Abstract
Purpose
Testosterone (T) plays an important role in men’s health and its deficiency is linked with poorer health. Yet, the role of nutritional and lifestyle factors in T regulation and production remains unclear. The objectives are to comprehensively test the cross-sectional associations of nutritional and lifestyle factors with T deficiency and to validate the associations in the NHANES survey.
Methods
We performed weighted multivariable logistic regression analysis to examine the association of 173 nutritional and lifestyle factors with T deficiency (total testosterone ≤ 3.5 ng/mL) in the NHANES III as the discovery set (mean age 41). We controlled for multiple comparisons with a false discovery rate (FDR) < 5% and replicated in the NHANES 1999–2004 (mean age 44).
Results
We identified seven nutritional factors to be inversely associated with T deficiency in NHANES 1999–2004, namely dietary intake of vitamin A, protein, saturated fatty acids, monounsaturated fatty acids, total fats, saturated fatty acid 16:0, and phosphorus. In a multivariable model, only vitamin A intake remained significantly associated with T deficiency (OR: 0.97, 95% CI: 0.94–0.99). Principal component analysis suggested two principal components 1) dietary fats, protein and phosphorous and 2) total vitamin A to be associated with T deficiency.
Conclusion
Our systematic evaluation provided new insight on the modifiable factors that could play a role in the regulation of T production. This study has the potential to contribute to the current body of literature to define a clinical definition for T deficiency after taking into account nutritional and lifestyle factors.
Keywords: testosterone, nutrition, EWAS, NHANES
Introduction
Testosterone (T) is linked with many physiologic effects, including improved sexual function, physical performance, muscle strength, lean body mass, cognitive function and an increased risk of cardiovascular mortality and morbidities [1–3]. Low levels of T is associated with cardiovascular diseases [4], cancer [5] and all-cause mortality [6]. In the United States alone, studies have reported that as many as 38.7% of men 45 years or older demonstrate low levels of T [7, 8] or T deficiency (total testosterone ≤ 350 ng/dL)[9] with a projection that by 2025 approximately 6.5 million men will have T deficiency [8, 10]. The obesity epidemic and the aging of the US male population have contributed, in part, to the increasing prevalence of T deficiency [11]. Yet, the role of other nutritional and environmental factors in the regulation of T production has not been widely investigated.
Recently, Patel et al. proposed a study design analogous to genome wide association studies (GWAS), “the environment-wide association study (EWAS)” to comprehensively test and analytically validate nutritional and environmental factors associated with chronic diseases and other clinical phenotypes [12]. Similar to GWAS, EWAS does not test only one or a few associations at a time. Rather, it simultaneously evaluates multiple environmental factors for association with proper adjustment for multiple comparisons. Subsequently, the identified significant associations are further validated across independent datasets [12]. Therefore, in this study, we propose to utilize an EWAS approach to comprehensively test nutritional and lifestyle factors in relation to T deficiency in the NHANES III (1988–1994) discovery set and further replicate significant associations in the continuous NHANES 1999–2004.
Methods
Study Population
The National Center for Health Statistics (NCHS) conducted the Third National Health and Nutrition Examination Survey (NHANES III), a cross-sectional study of the United States (US) civilian noninstitutionalized population aged two months or older, between 1988 and 1994. NHANES III used a multistage, stratified and clustered probability sampling in which Mexican-Americans, non-Hispanic blacks, the elderly and young children were oversampled to ensure adequate samples sizes [13]. NHANES III had two phases (1988–1991 and 1991–1994) from which independent, unbiased national estimates of health and nutrition can be calculated. Participants were interviewed at home and asked detailed demographic and health-related questions. Participants also underwent extensive physical and laboratory examinations in a mobile examination center or at a home visit. Trained-personnel collected blood samples from participants after an overnight fast under standardized conditions [13]. For studies on sex steroid hormones, we selected men who participated in the morning session to reduce diurnal variation in serum levels conditions. Men with a self-reported history of cardiovascular disease or prostate cancer were excluded because certain treatments may affect hormones levels. From this population, we selected 1,316 male participants aged 20 and older in the first phase of NHANES III as the discovery set, and findings from this data set were replicated in the continuous NHANES 1999–2004 (n = 890).
The continuous NHANES 1999–2004 is a program of studies undertaken by the NCHS of the US Centers for Disease Control and Prevention (CDC) to assess the health and nutritional status of adults and children in the United States. This study includes data from 890 male participants in the 1999–2000, 2001–2002 and 2003–2004 NHANES cycles with complete sex hormone data. Similar to NHANES III, continuous NHANES uses a multistage, stratified and clustered probability sampling strategy in which minority populations and the elderly are oversampled to ensure adequate representativeness of the total US civilian, noninstitutionalized population. Continuous NHANES includes an interview and an examination component that includes morning blood collection to reduce diurnal variation of hormones, and men with a history prostate cancer and younger than 20 years of age were excluded as well. Investigators are allowed to access surplus sera for approved studies. Details of the survey design, methods and data collections are available on the NHANES website (https://www.cdc.gov/nchs/nhanes/index.htm. Accessed May 2018).
The NHANES program is approved by the Institutional Review Board of the NCHS at CDC. Informed consent was obtained from all participants. Addressing questions about hormones and men’s health in NHANES was approved by the National Institutes of Health Office of Human Subjects Research and the NCHS Ethics Review Board at CDC.
Assessment of testosterone, estradiol and SHBG
In NHANES III, serum concentrations of total T, total estradiol, and SHBG were previously measured in Dr. Nader Rifai’s laboratory at Children’s hospital (Boston, MA) by electrochemiluminescence immunoassays on a 2010 Elecsys autoanalyzer (Roche Diagnostics, Indianapolis, IN). The lowest limits (LOD) of detection were 2 ng/mL for T, 5 pg/mL for estradiol and 3 nmol/L for SHBG; no samples had concentrations below LOD for these analytes. Values of total T below or equal to 350 ng/dL [12.15 nmol L−1] are considered T deficiency [9].
