Abstract
The spectrum of clinical manifestations of coronavirus disease 2019 in children is yet to be fully elucidated. We report the case of an infant who tested positive for severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and developed mild cardiovascular inflammation, a novelty for patients of very young age, that contributes to defining the puzzling nature of this disease in pediatric patients. The potential cardiovascular involvement of SARS‐CoV‐2 in children should always be taken into account.
Keywords: coronavirus disease 2019, heart, inflammation, SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
Abbreviations
- ACE2
angiotensin‐converting enzyme 2
- COVID‐19
coronavirus disease 2019
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
1. CASE REPORT
Coronavirus disease 2019 (COVID‐19) is milder in children, with much fewer reported severe and critical cases (around 6%) than in adult patients (18.5%). This proportion appears to be slightly more elevated in infants (<1 year of age), reaching 10.6%. 1
COVID‐19 may indeed have cardiac complications, including myocarditis, 2 and up to 31% of children have myocardial enzyme elevation, mainly creatine kinase MB, despite no specific sign or symptom of clinical cardiac disease. Nevertheless, correlation between D‐dimer and myocardial enzymes elevation (particularly creatine kinase MB) and disease severity has been described. 3
No specific morphological and functional cardiac assessment has yet been performed in COVID‐19 children with elevated myocardial enzymes.
For the first time, we report the case of an infant affected by COVID‐19 with documented mild cardiac involvement.
A full term, formula‐fed, 38‐day old male presenting with mild fever (37.6°C), rhinitis and modest hyporeactivity was admitted on 27th March for clinical evaluation. The pregnancy had been unremarkable, and the mother had been vaccinated against influenza. Both parents were diagnosed with COVID‐19 and household contagion was presumed. History was otherwise irrelevant and the infant did not show any sign or symptom of acute respiratory distress. A full workup was performed.
Complete blood count showed fluctuations during the hospital stay, but lymphocytopenia was never observed (min, 7100/μL); mild thrombocytosis (max, 525 000 /μL) was present, with normal values of hemoglobin. C‐reactive protein and erythrocyte sedimentation rate were confirmed negative throughout the hospital stay, while a persistent increase in procalcitonin levels was observed (max, 3.28 ng/mL; nv < 0.5). Electrolytes were in normal range and lactate dehydrogenase was mildly increased. Liver transaminases were normal.
Nasal and pharyngeal swab specimens tested positive for severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) nucleic acid by using real‐time reverse‐transcriptase polymerase chain reaction assay on 28th March, while Allplex Seegene Respiratory Panel for 18 viruses resulted negative.
Blood culture, urine culture, and pharyngeal swab for S. pyogenes were also negative.
Chest X‐ray showed mild thickening of bronchovascular markings, but no pulmonary parenchymal opacities. The chest computed tomograpghy scan was not performed, thus avoiding the exposure to ionizing radiations in an infant without overt respiratory involvement.
An increase in troponin T was observed (max, 8.2 ng/dL; reference range for age <5), as well as a slightly elevated creatine kinase‐MB (max, 9.8 μg/L; reference range ≤4.8). 4 Pro‐brain natriuretic peptide (208 pg/mL) was normal. INR and partial thromboplastin time were normal, while D‐dimer was found to be increased (max, 13.3 μg/mL, reference range <0.87) in two consecutive measurements, and subsequent spontaneous resolution. Fibrinogen transiently diminished (1.28 g/L, nv > 1.5) without associated platelet consumption. A graphical representation of laboratory tests trends is shown in Figure 1.
Figure 1.
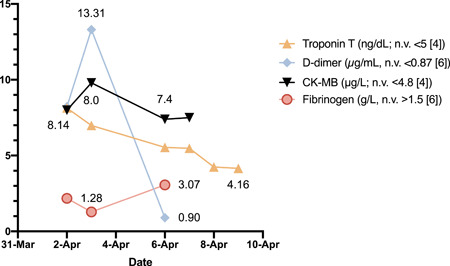
Time course of relevant cardiac and vascular biomarkers. Maximum and minimum values are reported next to the corresponding symbol
Continuous monitoring of vital parameters documented resting heart rate of 140/min, with peak frequency of 200/min. Serial electrocardiograms and a 24‐hour Holter electrocardiogram confirmed only mild sinus tachycardia and a first cardiac ultrasonography was normal (Figure 2, panel A). A cardiac magnetic resonance was also performed with the “feed and sleep” approach, which excluded edema of the myocardium and showed a minimal amount of pericardial effusion (Figure S1).
