Abstract
The detection data of IgM and IgG antibodies in 169 patients with coronavirus disease‐2019 (COVID‐19) were analyzed to evaluate differences in clinical performance between the colloidal gold method and chemiluminescence method. In this study, chemiluminescence detection of IgM antibody showed a positive conversion earlier (about 1‐2 days earlier), positive conversion rates higher in different stages of disease, and a trend of declining positive rate later than colloidal gold method. For IgG antibody, the chemiluminescence method showed a positive conversion earlier and the positive rate climbing more quickly than the colloidal gold method. No obvious negative‐converting tendency of IgG detection was observed within 35 days after the onset of disease. Although colloidal gold method is generally less sensitive than chemiluminescence method, it shows advantages of shorter turn‐around time, more simple procedure, and no special equipment required. The two methodologies can be chosen according to different laboratory conditions.
A reasonable understanding of the performance of reagents with different methodologies can help in clinical disease diagnosis effectively and assist in the diagnosis of the progression of COVID‐19, for which the dynamic changes of antibody will provide reliable evidence.
Keywords: COVID‐19, immunoassay, SARS‐CoV‐2, virus infection
1. INTRODUCTION
Currently, the worldwide outbreak of coronavirus disease‐2019 (COVID‐19) has become a public health event of international concern. 1 Effective and timely detection of novel coronavirus (severe acute respiratory syndrome coronavirus 2 [SARS‐CoV‐2]) infections will help patients to access timely treatment, prevent further transmission, and ultimately achieve effective control of the epidemic. At present, the diagnosis of SARS‐CoV‐2 infection mainly relies on the detection of viral nucleic acid in upper and lower respiratory tract samples. 2 , 3 , 4 However, due to the uneven sampling quality of the respiratory tract samples, especially upper respiratory tract samples, the different viral load of different respiratory tract samples and, at the same time, the insufficient understanding of the characteristics of the spread of SARS‐CoV‐2, nucleic acid tests are frequently reported as false negatives. 5 , 6 , 7 , 8 If other clinical and laboratory diagnostic methods can be effectively combined, it will help to ensure the timely diagnosis and timely treatment of diseases. 9 , 10 Serological testing, as a common method, plays an important role in the diagnosis of many pathogen infections. Moreover, the serological tests require more simple procedure and need no special equipment. The standard blood sample collection process ensures the quality of subsequent antibody detection and reduces the risk of virus transmission. With the good detection performance, serological testing can be used as supplementary diagnosis of COVID‐19 suspect cases with nucleic acid negative. 11
The human body will produce specific antibodies after the virus invades. The specific IgM antibody appears first, and then the titer of IgG antibody will continue to rise. Serological tests indirectly determine whether there is a viral infection by detecting the presence and changes of specific antibody in blood samples.
At present, during the COVID‐19 epidemic, research on antibody production and changes in patients of COVID‐19 is lack of systematization, and it is difficult to acquire clinical data of large‐scale cohort studies when clinical diagnosis and treatment are in emergency condition. In the clinical trials for the purpose of premarket registration, the SARS‐CoV‐2 antibody detection kits with different methodologies in China were evaluated for the clinical performance by adopting a unified clinical trial protocol based on the requirements of the published “Key Points of Technical Review for Registration of SARS‐CoV‐2 Antigen/Antibody Detection Reagents (Trial version).” 12 These clinical trials included not only the data for the evaluation of sensitivity and specificity of the reagents but also the research data of the continuous monitoring of enrolled subjects at different time points of disease. Summarizing the data of serial monitoring in the clinical research, and investigating the positive rate of IgM and IgG antibodies at different time points in the course of SARS‐CoV‐2 infection by reagents with different methodologies will have a positive effect on understanding the changes and outcomes of SARS‐CoV‐2‐related antibodies after human infection, the performance of different antibody detection methodologies, and thus further clarifying the role and significance of different serological detection methods in the diagnosis of COVID‐19.
It should be emphasized that all the clinical trials mentioned here have been conducted in compliance with the consistent protocol according to the “Key Points of Technical Review for Registration of SARS‐CoV‐2 Antigen/Antibody Detection Reagents (Trial version),” 12 issued by the Center for Medical Device Evaluation of National Medical Products Administration of China, as a result of which the clinical trial inclusion criteria, definition of the stage of disease and the test result reporting methods are all consistent among the different clinical trials, so it is acceptable to conduct comprehensive analysis with the data collected from all these clinical trials.