In continuous NHANES 1999–2004, details on the blood draw, process, storage and shipping methods are provided elsewhere [14]. As in NHANES III, total T, estradiol and SHBG were measured using the Elecsys 2010 system (Roche Diagnostics, Laval, QC, Canada). The laboratory technicians were blinded to the participant characteristics of the samples. The LOD of the assays were 2 ng/dL for T, 5 pg/mL for estradiol and 3 nmol/L for SHBG. One sample had a concentration below the LOD for T and 10 samples for estradiol, which were assigned to half the limit of detection. T deficiency was also defined as total T below or equal to 350 ng/dL [12.15 nmol L−1] [9]. In this study, the term T deficiency does not imply that a deficit needs to be replaced, therefore, its use is analogous to low T, but we avoided it to not be confused with the business centers with the same words. Twenty-one samples were assayed in duplicate for quality control purposes, and the coefficients of variation were 4.8% for testosterone, 21.4% for estradiol and 5.6% for SHBG. Testosterone deficiency with calculated free testosterone (< 225 pM) was also calculated [9].
Assessment of exposures and covariates
There were a total of 173 factors, including 133 nutritional and 40 lifestyle factors, in our EWAS in NHANES III (Supplementary Table S1, Supplementary Data). Data collected ranged from information obtained through the interview, such as smoking history, as well as physical and laboratory examination, for example serum vitamin C concentrations. Examples of markers and categories are presented in Table 1. These factors were assessed either as continuous or categorical variables. The majority of continuous variables had a right-skewed distribution. We transformed these variables into standardized z-scores by subtracting the mean and dividing by the standard deviation (SD) of the population. For categorical variables, we consistently defined one value as the referent category or the ‘negative’ result, for example ‘never smoker’ as the reference for ‘current smoker’. Vigorous physical activity (yes, no) was defined as participating three or more times per week in leisure-time physical activities with metabolic equivalent ≥ 6 for those aged 60 and older, and metabolic equivalent ≥7 for those younger than 60. Secondary exposure to smoking among never smokers (never smoked ≥100 cigarettes) was defined as exposure to smoke at home (≥1 person smoke at home) or at work (≥1 h smoke exposure at work). All exposure variables were assessed with standard procedures as detailed in the NHANES III documentation [13].
Table 1.
Number and examples of nutrition and lifestyle factors in NHANES III
| Factor category | Number of variables† | Examples |
|---|---|---|
| Nutrition | ||
| Food nutrient recall | 104 | Dietary fibre (continuous) |
| Aspartame (continuous) | ||
| Energy from protein (continuous) | ||
| Healthy Eating Index (HEI) | 10 | Total HEI score (continuous) |
| Nutrients and minerals (serum and urine) | 17 | Serum vitamin A (continuous) Serum selenium (continuous) |
| Alcohol use | 1 | Drink alcohol twice or more a day (yes/no) |
| Caffeinated beverages | 1 | Drink caffeinated beverages twice or more a day (yes/no) |
| Lifestyle factors | ||
| Personal smoking | 14 | Current smoker (reference: never smoker) |
| Ever smoked 20 cigars in life (yes/no) | ||
| Serum cotinine (continuous) | ||
| Environmental smoking | 4 | Does anyone smoke in the home (yes/no) |
| At work, hours per day can smell smoking (ordinal) | ||
| Physical activity | 1 | Vigorous physical activity (yes/no) |
| Social support | 2 | How many times talking on the phone with family, friends or neighbors per week (ordinal) |
| Environmental pollutants | 2 | Serum lead (continuous) |
| Urine cadmium (continuous) | ||
| Bacterial infection | 2 | Helicobacter pylory antibody (continuous) |
| Viral infection | 8 | Herpes Simplex Virus I antibody (positive/negative) |
| Hepatitis A antibody (positive/negative) | ||
| Parasite infection | 1 | Toxoplasma antibody (continuous) |
| Painkiller use | 6 | Taken aspirin in the past month (yes/no) |
| # NSAIDs taken in the past month (ordinal) |
This table summarizes the number of variables available for each factor category from the Supplementary Table S1 (e.g. there were 104 available variables under the Nutrition category, and 10 variables to define the Healthy Eating Index).
The following variables have been suggested to strongly affect nutritional and environmental factors and T levels and were therefore considered as confounders in our study: age, race/ethnicity, education, body mass index (BMI), estradiol, SHBG, and socioeconomic status (SES). Race/ethnicity was categorized into Non-Hispanic white, Non-Hispanic black, Mexican-American and other. We classified educational attainment as less than high school, high school equivalent, and higher than high school. BMI was calculated from measured weight and height (weight in kilograms divided by height in meteres squared). SES was estimated with poverty-to-income ratio (PIR), a ratio of total family income to the official poverty threshold at the family level. A PIR <1 indicated that income was less than the level of poverty. We categorized PIR into <1, 1-<2, 2-<3, and ≥ 3, indicating the lowest to highest SES as previously described [15].
Detailed information on the laboratory methods used and quality measures for metabolic factors/disorders in NHANES III have been reported previously [13, 16]. Briefly, hypertension was defined by blood pressure of ≥130 / ≥85 mmHg or any use of antihypertensive drugs. Diabetes was defined as fasting glucose ≥110 mg/dL or any use of insulin or glucose-lowering drugs. Any HDL-cholesterol levels <40 mg/dL for men was considered as low. Triglyceridemia was defined as any levels of triglycerides ≥150 mg/dL. Blood pressure was measured with mercury manometer and the average of the second and third blood pressure measurements was taken. Fasting plasma glucose levels were measured by using a modified hexokinase enzymatic method (Roche Diagnostic Systems, Inc., Montclair, NJ, USA). Blood lipids were enzymatically measured using the Hitachi 704 Analyzer (Boehringer Mannheim Diagnostics, Indianapolis, IN, USA).
