Abstract
On April 17 2020, the United States Food and Drug Administration granted Coronavirus Disease 2019 (COVID‐19) emergency use authorizations for the Seraph 100 Microbind Affinity Blood Filter. The medical device is aimed to treat critically ill COVID‐19 patients with confirmed or imminent respiratory failure. The aim of this life size in vitro pharmacokinetic study was to investigate the in vitro adsorption of chloroquine and hydroxychloroquine from human plasma using equipment that is also used at the bedside. After start of the hemoperfusion, Pre (Cpre) Seraph plasma levels were obtained at 5 (C5), 10 (C10), 15 (C15), 30 (C30), 60 (C60), and 120 (C120) minutes into the procedure. At two timepoints (5 and 120 minutes) post (Cpost) Seraph plasma levels were determined that were used to calculate the plasma clearance of the Seraph. Both drugs were determined using a validated HPLC method. Median [IQR] plasma clearance of the Seraph for chloroquine/hydroxychloroquine was 1.71 [0.51‐4.38] mL/min/1.79 [0.21‐3.68] mL/min respectively. The lack of elimination was also confirmed by the fact that plasma levels did not change over the 120 minutes treatment. As neither chloroquine nor hydroxychloroquine were removed by the treatment with the Seraph dose adjustments in COVID‐19 patients undergoing this treatment are not necessary.
Keywords: blood purification, COVID‐19, extracorporeal therapy
1. INTRODUCTION
In July 1934, Johann “Hans” Andersag synthesized chloroquine by replacing the acridine ring of atabrine with a quinoline ring. 1 He then developed the drug into a salt of the base using 2,4‐dihydroxy‐benzoic acid, naming it RESOCHIN as it was the RESOrcinate of a 4‐aminoCHINolin. After an initial clinical test in four psychiatric patients by BAYER pharmaceutical company in 1935 it was found to be “too toxic for practical use in humans”. 1 It took 10 years before chloroquine was back in the clinical arena in the context of a malaria trial sponsored by the US government. Today it is on the World Health Organization's list of essential medicines; that is, it is considered to belong to the safest and most effective medicines needed in a health care system. Chloroquine and hydroxychloroquine are used to treat malaria, rheumatoid arthritis, lupus erythematosus and Morbus Boeck (sarcoidosis). The two drugs are also used as malaria prophylaxis. Both 4‐aminoquinolines have recently gained a lot of attention. Dozens of clinical trials of chloroquine, hydroxychloroquine, or both, sometimes in combination with other drugs like remdesivir, are currently under way. The animated controversy about its potential benefits and risks in treating COVID‐19 patients is aggravated by methodological flaws 2 in high‐impact publications. 3 The underlying rational for their use is the fact that they were found to block COVID‐19 infection at low‐micromolar concentration. 4 Especially hydroxychloroquine is assumed to attenuate the progression of COVID‐19 through inhibiting the cytokine storm by reducing CD154 expression in T cells. 5
In April 2020, the United States Food and Drug Administration granted Coronavirus Disease 2019 (COVID‐19) emergency use authorizations for medical devices, including extracorporeal blood purification devices. For none of those devices it is known whether or not they decrease the concentration of both, chloroquine and hydroxychloroquine. One of the devices is the Seraph 100 Microbind Affinity Blood Filter, produced by ExThera Medical (Martinez, CA), first licensed in the European Economic Area in 2019. 6 The aim of this study was to investigate the in vitro adsorption of chloroquine and hydroxychloroquine from human plasma in a life size approach using equipment that is also used at the bedside.
2. MATERIALS AND METHODS
2.1. Patients and study protocol
Blood plasma was obtained from five voluntary donors during regular therapeutic plasma exchange (TPE) procedures due to various indications. Patients received neither chloroquine nor hydroxychloroquine.
The removed plasma of a single TPE treatment was stored at 4°C and used within 24 hours. 50 mg of chloroquine‐phosphate and 50 mg hydroxychloroquine‐sulfate, diluted in 10 mL of normal saline each, was injected into about 2200 mL plasma that was placed into an empty bag with Luer‐Lock connectors. This bag served as the patient dummy, with nominal concentrations of about 14.0 μg/mL chloroquine and 17.5 μg/mL hydroxychloroquine each. The fluid was mixed well for >3 minutes. For anticoagulation 2500 I.U. unfractionated heparin was added.
We primed a standard hemoperfusion blood tubing system (Fresenius Medical Care, Germany) as well as the Seraph 100 Microbind Affinity Blood Filter (Exthera Medical, CA, USA) with a total filling volume of about 200 mL with plasma and connected the dummy patients (plasma bag with Luer‐Lock tubing) with the hemoperfusion circuit. The Multifiltrate (Fresenius Medical Care GmbH, Bad Homburg, Germany) was used to pump the plasma through the adsorber as described previously. 7 In brief, using a standard tubing set of the multiFiltrate a roller pump propelled the plasma from one port of the plasmabag, through the Seraph back into the second port of the plasma bag at a rate of a 250 mL/min. The plasma bag was continuously shaken during the procedure to ensure mixture of the plasma. The experiment was repeated five times with different plasma bags from different donors. Visual controls were performed on the plasma bag as well as all the drawn samples to detect possible drug precipitations.
