Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care. 1999;22(3):382‐387. [DOI] [PubMed] [Google Scholar]
- 14. Silventoinen K, Magnusson PK, Tynelius P, Batty GD, Rasmussen F. Association of body size and muscle strength with incidence of coronary heart disease and cerebrovascular diseases: a population‐based cohort study of one million Swedish men. Int J Epidemiol. 2008;1:1‐8. [DOI] [PubMed] [Google Scholar]
- 15. Jacobs RL, Andrews CP, Coalson J. Organic antigen‐induced interstitial lung disease: diagnosis and management. Ann Allergy Asthma Immunol. 2002;88(1):30‐41. [DOI] [PubMed] [Google Scholar]
- 16. Uhal BD, Dang M, Dang V, et al. Cell cycle dependence of ACE‐2 explains downregulation in idiopathic pulmonary fibrosis. Eur Respir J. 2013;42(1):198‐210. [DOI] [PubMed] [Google Scholar]
- 17. Li X, Zhuang J, Rayford H, Zhang H, Shu R, Uhal BD. Attenuation of bleomycin‐induced pulmonary fibrosis by intratracheal administration of antisense oligonucleotides against angiotensinogen mRNA. Curr Pharm Des. 2007;13(12):1257‐1268. [DOI] [PubMed] [Google Scholar]
- 18. Li F, Li W, Farzan M, Harrison SC. Structure of SARS coronavirus spike receptor‐binding domain complexed with receptor. Science. 2005;309(5742):1864‐1868. [DOI] [PubMed] [Google Scholar]
- 19. Wu C, Wang Q, He L, Yang E, Zeng X. Hospitalization mortality and associated risk factors in patients with polymyositis and dermatomyositis: a retrospective case‐control study. PLoS One. 2018;13(2):e0192491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kawasumi H, Gono T, Kawaguchi Y, et al. IL‐6, IL‐8, and IL‐10 are associated with hyperferritinemia in rapidly progressive interstitial lung disease with polymyositis/dermatomyositis. BioMed Res Int. 2014;2014(1):815245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barlo NP, van Moorsel CHM, Korthagen NM, et al. Genetic variability in the IL1RN gene and the balance between interleukin (IL)‐1 receptor agonist and IL‐1β in idiopathic pulmonary fibrosis. Clin Exp Immunol. 2011;166:166‐351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kline JN, Schwartz DA, Monick MM, Floerchinger CS, Hunninghake GW. Relative release of lnterleukin‐1β and lnterleukin‐1 receptor antagonist by alveolar macrophages:a study in asbestos‐induced lung disease, sarcoidosis, and idiopathic pulmonary fibrosis. Chest. 1993;104(1):47‐53. [DOI] [PubMed] [Google Scholar]
- 23. Gono T, Kaneko H, Kawaguchi Y, et al. Cytokine profiles in polymyositis and dermatomyositis complicated by rapidly progressive or chronic interstitial lung disease. Rheumatology. 2014;53(12):2196‐2203. [DOI] [PubMed] [Google Scholar]
- 24. Lynch JP, Standiford TJ, Rolfe MW, Kunkel SL, Strieter RM. Neutrophilic alveolitis in idiopathic pulmonary fibrosis: the role of interleukin‐8. Am Rev Respir Dis. 1992;145(6):1433‐1439. [DOI] [PubMed] [Google Scholar]
- 25. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]