Statistical analysis
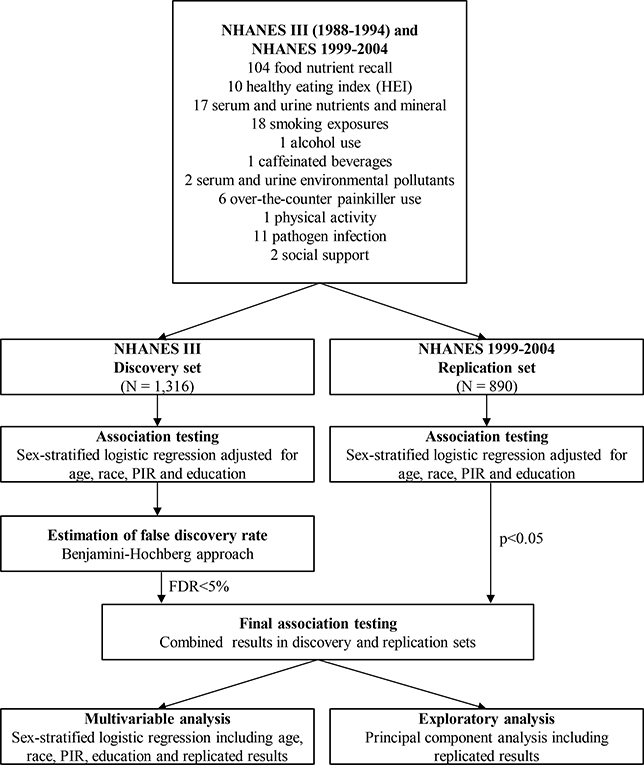
Sampling weights specific for each phase were applied to take into account selection probabilities, over-sampling, non-response, and differences between the sample and the total US population. Figure 1 depicts the analytical steps in this study which were similar to previously published nutrient- and environment-wide studies [12, 17]. T deficiency was used in the dichotomized format as the main outcome[9] given that this definition is becoming widely known and accepted due to its clinical relevance with health outcomes, including chronic diseases [9, 18]. We selected factors corresponding to categories of environmental exposures used in previous EWAS studies as summarized in Table 1 [12, 17]. First, each of the 173 nutrition and lifestyle factors was assessed in relation to T deficiency in the discovery set, phase I of the NHANES III. Survey-weighted logistic regression was used to examine the association of continuous and dichotomous nutrition and lifestyle factors in men. All models were linearly adjusted for age, race/ethnicity, education, BMI and PIR by adding each term into the regression model. Pearson correlation coefficients were calculated for environmental and nutritional factors visualized with a heatmap in Supplementary Figure S2.
Figure 1.
Diagram representing environment-wide analysis of nutrition and lifestyle factors in the NHANES III and NHANES 1999–2004.
We estimated the false discovery rate (FDR) among findings in the discovery set. FDR is the expected proportion of false discoveries, among all significant findings at a given significance level[19]. We estimated FDR using the Benjamini-Hochberg step down method to select factors with statistically significant association with T deficiency and FDR < 5% in the discovery set [20].
Replicated analysis of assessed nutrition and lifestyle factors was subsequently performed by re-running similar logistic regression models in the second dataset, namely NHANES 1999–2004 for 16 out of 28 factors identified in the discovery dataset (because not all of them were available). Only nutrition or lifestyle factors with both FDR < 5% in the first dataset and P value < 0.05 in the replication set were considered valid. Furthermore, analysis for replicated factors in the overall study population was repeated in additional multivariable models adjusting for age, race, PIR, education, SHBG, and estradiol and models incorporating all replicated factors.
Finally, we conducted principal components analysis to understand any underlying factors associated with T deficiency based on inter-correlation among tentatively replicated nutrition and lifestyle factors in the overall study population. Principal component analysis was performed with an orthogonal varimax rotation procedure. An eigenvalue of 41 was used to define the number of principal components to be extracted from our data [21]. Proportion of variance in abdominal obesity explained by each principal component was estimated, and 95% confidence intervals of this estimation were obtained using 1000 bootstrap resampling [22]. We further estimated the value of principal components identified and assessed them in relation to T deficiency using similar multivariable approach.
The NHANES III datasets were prepared with Statistical Analysis Software release 9.3 (Statistical Analysis Software Institute, Cary, NC, USA). All analyses were performed with R version 3.1.2 (R Foundation for Statistical Computing) [23]. The survey package was used to account for sampling weights and the psych package was used to perform principal component analysis. Circlize package was used to generate the circle plot.
Results
Descriptive characteristics of the study participants are shown in Table 2 (NHANES III and NHANES 1999–2004), and the means (s.d.) and frequencies of investigated nutrition (n = 133) and lifestyle factors (n = 40) are presented in Supplementary Table S1. There were approximately 19% of men with T deficiency, who were older, Non-Hispanic white, had higher BMI, hypertension, diabetes, low-HDL cholesterol and triglyceridemia, but lower poverty-to-income ratio, SHBG and estradiol levels compared to men without deficiency (Table 2). Similar characteristics were observed for men in NHANES 1999–2004 with the exception of SHBG that was not statistically different between the groups and that poverty-to-income ratio was higher among men with T deficiency (Table 2). The distribution of these characteristics were also observed by calculated free testosterone deficiency and differences were similar (Supplementary Table S3).
Table 2.