2.2. Sampling and analysis
A plasma sample of the whole plasma (C0) was obtained after mixing the chloroquine and hydroxychloroquine with the plasma. After start of the hemoperfusion Pre (Cpre) Seraph plasma levels were obtained at 5 (C5), 10 (C10), 15 (C15), 30 (C30), 60 (C60), and 120 (C120) minutes into the procedure. At two timepoints (5 and 120 minutes) post (Cpost) Seraph plasma levels were determined that were used to calculate the plasma clearance. One aliquot of the whole plasma sample was stored at room temperature without passing through the Seraph to determine drug stability. Plasma levels from this control samples were determined at the same intervals described above.
2.3. Ethics
Written informed consent was obtained from the voluntary plasma donors. The study was performed in accordance with the Declaration of Helsinki and German federal guidelines.
2.4. Chemical assays
Plasma concentrations of chloroquine and hydroxychloroquine were measured by a validated HPLC method applying UV‐detection. Plasma samples (100 μL) were prepared for analysis by addition of 100 μL 0.1% trifluoroacetic acid and 20 μL of 2.5 M perchloric acid for plasma protein precipitation. After 5 minutes centrifugation at 10 000 × g, 20 μL of the clear supernatant was injected into the HPLC system. Chloroquine and hydroxychloroquine were separated by gradient elution on an Agilent Zorbax SB‐C18 5 μm 2.1 × 150 mm column. The gradient started with 95% 0.1% trifluoroacetic acid and 5% methanol, evolved in 8 minutes to 50% methanol and returned to the starting conditions after 9 minutes, applying a constant flow rate of 0.4 mL/min. The analytes were detected at a wavelength of 343 nm. Under these conditions, chloroquine and hydroxychloroquine were specifically separated from each other and from endogenous plasma constituents. Precisions and accuracies of the method were 1.16% and 6.64% for chloroquine and 1.25% and 5.39% for hydroxychloroquine, respectively.
2.5. Statistical analysis
The Seraph plasma clearance (CL) of both drugs was assessed at 5 and 120 minutes into the procedure was calculated based on the plasma flow (Qe) and extraction ratio, using the equation:
CLdrug = Qe × (Cpre − Cpost)/Cpre. Calculations were performed using GraphPad Prism 8 (San Diego, CA, USA).
3. RESULTS
All five in vitro runs using the hydroxychloroquine and chloroquine spiked plasma could be carried out as planned for 120 minutes. We found no significant drug plasma clearance during the 120 min procedure. Median [IQR] plasma clearance of the Seraph for hydroxychloroquine was 1.79 [0.21‐3.68] mL/min (Figure 1). For chloroquine the median plasma clearance of the Seraph was 1.71 [0.51‐4.38] mL/min (Figure 1). A slight decrease in plasma levels of both drugs after connecting our dummy patient (plasma bag with about 2200 mL spiked plasma) from the start of the procedure to the first drug level into the procedure at 5 minutes. This decrease was <10% for both investigated substances and due to the fact that the dummy patient was connected to the plasma filled tubing system including the Seraph (about 200 mL) that contained neither hydroxychloroquine nor chloroquine. In other words, the first drop in plasma concentration was dilution. No additional decrease in drug plasma levels was observed during the experiment for either of the drugs. On visual control neither the plasma bag nor the drawn samples showed signs of drug precipitations.
FIGURE 1.
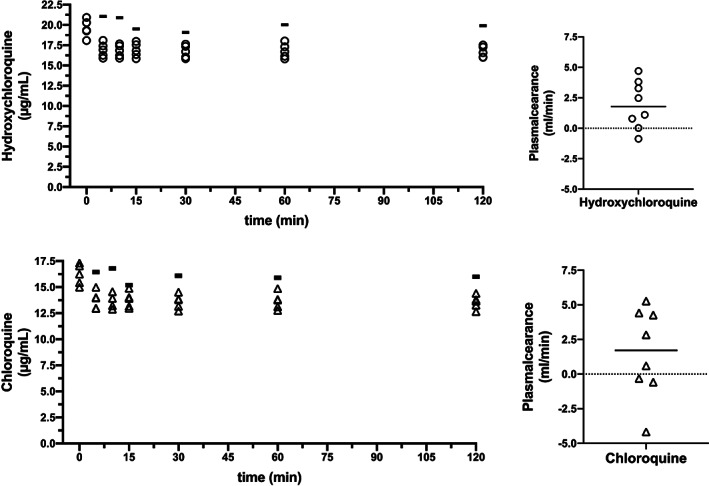
Time course of hydroxycholoroquine and chloroquine plasma concentrations during hemoperfusion using the Seraph 100 Microbind Affinity Blood Filter (left upper and lower graph; n = 5). The horizontal bar in both graphs is depicting the control sample that was not pumped through the Seraph. The right upper and lower graph depict the calculated plasma clearance of the Seraph for hydroxycholoroquine and chloroquine (n = 8)
4. DISCUSSION AND CONCLUSION
On April 17 2020, the US FDA issued an emergency use authorization for the Seraph 100 Microbind Affinity Blood Filter in patients with COVID‐19 admitted to the ICU with confirmed or imminent respiratory failure. Removal of drugs that are used to treat COVID‐19 patients had not been reported for any of the devices authorized for emergency use. To our knowledge, this is the first in vitro measurement of the hydroxychloroquine and chloroquine elimination characteristics of the Seraph using a life size adsorber and human plasma with a plasma flow rate compatible with the clinical setting.