2. MATERIALS AND METHODS
Based on the clinical trials of four SARS‐CoV‐2 antibody detection kits submitted and confirmed for registration in China between February 2020 and May 2020, including both colloidal gold method and chemiluminescence method, multitime‐point surveillance data with the information of sampling time were collected and analyzed in this study, to observe the production and conversion of SARS‐CoV‐2‐specific antibodies and evaluate the positive rates of antibodies detected by reagents with different methodologies.
The clinical trials were conducted at seven clinical institutions in compliance with the consistent protocol based on the “Key Points of Technical Review for Registration of SARS‐CoV‐2 Antigen/Antibody Detection Reagents (Trial version).” 12 All the clinical trials complied with the provisions of the Declaration of Helsinki and the International Conference on Harmonization on the clinical trial management and were approved by the ethical review committee of clinical trial institutions.
This study includes the continuous monitoring data of IgM and IgG in 169 patients with COVID‐19 from the sample sets of the clinical trials mentioned above. All the enrolled patients were confirmed according to the valid version of “Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia” 13 issued by the National Health Commission of China. Details about the inclusion criteria are shown in part 2.2.
In addition to the investigation of the multitime‐point surveillance data included in this study, the clinical trials mentioned above were also conducted to evaluate the sensitivity and specificity of the four reagents with the samples of COVID‐19 confirmed cases and excluded cases. It was demonstrated that there was no cross reaction with other respiratory viruses, including other coronaviruses for these four reagents (data not shown). All the confirmed cases and excluded cases were diagnosed according to the “Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia.” 13
2.1. Reagents and instruments
SARS‐CoV‐2 IgM and IgG antibody chemiluminescence detection kits were from Maccura Biotechnology Co, Ltd and Bioscience (Tianjin) Diagnostic Technology Co, Ltd. They finished the tests with the chemiluminescence immunoassay instruments specified in the manuals of the in‐vitro Diagnostic (IVD) kits; SARS‐CoV‐2 colloidal gold test kits came from Innovita (Tangshan) Biological Technology Co, Ltd and Zhuhai Livzon Diagnostics Inc, of which the test process and test result interpretation did not require any test instruments (Table 1).
Table 1.
Information for the SARS‐CoV‐2 IgM and IgG antibody detection kits
| Company | Method | Instrument |
|---|---|---|
| Maccura Biotechnology Co, Ltd | Capture immunoassay for IgM and indirect Immunoassay for IgG; chemiluminescence | Chemiluminescence immunoassay analyzer |
| Bioscience (Tianjin) Diagnostic Technology Co, Ltd | Indirect immunoassay; chemiluminescence | Chemiluminescence immunoassay analyzer |
| Innovita (Tangshan) Biological Technology Co, Ltd | Capture immunoassay; colloidal gold | NA |
| Zhuhai Livzon Diagnostics Inc | Indirect immunoassay; colloidal gold | NA |
Abbreviation: SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
2.2. Inclusion criteria and sample collection
All the subjects included in this study were COVID‐19 confirmed cases with multitime‐point surveillance data of different stage of disease.
COVID‐19 confirmed cases are defined as COVID‐19 suspect cases with one of the following etiological evidence: real‐time fluorescent reverse transcription‐polymerase chain reaction indicates positive for SARS‐CoV‐2 nucleic acid, or/and viral gene sequence is highly homologous to known SARS‐CoV‐2. Suspect cases refer to patients possessing both the epidemiological history (traveling to or residence in Wuhan, in contact with SARS‐CoV‐2 infected people etc) and clinical manifestations (fever, respiratory symptoms, imaging characteristics of COVID‐19, normal or decreased WBC count and/or lymphocyte count, etc) in the early stage of onset.
The definition of COVID‐19 “suspect cases” and “confirmed cases” are determined according to the valid version of “Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia.” 13
The serum or plasma samples were collected at two or more time points of different stages of disease for each case. The stage of disease was determined according to the interval between the time of sample collection and the onset day of disease with the symptom of fever, dry cough, and so forth, which was set as the 0 day. The time of sample collection was determined according to the needs of clinical diagnosis and treatment, but not continuous collection every day.
2.3. Sample detection
Sample detection was done respectively referring to the instruction for use of the products. Each sample was tested for IgM and IgG simultaneously.