Descriptive characteristics of study participants in NHANES III (discovery set) and NHANES 1999–2004
| NHANES III | NHANES 1999–2004 | ||||||
|---|---|---|---|---|---|---|---|
| Testosterone deficiency (N=243) | No testosterone deficiency (N=1073) | P-value | Testosterone deficiency (N=213) | No testosterone deficiency (N=677) | P-value | ||
| Age – mean (SE) | 51.40 (1.08) | 41.36 (0.73) | <0.0001 | 50.50 (1.62) | 41.35 (0.79) | 0.0003 | |
| Race (%) | 0.57 | 0.06 | |||||
| Non-Hispanic white | 76.72 | 78.89 | 74.95 | 71.05 | |||
| Non-Hispanic black | 9.54 | 9.52 | 6.73 | 11.37 | |||
| Mexican-American | 4.03 | 4.65 | 12.82 | 13.16 | |||
| Others | 9.71 | 6.94 | 5.50 | 4.42 | |||
| Poverty-to-income ratio (PIR) (%) | 0.11 | 0.98 | |||||
| <1 | 3.94 | 10.76 | 10.77 | 10.76 | |||
| 1–2 | 21.16 | 18.30 | 17.30 | 22.38 | |||
| 2–3 | 32.09 | 19.74 | 10.78 | 14.62 | |||
| ≥3 | 42.81 | 51.20 | 61.16 | 52.24 | |||
| Body mass index (kg/m2) – mean (SD) | 28.70 (0.70) | 25.93 (0.70) | 0.0003 | 31.82 (0.80) | 26.67 (0.24) | <0.0001 | |
| Metabolic disorder (%) | |||||||
| Hypertension | 61.69 | 37.37 | 0.0003 | 59.25 | 40.82 | <0.0001 | |
| Diabetes | 19.17 | 9.94 | 0.02 | 26.84 | 11.59 | 0.0001 | |
| Low HDL-cholesterol | 40.73 | 30.89 | 0.04 | 34.96 | 28.71 | 0.07 | |
| Triglyceridemia | 49.02 | 28.84 | 0.0003 | 44.62 | 30.89 | 0.0005 | |
| Hormone levels – mean (SD) | |||||||
| Testosterone (ng/mL) | 2.62 (0.10) | 5.92 (0.10) | <0.0001 | 2.66 (0.07) | 5.82 (0.10) | <0.0001 | |
| SHBG (nmol/L) | 32.30 (1.95) | 39.24 (0.78) | 0.008 | 23.24 (1.99) | 32.33 (1.17) | 0.13 | |
| Estradiol (ng/mL) | 31.33 (1.64) | 38.22 (0.63) | 0.0001 | 28.89 (1.75) | 39.24 (1.00) | <0.0001 | |
We performed a systematic screening of the relationships of the 173 nutrition and lifestyle factors with T deficiency in men. A total of 28 factors with FDR< 5% in the discovery set were examined for significance (P < 0.05) in the replication set but only 16 were available to replicate (Supplementary Figure S1 and Supplementary Table S2). Supplementary Figure 1 shows the distribution of P-values for each investigated factors and effect sizes in a circle Manhattan plot and the detailed results on these associations are presented in Supplementary Table S2. This resulted in seven tentatively replicated factors showing significant inverse associations for vitamin A intake (OR = 0.64, 95% CI: 0.46–0.89, P value = 0.01), protein intake (OR = 0.60, 95% CI: 0.45–0.81, P value = 0.003), saturated fatty acids intake (OR = 0.67, 95% CI: 0.51–0.89, P value = 0.01), monounsaturated fatty acids intake (OR = 0.68, 95% CI: 0.52–0.88, P value = 0.008), total fat intake (OR = 0.70, 95% CI: 0.54–0.91, P value = 0.01), SFA 16:0 (OR = 0.68, 95% CI: 0.52–0.90, P value = 0.01), phosphate intake (OR = 0.61, 95% CI: 0.42–0.87, P value = 0.01) with T deficiency (Table 3).
Table 3.
Associations between replicated nutrition and lifestyle factors in relation to T deficiency in the discovery and replication datasets.
| Factors | Discovery dataset (NHANES III) | Replication dataset (NHANES 1999–2004) | |||||
|---|---|---|---|---|---|---|---|
| N = T deficiency | N = Total | OR (95% CI) | N = T deficiency | N = Total | OR (95% CI) | P-value | |
| Vitamin A intake (g) | 234 | 1273 | 0.90 (0.71–0.91 | 140 | 565 | 0.64 (0.46–0.89) | 0.01 |
| Protein intake (g) | 234 | 1273 | 0.67 (0.53–0.85 | 140 | 565 | 0.60 (0.45–0.81) | 0.003 |
| Saturated fatty acids intake (g) | 234 | 1273 | 0.71 (0.58–0.87) | 140 | 565 | 0.67 (0.51–0.89) | 0.01 |
| Monounsaturated fatty acids intake (g) | 234 | 1273 | 0.76 (0.64–0.89) | 140 | 565 | 0.68 (0.52–0.88) | 0.008 |
| Total fat intake (g) | 234 | 1273 | 0.75 (0.63–0.89) | 140 | 565 | 0.70 (0.54–0.91) | 0.01 |
| SFA 16:0 (g) | 234 | 1273 | 0.71 (0.58–0.88) | 140 | 565 | 0.68 (0.52–0.90) | 0.01 |
| Phosphate intake (mg) | 234 | 1273 | 0.70 (0.56–0.87) | 140 | 565 | 0.61 (0.42–0.87) | 0.01 |
All models were adjusted for age, BMI, race/ethnicity, education, PIR, SHBG and estradiol. Benjamini–Hochberg adjusted P-values for FDR < 5% are presented for the discovery dataset, and P-values from significance testing are presented for replication dataset.