Extracorporeal procedures can remove therapeutic substances making dose adjustments necessary. 8 Neither chloroquine (molecular weight 320 Da; volume of distribution >20 L/kg; protein‐binding 60%) nor hydroxychloroquine (molecular weight 336 Da; volume of distribution 27 L/kg; protein‐binding 75%) are considered to be dialysable. 9 Yet in the case of charcoal hemoperfusion removal could be shown in vitro. 10 The lack of drug removal of the Seraph can be explained by the distinct structure, that is, ultra‐high molecular weight polyethylene beads with endpoint‐attached heparin to which bacteria, viruses, fungi and toxins have been shown to bind. Interestingly there are no data available on other medical devices that received emergency use clearance by the FDA for the treatment of COVID‐19, thus it is there unknown whether chloroquine and hydroxychloroquine have to be adapted in their dose due to removal.
We wish to point out an important limitation of this in vitro study. The investigated drugs medium was human plasma rather than whole blood. Therefore, interactions between cellular blood components, the Seraph, chloroquine and hydroxychloroquine could not be investigated by this study. However, hydroxychloroquine is not significantly bound to red blood cells but only to white blood cells that just make up 1% of the blood volume. 11
In summary, our work indicates that the Seraph removes neither hydroxychloroquine nor chloroquine hence, there is no need for a dose adaption or an additional dose in patient treated with the Seraph.
CONFLICTS OF INTEREST
The authors declare that there is no conflicts of interest regarding the publication of this paper.
ACKNOWLEDGMENTS
MTS, JJS, GE and JTK received research funding from ExThera Medical. MTS and JTK received travel support from ExThera Medical. All other authors: none to declare.
Seffer M‐T, Martens‐Lobenhoffer J, Schmidt JJ, Eden G, Bode‐Böger SM, Kielstein JT. Clearance of chloroquine and hydroxychloroquine by the Seraph® 100 Microbinda Affinity Blood Filter ‐a device approved for the treatment of COVID‐19 patients. Ther Apher Dial. 2021;25:237–241. 10.1111/1744-9987.13549
Stefanie M. Bode‐Böger and Jan T. Kielstein contributed equally and should be considered last authors.
REFERENCES
- 1. Coatney GR. Pitfalls in a discovery: The chronicle of chloroquine. Am J Trop Med Hyg. 1963;12:121–128. [DOI] [PubMed] [Google Scholar]
- 2. The Lancet Editors . Expression of concern: Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID‐19: A multinational registry analysis. Lancet. 2020;395:e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mehra MR, Desai SS, Ruschitzka F, Patel AN. Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID‐19: A multinational registry analysis. Lancet. 2020;S0140‐6736(20)31180‐6. 10.1016/S0140-6736(20)31180-6 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4. Gao J, Tian Z, Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID‐19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72–73. [DOI] [PubMed] [Google Scholar]
- 5. Zhou D, Dai SM, Tong Q. COVID‐19: A recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. J Antimicrob Chemother. 2020;75(7):1667–1670. 10.1093/jac/dkaa114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Seffer MTD, Forni LG, Kielstein JT. Heparin 2.0—A new approach to the infection crisis. Blood Purif. 2020. 10.1159/000508647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schmidt JJ, Eden GS, Seffer MT, Winkler M, Kielstein JT. In vitro elimination of anti‐infective drugs by the Seraph 100 Microbind Affinity Blood Filter. Clin Kidney J. 2020;sfaa063. 10.1093/ckj/sfaa063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Matzke GR, Aronoff GR, Atkinson AJ Jr, et al. Drug dosing consideration in patients with acute and chronic kidney disease‐a clinical update from kidney disease: Improving global outcomes (KDIGO). Kidney Int. 2011;80:1122–1137. [DOI] [PubMed] [Google Scholar]
- 9. Van Stone JC. Hemodialysis and chloroquine poisoning. J Lab Clin Med. 1976;88:87–90. [PubMed] [Google Scholar]
- 10. Gottschall S, Falkenhagen D, Schmitt E, Courtney JM, Klinkmann H. Activated charcoal and resin haemoadsorbents: in vitro drug removal and blood compatibility studies. Biomed Biochim Acta. 1984;43:245–248. [PubMed] [Google Scholar]
- 11. Brocks DR, Skeith KJ, Johnston C, et al. Hematologic disposition of hydroxychloroquine enantiomers. J Clin Pharmacol. 1994;34:1088–1097. [DOI] [PubMed] [Google Scholar]


