1.
The SARS‐CoV‐2 pandemic has led to worldwide research aiming to identify the risk factors for developing critical illness and mortality caused by COVID‐19. It quickly became apparent that besides older age, obesity is one of the most important risk factors for a more severe course of COVID‐19, although the mechanisms remain largely unknown. 1 , 2 , 3 Notably, with respect to acute respiratory distress syndrome (ARDS) and acute lung injury (ALI), evidence is mounting that obesity is a risk factor for ARDS/ALI, but among people with ARDS/ALI, obesity is associated with better outcome, a phenomenon which has been called the “obesity paradox”. 4 , 5 Whether such a phenomenon also holds true for severe lung disease following SARS‐CoV‐2 infection is not yet clear. 6 Obesity is commonly recognized to reflect a state of low‐grade chronic inflammation. 7 Adipose tissue produces a great number of adipokines that act as signalling molecules with a wide array of effects on many organ systems, including the lungs. A potential underlying pathophysiological mechanism explaining the effect of obesity on the severity of COVID‐19 infection may, therefore, be conferred by abnormalities in the production of adipokines by adipose tissue, of which leptin and adiponectin have received most attention. 8 , 9 Leptin is a primarily proinflammatory adipokine that influences both innate and adaptive immune responses by stimulating the production of proinflammatory cytokines (interleukin (IL)‐2, interferon‐ γ and tumour necrosis factor alpha) and suppressing the production of anti‐inflammatory cytokines (IL‐4 and IL‐5). 10 In contrast, adiponectin is a predominantly anti‐inflammatory adipokine that inhibits proinflammatory cytokines (TNF‐α, IL‐6 and nuclear factor‐κB) and induces anti‐inflammatory cytokines (IL‐10 and IL‐1 receptor antagonist). 10 It is commonly appreciated that systemic leptin concentrations are upregulated, whereas adiponectin concentrations are paradoxically downregulated in obese individuals. 11 , 12 In obese mice, adiponectin deficiency is associated with an exaggeration of inflammation during early sepsis, whereas adiponectin treatment has been shown to attenuate the inflammatory response. 13 In humans, the leptin/adiponectin ratio has been suggested to reflect a state of adipose tissue dysfunction 14 and may be associated with incident cardiovascular events. 15 Nonetheless, low plasma adiponectin levels may not confer worse cardiovascular outcome in population studies. 16
Interestingly, plasma adiponectin is decreased in response to a low sodium diet as well to angiotensin II infusion. 17 This lends support to the hypothesis that adiponectin could be involved in potentially adverse effects of a low sodium balance on tissue ACE2 expression and, hence, on pulmonary viral load, as was proposed recently. 18 In line, serum sodium was lower in patients with severe SARS‐Cov‐2 infection, but in that study, potassium and calcium were also modestly decreased. 19 On the other hand, leptin may affect renal sodium handling in rat studies, 20 but circulating leptin levels have—to our knowledge—not been shown to be affected by a low sodium diet. Of further interest, a low sodium diet associates with enhanced low‐grade chronic inflammation. 21
Indeed, imbalanced production of adipokines could provide an attractive mechanistic explanation for the obesity‐associated risk of severe COVID‐19 infections (Figure 1). However, data with respect to the association of leptin and adiponectin with sepsis severity and outcome are inconclusive. 22 Adipose tissue also produces a great number of other adipokines, including resistin and visfatin, 23 , 24 which affect the immune system and may be associated with adverse outcome of sepsis. 22 , 25 , 26 Of further relevance, at least with respect to leptin and adiponectin, it is known that plasma levels of both are decreased during severe sepsis, 27 making that the timing of measurement of plasma adipokines is of extreme importance.
Figure 1.
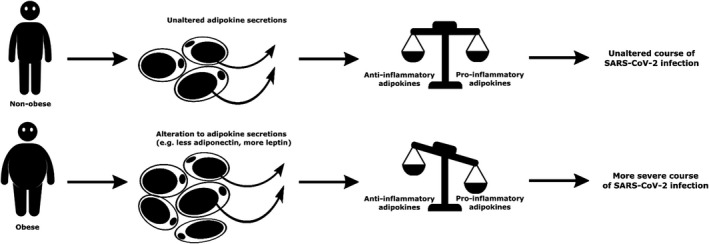
A schematic overview of the hypothesized difference between nonobese and obese individuals in the severity of SARS‐CoV‐2 infection
Taken together, we propose that a comprehensive approach, not merely pinpointing on a small number of easily measurable plasma adipokine concentrations but including a wide array of adipokines and inflammation markers as well metabolomic profiling, is required to determine the predictive effects of alterations in the “adipokinome” on outcome of severe COVID‐19 infections, before implementing their measurement in clinical practice. Moreover, such a strategy could be of help to develop specific adipokine targeted treatment strategies that can be implemented in the acute setting. Avoiding low sodium status could be one such strategy.
CONFLICT OF INTEREST
None.