2.4. Data analysis
The detection data collected in this study came from 169 COVID‐19 confirmed cases, 92 males and 77 females; the age of the subjects ranged from 19 to 90 years old. The demographic information and basic characteristics of the study population are shown in Table 2. It reveals that there is no significant difference between the basic characteristics of the chemiluminescence set, colloidal gold set, and the total set of subjects.
Table 2.
Demographic information and basic characteristics of the study population
| All subjects | Group of chemiluminescence immunoassay | Group of colloidal gold | ||
|---|---|---|---|---|
| Total Number | ||||
| Subjects number | 169 | 109 | 60 | |
| Male (%) | 92 (54%) | 60 (55%) | 32 (53%) | |
| Female (%) | 77 (46%) | 49 (45%) | 28 (47%) | |
| Age | ||||
| Minimum | 19 y old | 19 y old | 20 y old | |
| Maximum | 90 y old | 90 y old | 85 y old | |
| Median | 55 y old | 54 y old | 57 y old | |
| 18‐50 y old | 69 (41%) | 46 (42%) | 23 (38%) | |
| 51 ~ 64 y old | 49 (29%) | 30 (28%) | 19 (32%) | |
| 65 y and over | 51 (30%) | 33 (30%) | 18 (30%) | |
| Antibody detection tests | ||||
| Number of samples for single patient min‐max (median) | 2‐11 (3) | 2‐11 (3) | 2‐4 (3) | |
| Proportion of IgM turned positive | 163/169 | 104/109 | 59/60 | |
| Proportion of IgG turned positive | 168/169 | 109/109 | 59/60 |
The test results of all the cases with continuous monitoring data in the clinical trials of each product were summarized to calculate the positive rate of IgM and IgG during the disease. The production and conversion of SARS‐CoV‐2‐specific IgM and IgG antibodies during the course of disease were observed, and the positive rates of antibodies detected by reagents with different methodologies were compared with evaluate the differences in antibody detection performance. Combining with the characteristics of different methods for clinical practice and interpretation of results, the clinical application and significance of reagents with different methodologies are discussed.
3. RESULTS
3.1. Performance comparison of two immunoassay methods for detection of SARS‐CoV‐2
Detection results of 169 COVID‐19 cases using four different IVD kits (including colloidal gold method and chemiluminescence method) in seven clinical sites were recorded. Samples were collected at multiple monitoring points. A total of 2 to 11 serum or plasma samples were collected at different time points from each case.
Among the 169 cases, 109 cases were detected using chemiluminescence method, and 60 cases were detected using colloidal gold method. For each case, the onset date of fever and other symptoms was recorded as the 0 day of the course of disease, and the longest monitored course of disease reached 35 days. By calculating the cumulative positive rate of IgM and IgG at different time points during the course of the disease, the production and conversion of IgM and IgG antibodies were observed.
The results of the cumulative positive conversion rates of IgM antibody by chemiluminescence and colloidal gold are shown in Figure 1, and the cumulative positive conversion rates of IgG antibody are shown in Figure 2.
Figure 1.
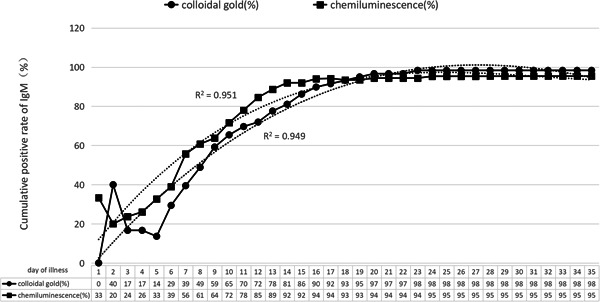
Cumulative positive rate of IgM antibody test
Figure 2.
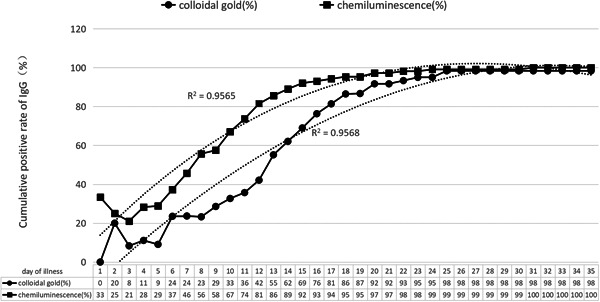
Cumulative positive rate of IgG antibody test
The continuous monitoring results of serum and plasma IgM antibody show that the cumulative positive conversion rate (proportion of positive cases in all tested cases) was more than 50% by the first week of the disease course by chemiluminescence method, and it was about 40% by colloidal gold method; in the second week of the disease course, the positive conversion rate of IgM continued to increase, and by the 14th day, the cumulative positive conversion rate had reached about 90% by chemiluminescence method, and 80% by colloidal gold method; after 3 weeks, the cumulative positive conversion rate was close to 100% by both methods.