Correlation between replicated factors is displayed as a heatmap in Supplementary Figure S2. The larger the correlation between a pair of variables, the closer in proximity they appear in the heatmap. We performed a principal component factor analysis to assess any structure underlying replicated factors and to identify common underlying factors. Two principal components (PC) were identified. The first PC1 consisted of total fat intake, SFA 16:0, saturated fat, monounsaturated fat, protein, and phosphate intake. The second PC2 was mainly comprised of vitamin A intake. The total variance of replicated variables explained by PC1 was 73% (95% CI: 72–75%) and 14% (95% CI: 13–15%) by PC2 (Supplementary Figure S3). For the final analysis, we obtained estimates for replicated nutritional and lifestyle factors in relation to T deficiency in multivariable analysis in the NHANES 1999–2004 dataset (Table 4). The only factor that remained statistically significant associated to T deficiency was Vitamin A (OR = 0.67, 95% CI: 0.49–0.92).
Table 4.
Multivariable† analysis of replicated factors and T deficiency in NHANES 1999–2004
| Weighted mean (SD) | OR (95% CI) per SD change | |
|---|---|---|
| Vitamin A intake (g) | 0.18 (0.05) | 0.67 (0.49–0.92) |
| Protein intake (g) | 0.67 (0.05) | 0.71 (0.39–1.29) |
| Saturated fatty acids intake (g) | 0.49 (0.06) | 0.55 (0.18–1.73) |
| Monounsaturated fatty acids intake (g) | 0.61 (0.05) | 0.38 (0.11–1.37) |
| Total fat intake (g) | 0.62 (0.05) | 2.48 (0.54–11.42) |
| SFA 16:0 (g) | 0.55 (0.06) | 1.49 (0.38–5.83) |
| Phosphate intake (mg) | 0.60 (0.05) | 1.08 (0.55–2.13) |
Adjusted for age, race, PIR, BMI, SHBG, and estradiol
Discussion
In a systematic screening of 173 nutrition and lifestyle factors, seven factors were identified and validated to be inversely associated with T deficiency after applying the EWAS methodology in a representative sample of the US population. These seven factors included intake of total vitamin A, protein, total saturated fatty acids, total monounsaturated fatty acids, total fats, saturated fatty acid 16:0, and phosphorus. In a multivariable model in NHANES 1999–2004, the seven replicated nutritional and lifestyle factors were investigated in relation to T deficiency and only vitamin A was significantly associated. On the basis of inter-correlation between these factors, two PC were found: (1) total fats, saturated fatty acid 16:0, total saturated fatty acids, total monounsaturated fatty acids, protein and phosphorous; and (2) total vitamin A. The two principal components also displayed significant associations with T deficiency.
To our knowledge, this is the first study that investigates the association of a large number of nutritional and lifestyle factors associated with T deficiency using a EWAS approach - a study design analogous to GWAS. Although EWAS has been previously used with other health outcomes, there was a research gap in studying the role of nutritional and lifestyle factors in the production and secretion of T levels using this method. The seven nutritional factors that were tested and replicated in this study have been previously suggested to be linked with serum T levels. Yet, the emerging body of literature related to these associations has provided conflicting results.
Therefore, several aspects of the findings of our investigation merit further discussion. First, in our study all associations between the nutritional factors and T deficiency were inverse, meaning that every unit increase of these nutritional factors had the potential to reduce the likelihood of T deficiency, possibly due to a direct increase in serum T levels. In our study, nutritional factors are dietary intakes that were compared with serum levels from previous papers in the paragraphs below, and although in some instances our sample size was larger compared to previous studies our dietary intake data is also more prone to recall and selection bias. Second, vitamin A was investigated in an experimental study conducted in guinea pigs and it was reported that low levels of vitamin A are accompanied with corresponding low levels of testosterone [24]. However, a population-based nutrition study, which included 155 male twins, identified an inverse association between vitamin A and T levels [25]. Years later, a review study, mainly conducted in animals, reported that low levels of vitamin A leads to decrease T production [26]. Further, a more recent experimental study by Yang et al. 2018 seemed to agree with the latter review and our study by reporting that low levels of vitamin A adversely affects T secretion through regulation of the Leydig cells differentiation in mouse and rat models [27]. However, this association should be interpreted with caution because dietary recalls and cross-sectional studies are more prone to recall and selection biases that could have influenced these findings.
Third, a number of studies have reported that dietary fat (e.g. total fat, saturated and unsaturated fats) plays a role in the production and secretion of T, yet these findings remain inconsistent as dietary fat has been found to have both positive and negative effects on circulating total T [28–32]. Our study observed an inverse association between total fat, saturated and unsaturated fats and T deficiency. This observation has plausibility as it has been previously shown that saturated fat intake increased total cholesterol concentration [33], and total cholesterol is a precursor for T [34, 35]. Another plausibility derived from a high-fat diet study, rich in saturated fatty acids, found that this diet caused a decrease in SHBG and consequently an increase in the levels of free T [36]. Yet, an aforementioned male twin study of 155 pairs also investigated the relationship between T levels and total fat and reported an inverse relationship [25]. Similarly, Nagata et al. 2000 found that intake of saturated, monounsaturated, and polyunsaturated fats was inversely correlated with serum total testosterone among Japanese men, but the correlation was statistically significant only for the polyunsaturated fat [37]. However, Key et al. 1990 found a positive correlation only between T and polyunsaturated fat intake among 40 men [29]. Another small population-based study confirmed that 43 healthy men had high concentrations of total T among those men who ate a high-fat diet [36]. Confirming these latter observations, two experimental studies conducted in rat models showed positive associations between T levels and unsaturated fatty acids [32, 38].
Fourth, dietary protein levels have been suggested to influence circulating T levels. Yet, the direction of this influence remains unclear due to contradicting results found in the literature. A few experimental studies have reported that a low-protein diet can lead to low levels of T in male rat models [39, 40], but an inverse association was found between protein and T levels in a number of population-based studies [25, 41]. Interestingly, more recent literature has focused mainly trying to compare and assess the effects on T levels by different types of proteins (e.g. soy vs whey protein which is the most common type of protein investigated). For instance, Kraemer et al. 2013 identified lower levels of T among 10 young men following a supplementation with soy protein, but not with whey protein [42]. However, a recent meta-analysis [43, 44] showed that soy protein doesn’t alter T profile, and it is not inferior to the positive effects of whey protein [45]. This finding was recently confirmed by a double-blind randomized clinical trial reporting no alteration in the T profile after soy or whey protein supplementation among young men with resistance exercise training [46].
