Dear Editor,
Dermatological manifestations of the Coronavirus Disease 2019 (COVID‐19) are being described progressively. Cutaneous findings include pseudo‐chilblains, petechial exanthems, urticarial exanthems, morbilliform exanthems, and livedoid changes. 1 , 2 , 3
With this letter, we want to acknowledge minor aphthae as a new mucous finding possibly related to COVID‐19. This letter reports four cases during the COVID‐19 pandemic in Madrid (Spain).
All four patients attended the emergency care unit with a history of typical COVID‐19 symptoms (anosmia, fever, headache, malaise, and dyspnea), and real‐time reverse transcription polymerase chain reaction (RT‐PCR) for Severe Acute Respiratory Syndrome Coronavirus‐2 (SARS‐CoV‐2) from a nasopharyngeal swab was positive in all of them. Chest radiographs, both posteroanterior and lateral, were performed. Only two of them had pulmonary infiltrates compatible with COVID‐19‐induced pneumonia. During the course of the disease, all of the patients developed minor aphthae (Fig. 1a‐d). The particularities of each case are summarized in Table 1.
Figure 1.
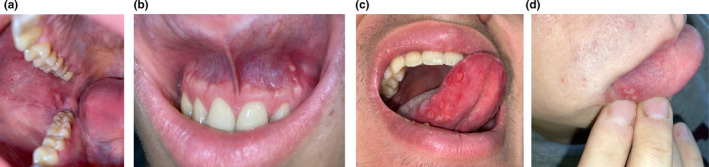
(a) Single ulcer in the right buccal mucosa, with erythematous peripheral rim. (b) Single aphthous ulcer in the superior mucogingival junction. (c) Seven aphthae in the ventral right side of the tongue mucosa. (d) Four clustered aphthae in the right side of the inferior labial mucosa
Table 1.
Summary of the features of our four cases of aphthosis in the context of SARS‐CoV‐2 infection
| Age and sex | Number of aphthae | Location | Latency from COVID‐19 symptoms onset | History of recurrent aphthous stomatitis | Other findings | |
|---|---|---|---|---|---|---|
| Case 1 | 43‐year‐old female | 1 | Right buccal mucosa | 4 days | Yes |
Bilateral pneumonia, mild lymphopenia |
| Case 2 | 33‐year‐old male | 1 | Superior mucogingival junction | 3 days | No |
Pneumonia of right lower pulmonary field, mild lymphopenia |
| Case 3 | 37‐year‐old male | 7 | Ventral right side of tongue mucosa | 5 days | No | |
| Case 4 | 19‐year‐old male | 4 | Right side of inferior labial mucosa | 0 days | No |
Tests for excluding secondary causes of aphthosis were performed in all four patients. PCR testing for herpes simplex virus from an ulcer swab rendered a negative result in all of them. Serologies for syphilis, hepatitis B, hepatitis C, human immunodeficiency virus, Epstein‐Barr virus, and cytomegalovirus were negative. Antinuclear antibodies, antineutrophil cytoplasmic antibodies, celiac disease antibodies, rheumatoid factor, and HLA‐B51 were negative. Complement levels were normal. Underlying deficiencies of vitamin B12, folate, and iron were excluded. Complete blood count was normal in all of them, except for mild lymphopenia in cases 1 and 2.
DISCUSSION
Minor aphthous ulcers are a common condition and can be seen in relation to many triggers and comorbidities such as trauma, smoking, infections, rheumatologic diseases, nutritional deficiencies, and drugs, among other causes. 4
Common features in our patients were a low number of aphthae (ranging from one to more than five) and a tendency to affect younger patients. All aphthae measured less than 1 cm, and most of them had a creamy‐white fibrin surface and an erythematous peripheral ring. They affected predominantly non‐keratinized mucosa. Only the patient in case 1 had a history of recurrent aphthous stomatitis, with this being the first episode of aphthae for the rest of them.
To this day, some mucosal lesions associated with COVID‐19 have been described, mainly blisters on the labial mucosa and ulcers on the hard palate. 5
It has been proven that tumor necrosis factor alpha(TNF‐α) is elevated in the serum of patients with recurrent aphthous stomatitis, leading to augmented endothelial cell adhesion and neutrophil chemotaxis, thus initiating the process of ulcer formation. 4 We believe that COVID‐19 may induce these aphthous flares due to the cytokine storm triggered by the virus (in which, among others, TNF‐α plays a major role), that would lead neutrophils to attack the oral mucosa. 6
Because oral aphthous ulcers are common occurrences and may arise from various causes, and due to the small number of patients in our report, the causal association of oral aphthous ulcers with COVID‐19 infection cannot be shown.
By sharing these cases, it is our hope to add more information to the scarce knowledge we have on this disease. More studies are needed to elucidate the relation between SARS‐CoV‐2 and oral aphthae.
Acknowledgments
The patients in this manuscript have given written informed consent to the publication of their case details.
All human and animal studies are approved by an Institutional Review Board.
Conflict of interest: None.
Funding source: None.
References
- 1. Diaz‐Guimaraens B, Dominguez‐Santas M, Suarez‐Valle A, et al. Petechial skin rash associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol 2020. 10.1001/jamadermatol.2020.1741 [DOI] [PubMed] [Google Scholar]
- 2. Suarez‐Valle A, Fernandez‐Nieto D, Diaz‐Guimaraens B, Dominguez‐Santas M, Carretero I. Perez‐Garcia B. Acro‐ischemia in hospitalized COVID‐19 patients. J Eur Acad Dermatol Venereol 2020. 10.1111/jdv.16592. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID‐19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol 2020. 10.1111/bjd.19163. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Akintoye SO, Greenberg MS. Recurrent aphthous stomatitis. Dent Clin North Am 2014; 58: 281–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Martín Carreras‐Presas C. Amaro Sánchez J, López‐Sánchez AF, Jané‐Salas E, Somacarrera Pérez ML. Oral vesiculobullous lesions associated with SARS‐CoV‐2 infection. Oral Dis 2020. 10.1111/odi.13382. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mehta P, McAuley DF, Brown M, et al. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet Lond Engl 2020; 395: 1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]