The continuous monitoring results of serum and plasma IgG antibody show that the cumulative positive conversion rate was about 45% by the first week of the disease course by chemiluminescence, and it was less than 30% by colloidal gold method; in the second week of the course, nearly 90% IgG positive could be detected by chemiluminescence, and only about 60% IgG positive could be detected by colloidal gold; after 23 days, the cumulative positive conversion rate of IgG reached more than 95%.
Comparing the results of Figures 1 and 2, the positive conversion time of IgG is 2 days later than IgM by chemiluminescence method on average, and it is 3 to 5 days later by colloidal gold method. After 3 weeks, positive conversion of IgG and IgM can be observed in almost all patients' blood samples by both methods.
Within 35 days of continuous monitoring, six patients did not show positive IgM antibody conversion, and one patient did not show IgG antibody positive conversion (included in the six cases of IgM nonpositive conversion). They were four males and two females, including five cases tested by chemiluminescence and one case tested by colloidal gold method. Three of these cases were sampled at the 17th day, 18th day, and 31th day, respectively for the first time, of which the reason for the absence of IgM positive conversion may be related to the late sampling time when IgM may have turned negative. The other three cases did not show IgM positive, including one which did not show IgG positive neither during the entire detection process, and this result may be due to the differences in immune responses to pathogens of different individuals.
3.2. Performance comparison of two immunoassay methods for detection of SARS‐CoV‐2 in different stages of illness
Based on the collected data of 169 cases, analysis of positive rate at different stages of COVID‐19 by reagents of colloidal gold method (60 cases) and chemiluminescence method (109 cases) was conducted, the difference in detection performance between the two methodologies are compared.
Figure 3 shows that, compared with the colloidal gold method, chemiluminescence detection of IgM antibody showed an earlier positive conversion (about 1‐2 days earlier), and showed a higher positive conversion rate at different course of disease. In the 2nd to 3rd week of the disease course, the highest positive rate of IgM antibody detected by chemiluminescence method was almost 100%, and the slightly lower detection rate was observed by colloidal gold method. After the third week of disease course, the positive rate of colloidal gold method began to show a downward trend, while the positive detection rate of chemiluminescence method remained at a high level (above 80%), which tended to turn downward after the fourth week. The reason may be that the colloidal gold method has lower sensitivity than the chemiluminescence method. When the titer of the IgM antibody in patients in the early and late stages of the disease is low, some samples may not be detected positive by the colloidal gold method.
Figure 3.
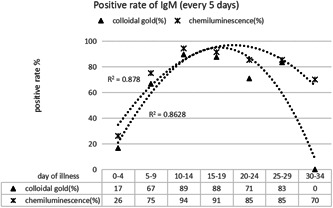
Comparison of positive rate of IgM detection by different methods at different stages
Similar to IgM, chemiluminescence detection of IgG antibody showed a positive reaction earlier, and reached the highest positive detection rate earlier than the colloidal gold method (Figure 4). The reason may also be related to the lower sensitivity of the colloidal gold method. Regardless of the method used, there was no downward trend in the positive rate of IgG detection during the observation period (35 days). It can be seen clearly, after reaching the peak, the positive rate of IgG can be maintained for a period of time (Figure 4).
Figure 4.
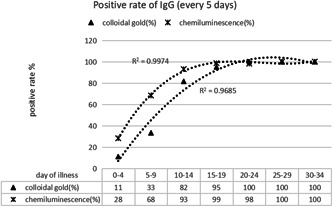
Comparison of positive rate of IgG detection by different methods at different stages
4. DISCUSSION
At present, there are many studies about serological testing of SARS‐CoV‐2 antibody published. Some studies have demonstrated the performance of antibody testing kits in clinical studies by analyzing sensitivity and specificity. 14 , 15 , 16 , 17 Li et al 17 believe that the test can be used in identifying SARS‐CoV‐2 carriers in hospitals, clinics, and testing laboratories. Yong et al's 18 findings indicate that the antibody detection could be used as an effective supplementary indicator of SARS‐CoV‐2 infection in suspected cases without detectable viral RNA.