Finally, there has been a small number of studies investigating the link between phosphorous and T levels. However, this link merits further scrutiny because high levels of phosphorous has been associated with a high risk of cardiovascular diseases[47], but low levels of T has been linked to a higher risk of cardiovascular disease [4, 48]. A community dwelling study of 1,346 older men reported that high serum T levels were associated with lower serum phosphorus levels in a model adjusted for age, race and estradiol levels [49]. This seems to concur with the aforementioned statements that low T levels increased cardiovascular diseases, but high phosphorous levels has the same effect. Interestingly, a recent cross-sectional study of 1,899 Korean men showed a non-significant correlation between serum phosphorus and T [50].
Our study has a number of strengths. NHANES is a program of studies that is representative of the civilian noninstitutionalized US population, which aids in the generalizability of these results. In addition, NHANES follows a rigorous protocol with extensive quality control procedures for the collection of the exposures, outcome of interest and potential confounding factors analyzed and adjusted in this study. We also mutually adjusted for testosterone, SHBG and estradiol. Robustness of the statistical associations between investigated markers and T deficiency was ascertained through replication analysis and adjustment for presence of major confounders. The systematic screening was able to eliminate factors with small effects which may be more prone to bias. Additionally, this method overcomes the limitation of selective reporting, which may be an issue with studies focusing on individual exposures.
Our findings should be interpreted in the context of the study design due to its inherit limitations. First, both NHANES III and NHANES 1999–2004 are cross-sectional studies; therefore, we cannot infer causality. Further, due to the cross sectional nature of the data, we cannot rule out the issue of reverse causality between T deficiency and several dietary and lifestyle factors. Second, although in our study we followed a systematic approach that can give a list of T deficiency correlates with strong statistical power, it will be required to conduct other study designs/models (e.g. causal inference modeling and randomized trials) to determine which of these correlates are the most important. Third, even though we took into account potential confounders and inter-correlation between replicated factors, residual confounding may have occurred because the information of these confounders was obtained by self-reporting. We were unable to exclude the potential role of other relevant factors apart from those assessed in NHANES III. Therefore, obtaining an equivalent definition of ‘genome-wide significance’ as one would be able to claim in a genome-wide association studies analysis may be impractical or otherwise requires more rigorous and thorough assessments of nutrition and lifestyle determinants. However, this study has the potential to contribute to the current body of literature used to define a clinical definition for T deficiency.
Conclusion
This is the first study that uses a EWAS approach to identify nutritional factors that were robustly associated with T deficiency. This study has the potential to contribute to the current body of literature used to define a clinical definition for T deficiency after taking into account nutritional and lifestyle factors.
Supplementary Material
Footnotes
Conflict of interest: The authors declare that they have no conflict of interest.
Submission declaration and verification: This work has not been published previously and it is not under consideration for publication elsewhere. The publication is approved by all authors and, if accepted, it will not be published elsewhere in the same form, in English or in any other language, including
Ethical approval: The protocols for the conduct of NHANES III and NHANES 1999–2004 were approved by the Institutional Review Board of the National Center for Health Statistics, US Centers for Disease Control and Prevention. Informed consent was obtained from all participants.
Informed Consent: We conducted a secondary data analysis using data from NHANES, which can be accessed by the public. NHANES obtained informed consents from participants.
References
- 1.Khera M, Adaikan G, Buvat J, Carrier S, El-Meliegy A, Hatzimouratidis K, McCullough A, Morgentaler A, Torres LO and Salonia A. Diagnosis and Treatment of Testosterone Deficiency: Recommendations From the Fourth International Consultation for Sexual Medicine (ICSM 2015). J.Sex.Med 2016;13:1787–1804. [DOI] [PubMed] [Google Scholar]
- 2.Buvat J, Maggi M, Guay A and Torres LO. Testosterone deficiency in men: systematic review and standard operating procedures for diagnosis and treatment. J.Sex.Med 2013;10:245–284. [DOI] [PubMed] [Google Scholar]
- 3.Corona G, Rastrelli G, Di Pasquale G, Sforza A, Mannucci E and Maggi M. Endogenous Testosterone Levels and Cardiovascular Risk: Meta-Analysis of Observational Studies. J.Sex.Med. 2018;15:1260–1271. [DOI] [PubMed] [Google Scholar]