REFERENCES
- 1. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospitalization and critical illness among 4,103 patients with COVID‐19 disease in New York City. medRxiv. 2020: 2020.04.08.20057794. [Google Scholar]
- 2. Simonnet A, Chetboun M, Poissy J, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) requiring invasive mechanical ventilation. Obesity. 2020. 10.1002/oby.22831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lighter J, Phillips M, Hochman S, et al. Obesity in patients younger than 60 years is a risk factor for Covid‐19 hospital admission. Clin Infect Dis. 2020. 10.1093/cid/ciaa415. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhi G, Xin W, Ying W, Guohong X, Shuying L. "obesity paradox" in acute respiratory distress syndrome: asystematic review and meta‐analysis. PLoS One. 2016;11:e0163677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ni Y‐N, Luo J, Yu HE, et al. Can body mass index predict clinical outcomes for patients with acute lung injury/acute respiratory distress syndrome? A meta‐analysis. Crit Care. 2017;21:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jose RJ, Manuel A. Does Coronavirus disease 2019 disprove the obesity paradox in acute respiratory distress syndrome? Obesity. 2020;28:1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Unamuno X, Gomez‐Ambrosi J, Rodriguez A, Becerril S, Fruhbeck G, Catalan V. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur J Clin Invest. 2018;48:e12997. [DOI] [PubMed] [Google Scholar]
- 8. Messina G, Polito R, Monda V, et al. Functional role of dietary intervention to improve the outcome of COVID‐ 19: a hypothesis of work. Int J Mol Sci. 2020;21(9):3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Salvator H, Grassin‐Delyle S, Naline E, et al. Contrasting effects of adipokines on the cytokine production by primary human bronchial epithelial cells: inhibitory effects of adiponectin. Front Pharmacol. 2020;11 10.3389/fphar.2020.00056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ali Assad N, Sood A. Leptin, adiponectin and pulmonary diseases. Biochimie. 2012;94:2180‐2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dallinga‐Thie GM, Dullaart RP. Do genome‐wide association scans provide additional information on the variation of plasma adiponectin concentrations? Atherosclerosis. 2010;208:328‐329. [DOI] [PubMed] [Google Scholar]
- 12. Gómez‐Ambrosi J, Salvador J, Silva C, et al. Increased cardiovascular risk markers in obesity are associated with body adiposity: role of leptin. Thromb Haemost. 2006;95:991‐996. [DOI] [PubMed] [Google Scholar]
- 13. Wang X, Buechler NL, Yoza BK, McCall CE, Vachharajani V. Adiponectin treatment attenuates inflammatory response during early sepsis in obese mice. J Inflamm Res. 2016;9:167‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Finucane FM, Luan J, Wareham NJ, et al. Correlation of the leptin:adiponectin ratio with measures of insulin resistance in non‐diabetic individuals. Diabetologia. 2009;52:2345‐2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kappelle PJ, Dullaart RP, van Beek AP, Hillege HL, Wolffenbuttel BH. The plasma leptin/adiponectin ratio predicts first cardiovascular event in men: a prospective nested case‐control study. Eur J Intern Med. 2012;23:755‐759. [DOI] [PubMed] [Google Scholar]
- 16. Menzaghi C, Trischitta V. The adiponectin paradox for all‐cause and cardiovascular mortality. Diabetes. 2018;67:12‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lely AT, Krikken JA, Bakker SJL, et al. Low dietary sodium and exogenous angiotensin II infusion decrease plasma adiponectin concentrations in healthy men. J Clin Endocrinol Metab. 2007;92:1821‐1826. [DOI] [PubMed] [Google Scholar]
- 18. Post A, Dullaart RPF, Bakker SJL. Is low sodium intake a risk factor for severe and fatal COVID‐19 infection? Eur J Intern Med. 2020;75:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lippi G, South AM, Henry BM. Electrolyte imbalances in patients with severe coronavirus disease 2019 (COVID‐19). Ann Clin Biochem. 2020;57:262‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Villarreal D, Reams G, Freeman R, Spear R, Tchoukina I, Samar H. Leptin blockade attenuates sodium excretion in saline‐loaded normotensive rats. Mol Cell Biochem. 2006;283:153‐157. [DOI] [PubMed] [Google Scholar]
- 21. Gruppen EG, Connelly MA, Vart P, Otvos JD, Bakker SJ, Dullaart RP. GlycA, a novel proinflammatory glycoprotein biomarker, and high‐sensitivity C‐reactive protein are inversely associated with sodium intake after controlling for adiposity: the prevention of renal and vascular end‐stage disease study. Am J Clin Nutr. 2016;104:415‐422. [DOI] [PubMed] [Google Scholar]
- 22. Karampela I, Christodoulatos GS, Dalamaga M. The role of adipose tissue and adipokines in sepsis: inflammatory and metabolic considerations, and the obesity paradox. Curr Obes Rep. 2019;8:434‐457. [DOI] [PubMed] [Google Scholar]
- 23. Al‐Suhaimi EA, Shehzad A. Leptin, resistin and visfatin: the missing link between endocrine metabolic disorders and immunity. Eur J Med Res. 2013;18:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miller L, Singbartl K, Chroneos ZC, Ruiz‐Velasco V, Lang CH, Bonavia A. Resistin directly inhibits bacterial killing in neutrophils. Intensive Care Med Exp. 2019;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Karampela I, Christodoulatos GS, Kandri E, et al. Circulating eNampt and resistin as a proinflammatory duet predicting independently mortality in critically ill patients with sepsis: a prospective observational study. Cytokine. 2019;119:62‐70. [DOI] [PubMed] [Google Scholar]
- 26. Koch A, Weiskirchen R, Krusch A, et al. Visfatin serum levels predict mortality in critically ill patients. Dis Markers. 2018;2018:7315356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Langouche L, Vander Perre S, Frystyk J, Flyvbjerg A, Hansen TK, Van den Berghe G. Adiponectin, retinol‐binding protein 4, and leptin in protracted critical illness of pulmonary origin. Crit Care. 2009;13:R112. [DOI] [PMC free article] [PubMed] [Google Scholar]