Figure 2.
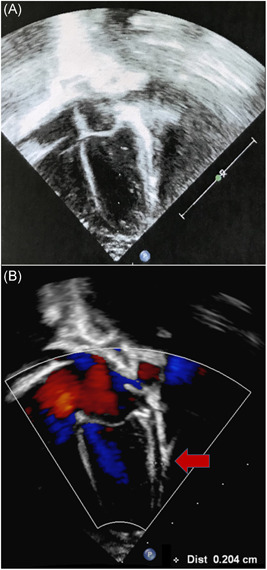
A, Four chamber view of the heart taken during the first cardiological evaluation shows normal structure and dimensions of cardiac chambers, without pericardial effusion. B, Four chamber view of the heart four days after the previous one shows a 2 to 3 mm pericardial effusion in the lateral‐posterior pericardial space (arrow)
Three days after the first cardiac ultrasound, a new sonographic evaluation documented a 2 mm pericardial effusion which did not evolve during follow‐up (Figure 2, panel B).
The patient remained asymptomatic with normal vital parameters, thus no specific treatment was undertaken. Serological tests ruled out other common causes of viral myopericarditis (Coxsackie virus, echovirus, cytomegalovirus, and Epstein‐Barr virus).
In consideration of the altered D‐dimer and fibrinogen, and given the recent reports of SARS‐CoV‐2 associated coagulopathy and cerebral infarcts, 5 the infant underwent a brain MR angiography with the “feed and sleep” approach which resulted normal. Lupus‐like anti coagulants, anti‐nuclear, and anti‐cardiolipin antibodies were undetectable.
Hospital stay was unremarkable; no oxygen or antiviral therapy was administered. After 14 days the child was discharged in good health and he tested negative for SARS‐CoV‐2 on 16th and 17th April. He was thus enrolled in a cardiological follow‐up to monitor the long‐term evolution of the clinical picture.
SARS‐CoV‐2 enters the cells through the angiotensin‐converting enzyme 2 (ACE2) receptor, which is displayed by a range of tissues, including the endothelium and the respiratory epithelium. 2 The reason why COVID‐19 is milder in children is unknown. Cardiac involvement by SARS‐CoV‐2 may be directly virus‐dependent, and/or secondary to inflammatory response. Given the immaturity of the immune system of our patient, we speculate that the former may be more relevant, at least in infants and neonates. The ubiquity of the ACE2 receptor may explain the cardiac and vascular involvement that have been observed in our case.
To the best of our knowledge this is the first report of cardiac involvement in an infant with SARS‐CoV‐2 infection, with comprehensive clinical, biochemical and multimodal imaging characterization. We suggest that SARS‐CoV‐2 cardiac involvement should always be taken into account also in children; while our case was mild, it might be of concern especially in patients with other underlying conditions. Follow‐up is necessary to detail the long‐term outcomes of cardiac involvement in affected patients.
Supporting information
Supporting information
Supporting information
Del Barba P, Canarutto D, Sala E, et al. COVID‐19 cardiac involvement in a 38‐day old infant. Pediatric Pulmonology. 2020;55:1879–1881. 10.1002/ppul.24895
REFERENCES
- 1. Dong Y, Mo X, Hu Y, et al. Epidemiological characteristics of 2143 pediatric patients with 2019 doronavirus disease in China. Pediatrics. 2020;145:e20200702.32179660 [Google Scholar]
- 2. Madjid M, Safavi‐Naeini P, Solomon SD, Vardeny O. Potential cffects of coronaviruses on the cardiovascular system. JAMA Cardiol. 2020. https://jamanetwork.com/journals/jamacardiology/fullarticle/2763846. Accessed 12 April 2020. [DOI] [PubMed] [Google Scholar]
- 3. Qiu H, Wu J, Liang H, Luo Y, Song Q, Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID‐19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020:1‐8. www.thelancet.com/infection. Accessed 11 April 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jehlicka P, Huml M, Rajdl D, et al. How to interpret elevated plasmatic level of high‐sensitive troponin T in newborns and infants? Physiol Res. 2018;67(2):191‐195. [DOI] [PubMed] [Google Scholar]
- 5. Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid‐19. N Engl J Med. 2020;382:e38. 10.1056/NEJMc2007575 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Supporting information