- 4.Lopez DS, Canfield S and Wang R. Testosterone replacement therapy and the heart: friend, foe or bystander? Transl.Androl.Urol 2016;5:898–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopez DS, Advani S, Tsilidis KK, Wang R and Canfield S. Endogenous and exogenous testosterone and prostate cancer: decreased-, increased- or null-risk? Transl.Androl.Urol 2017;6:566–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Platz EA. Low testosterone and risk of premature death in older men: analytical and preanalytical issues in measuring circulating testosterone. Clin.Chem. 2008;54:1110–1112. [DOI] [PubMed] [Google Scholar]
- 7.Mulligan T, Frick MF, Zuraw QC, Stemhagen A and McWhirter C . Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int.J.Clin.Pract 2006;60:762–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Araujo AB, Esche GR, Kupelian V, O’Donnell AB, Travison TG, Williams RE, Clark RV and McKinlay JB. Prevalence of symptomatic androgen deficiency in men. J.Clin.Endocrinol.Metab. 2007;92:4241–4247. [DOI] [PubMed] [Google Scholar]
- 9.Paduch DA., Brannigan RD., Fuchs EF., Kim ED., Marmar JL., Sandlow JI. The Laboratory Diagnosis of Testosterone Deficiency. Available from http://university.auanet.org/common/pdf/education/clinical-guidance/Testosterone-Deficiency-WhitePaper.pdf. White Paper 2017. [DOI] [PubMed] [Google Scholar]
- 10.Malik RD, Lapin B, Wang CE, Lakeman JC and Helfand BT. Are we testing appropriately for low testosterone?: Characterization of tested men and compliance with current guidelines. J.Sex.Med. 2015;12:66–75. [DOI] [PubMed] [Google Scholar]
- 11.Rohrmann S, Shiels MS, Lopez DS, Rifai N, Nelson WG, Kanarek N, Guallar E, Menke A, Joshu CE, Feinleib M, Sutcliffe S and Platz EA. Body fatness and sex steroid hormone concentrations in US men: results from NHANES III. Cancer Causes Control 2011;22:1141–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel CJ, Rehkopf DH, Leppert JT, Bortz WM, Cullen MR, Chertow GM and Ioannidis JP. Systematic evaluation of environmental and behavioural factors associated with all-cause mortality in the United States national health and nutrition examination survey. Int.J.Epidemiol 2013;42:1795–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Center for Health Statistics 1994. Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–94. Series 1: programs and collection procedures. Vital Health Stat 1: 1–407. 1994. [PubMed] [Google Scholar]
- 14.Nyante SJ, Graubard BI, Li Y, McQuillan GM, Platz EA, Rohrmann S, Bradwin G and McGlynn KA. Trends in sex hormone concentrations in US males: 1988–1991 to 1999–2004. Int.J.Androl. 2012;35:456–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loucks EB, Rehkopf DH, Thurston RC and Kawachi I. Socioeconomic disparities in metabolic syndrome differ by gender: evidence from NHANES III. Ann.Epidemiol. 2007;17:19–26. [DOI] [PubMed] [Google Scholar]
- 16.Ma Z, Gingerich RL, Santiago JV, Klein S, Smith CH and Landt M. Radioimmunoassay of leptin in human plasma. Clin.Chem. 1996;42:942–946. [PubMed] [Google Scholar]
- 17.Wulaningsih W, Van Hemelrijck M, Tsilidis KK, Tzoulaki I, Patel C and Rohrmann S. Investigating nutrition and lifestyle factors as determinants of abdominal obesity: an environment-wide study. Int.J.Obes.(Lond) 2017;41:340–347. [DOI] [PubMed] [Google Scholar]
- 18.Lopez DS, Qiu X, Advani S, Tsilidis KK, Khera M, Kim J, Morgentaler A, Wang R and Canfield S. Double Trouble: co-occurrence of testosterone deficiency and body fatness associated with all-cause mortality in US men. Clin.Endocrinol.(Oxf) 2017;. [DOI] [PubMed] [Google Scholar]
- 19.Storey JD and Tibshirani R. Statistical significance for genomewide studies. Proc.Natl.Acad.Sci.U.S.A. 2003;100:9440–9445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benjamini Y and Hechtlinger Y. Discussion: An estimate of the science-wise false discovery rate and applications to top medical journals by Jager and Leek. Biostatistics 2014;15:13–6; discussion 39–45. [DOI] [PubMed] [Google Scholar]
- 21.Lee JY, Chen HI, Urban C, Hojat A, Church E, Xie SX and Farrar JT. Development of and psychometric testing for the Brief Pain Inventory-Facial in patients with facial pain syndromes. J.Neurosurg. 2010;113:516–523. [DOI] [PubMed] [Google Scholar]
- 22.Timmerman ME, Kiers HA and Smilde AK. Estimating confidence intervals for principal component loadings: a comparison between the bootstrap and asymptotic results. Br.J.Math.Stat.Psychol 2007;60:295–314. [DOI] [PubMed] [Google Scholar]
- 23.Survey Lumley T. : Analysis of Complex Survey Samples. R Package version 3.14 (2009). Available at: https://cran.r-project.org/web/packages/survey/index.html. (Accessed: 15th April 2015). [Google Scholar]
- 24.Nayyar T, Mukherjee S and Das SK. Alterations in binding characteristics of peripheral benzodiazepine receptors in testes by vitamin A deficiency in guinea pigs. Mol.Cell.Biochem. 2000;211:47–50. [DOI] [PubMed] [Google Scholar]
- 25.Bishop DT, Meikle AW, Slattery ML, Stringham JD, Ford MH and West DW. The effect of nutritional factors on sex hormone levels in male twins. Genet.Epidemiol. 1988;5:43–59. [DOI] [PubMed] [Google Scholar]
- 26.Livera G, Rouiller-Fabre V, Pairault C, Levacher C and Habert R. Regulation and perturbation of testicular functions by vitamin A. Reproduction 2002;124:173–180. [PubMed] [Google Scholar]
- 27.Yang Y, Luo J, Yu D, Zhang T, Lin Q, Li Q, Wu X, Su Z, Zhang Q, Xiang Q and Huang Y. Vitamin A Promotes Leydig Cell Differentiation via Alcohol Dehydrogenase 1. Front.Endocrinol.(Lausanne) 2018;9:644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howie BJ and Shultz TD. Dietary and hormonal interrelationships among vegetarian Seventh-Day Adventists and nonvegetarian men. Am.J.Clin.Nutr. 1985;42:127–134. [DOI] [PubMed] [Google Scholar]
- 29.Key TJ, Roe L, Thorogood M, Moore JW, Clark GM and Wang DY. Testosterone, sex hormone-binding globulin, calculated free testosterone, and oestradiol in male vegans and omnivores. Br.J.Nutr. 1990;64:111–119. [DOI] [PubMed] [Google Scholar]
- 30.Belanger A, Locong A, Noel C, Cusan L, Dupont A, Prevost J, Caron S and Sevigny J. Influence of diet on plasma steroids and sex hormone-binding globulin levels in adult men. J.Steroid Biochem. 1989;32:829–833. [DOI] [PubMed] [Google Scholar]
- 31.Raben A, Kiens B, Richter EA, Rasmussen LB, Svenstrup B, Micic S and Bennett P. Serum sex hormones and endurance performance after a lacto-ovo vegetarian and a mixed diet. Med.Sci.Sports Exerc 1992;24:1290–1297. [PubMed] [Google Scholar]
- 32.Marra CA and de Alaniz MJ. Influence of testosterone administration on the biosynthesis of unsaturated fatty acids in male and female rats. Lipids 1989;24:1014–1019. [DOI] [PubMed] [Google Scholar]
- 33.Grundy SM and Vega GL. Causes of high blood cholesterol. Circulation 1990;81:412–427. [DOI] [PubMed] [Google Scholar]
- 34.Huff T and Jialal I. Physiology, Cholesterol In: Anonymous StatPearls. Treasure Island (FL): StatPearls Publishing LLC; 2018. [PubMed] [Google Scholar]
- 35.Zhang X, Li J, Zhou X, Guan Q, Zhao J, Gao L, Yu C, Wang Y and Zuo C. Simvastatin Decreases Sex Hormone Levels in Male Rats. Endocr.Pract. 2017;23:175–181. [DOI] [PubMed] [Google Scholar]
- 36.Dorgan JF, Judd JT, Longcope C, Brown C, Schatzkin A, Clevidence BA, Campbell WS, Nair PP, Franz C, Kahle L and Taylor PR. Effects of dietary fat and fiber on plasma and urine androgens and estrogens in men: a controlled feeding study. Am.J.Clin.Nutr. 1996;64:850–855. [DOI] [PubMed] [Google Scholar]
- 37.Nagata C, Takatsuka N, Kawakami N and Shimizu H. Relationships between types of fat consumed and serum estrogen and androgen concentrations in Japanese men. Nutr.Cancer 2000;38:163–167. [DOI] [PubMed] [Google Scholar]
- 38.Gromadzka-Ostrowska J, Przepiorka M and Romanowicz K. Influence of dietary fatty acids composition, level of dietary fat and feeding period on some parameters of androgen metabolism in male rats. Reprod.Biol. 2002;2:277–293. [PubMed] [Google Scholar]
- 39.Hanai M and Esashi T. The interactive effect of dietary protein and vitamin levels on the depression of gonadal development in growing male rats kept under disturbed daily rhythm. J.Nutr.Sci.Vitaminol.(Tokyo) 2007;53:138–144. [DOI] [PubMed] [Google Scholar]
- 40.Hanai M and Esashi T. Effect of dietary protein levels on sex hormones in growing male rats kept under constant darkness. Exp.Anim. 2012;61:555–561. [DOI] [PubMed] [Google Scholar]
- 41.Anderson KE, Rosner W, Khan MS, New MI, Pang SY, Wissel PS and Kappas A. Diet-hormone interactions: protein/carbohydrate ratio alters reciprocally the plasma levels of testosterone and cortisol and their respective binding globulins in man. Life Sci. 1987;40:1761–1768. [DOI] [PubMed] [Google Scholar]
- 42.Kraemer WJ, Solomon-Hill G, Volk BM, Kupchak BR, Looney DP, Dunn-Lewis C, Comstock BA, Szivak TK, Hooper DR, Flanagan SD, Maresh CM and Volek JS. The effects of soy and whey protein supplementation on acute hormonal reponses to resistance exercise in men. J.Am.Coll.Nutr 2013;32:66–74. [DOI] [PubMed] [Google Scholar]
- 43.Hamilton-Reeves JM, Vazquez G, Duval SJ, Phipps WR, Kurzer MS and Messina MJ. Clinical studies show no effects of soy protein or isoflavones on reproductive hormones in men: results of a meta-analysis. Fertil.Steril. 2010;94:997–1007. [DOI] [PubMed] [Google Scholar]
- 44.Messina M, Hamilton-Reeves J, Kurzer M and Phipps W. Effect of soy protein on testosterone levels. Cancer Epidemiol.Biomarkers Prev. 2007;16:2795. [DOI] [PubMed] [Google Scholar]
- 45.Kalman D, Feldman S, Martinez M, Krieger DR and Tallon MJ. Effect of protein source and resistance training on body composition and sex hormones. J.Int.Soc.Sports Nutr. 2007;4:4–2783-4–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reidy PT, Borack MS, Markofski MM, Dickinson JM, Deer RR, Husaini SH, Walker DK, Igbinigie S, Robertson SM, Cope MB, Mukherjea R, Hall-Porter JM, Jennings K, Volpi E and Rasmussen BB. Protein Supplementation Has Minimal Effects on Muscle Adaptations during Resistance Exercise Training in Young Men: A Double-Blind Randomized Clinical Trial. J.Nutr. 2016;146:1660–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Foley RN, Collins AJ, Herzog CA, Ishani A and Kalra PA. Serum phosphorus levels associate with coronary atherosclerosis in young adults. J.Am.Soc.Nephrol. 2009;20:397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kloner RA, Carson C, 3rd,, Dobs A, Kopecky S and Mohler ER 3rd. Testosterone and Cardiovascular Disease. J.Am.Coll.Cardiol 2016;67:545–557. [DOI] [PubMed] [Google Scholar]
- 49.Meng J, Ohlsson C, Laughlin GA, Chonchol M, Wassel CL, Ljunggren O, Karlsson MK, Mellstrom D, Orwoll ES, Barrett-Connor E, Ix JH and Osteoporotic Fractures in Men (MrOs) Study Group. Associations of estradiol and testosterone with serum phosphorus in older men: the Osteoporotic Fractures in Men study. Kidney Int. 2010;78:415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Min SK, Choi K, Kim SK, Lee GI and Cho IC. Phosphorus as predictive factor for erectile dysfunction in middle aged men: A cross sectional study in Korea. Investig.Clin.Urol 2016;57:442–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.