Besides the sensitivity and specificity study, the continuous monitoring of SARS‐CoV‐2 antibody will help to understand the antibody production and conversion in patients with COVID‐19 and thus evaluate the importance of serological test for SARS‐CoV‐2 antibody in epidemic prevention and control. In this study the multitime‐point surveillance data of SARS‐CoV‐2‐specific IgM and IgG in 169 COVID‐19 confirmed cases were analyzed and the performance comparison of the colloidal gold method and chemiluminescence method in SARS‐CoV‐2 antibody detection was conducted.
Based on the data in this study, similar to the reported outcomes of SARS‐CoV‐2 antibody detection, a few cases have begun to produce IgM antibodies within the first week of the disease course, but the overall positive rate is only around 50% or less. In the second week of the disease course, the IgM antibody positive rate significantly increased, and reached the peak in the third week, when more than 90% of patients showed a positive reaction. During the above process, the chemiluminescence detection reagents showed a significant advantage in sensitivity compared with the colloidal gold method, with that the positive conversion time was advanced by 1‐2 days and reached the peak positive rate earlier. Similar results were also reported in others' studies. 19 , 20 The positive rate of colloidal gold IgM antibody detection shows a downward trend after 3 weeks, while the chemiluminescence method which have better sensitivity can still maintain a positive detection rate of more than 80%, but the overall IgM positive rate shows a significant downward trend by the end of the fourth week.
The time of appearance and peak time of IgG antibody was about 2 to 5 days later than IgM by different test methodologies. After 23 days of disease course, the IgG antibody detections for almost all of the cases were positive. After the production, IgG antibodies can continue to be positive for at least 1 month and probably a much longer period. The chemiluminescence method also showed higher detection rate and earlier detection time than the colloidal gold method for SARS‐CoV‐2 IgG. As for other pathogens, the outcome of IgM−/IgG−, IgM+/IgG−, IgM+/IgG+, and IgM−/IgG+ will appear in turn throughout the course of disease. At different stages of disease, different results will be obtained by detecting antibodies. Proper use of antibody detection will play a positive role in the diagnosis of SARS‐CoV‐2 infections and the determination of disease progression.
It is worth noting that overall, the positive conversion rate obtained by multitime‐point sampling monitoring may be lower than the positive rate calculated with single‐point sampling cases. The main reason is that the sampling interval of series samples from one patient is not strictly controlled. When the interval is large, the positive conversion rate in this period may be underestimated.
In the diagnosis of COVID‐19, positive viral nucleic acid test as direct etiological evidence is regarded as one of the key indicators. In the past few months of clinical practice and research, SARS‐CoV‐2 nucleic acid detection technology has proved to have a good positive detection rate, especially in the first 2 weeks during disease, and can effectively diagnose related viral infections. But so far, the false negatives of nucleic acid tests are still frequently reported, and the main factors include patient sampling quality, viral load and distribution, and the standardization of the detection process, and so forth. Serological testing indirectly determines whether virus infection occurs and shows the stages of the infection by detecting the production and changes of specific antibody in the blood sample. The sampling of serum or plasma is more convenient and easier to be standardized, which is useful for dynamic monitoring of the patient's condition. The serological sample is more homogenized and proved to be highly reproducible in assays. For patients with negative nucleic acid results, serological antibody test can be used as a supplementary method to increase the positive rate of the diagnosis, help to confirm the disease state and further disease treatment and epidemic control.
In the first 2 weeks of disease progression, IgM antibody is produced and peaked early, which is a marker of the acute phase of viral infection. In addition, the four‐fold increase in IgG antibody titer can also be used as one of the judgment methods for the acute phase of infection. These serological markers can be used as an effective supplement for nucleic acid detection and used to confirm the disease state. Two weeks later, with the effect of antiviral therapy, the positive rate of nucleic acid detection gradually decreased, 6 , 11 the antibody tended to reach the highest detection level, and most of the infected patients can be tested as IgM and IgG positive. After about 3 weeks of disease, the positive rate of IgM begins to show a downward trend and the continued positive IgG antibody is an effective indicator of previous infections.
It is worth noting that for patients with suspected symptoms and close contacts, when IgM−/IgG− is tested for the first time, it is recommended to continue serological testing. Close attention should be paid if IgM+/IgG− antibody conversion results are detected, and effective isolation measures and continuing nucleic acid and antibody testing should be given. When IgM appears positive again, and IgG antibody titer increases significantly, the patient can be considered as SARS‐CoV‐2 infection. It is not recommended to use a single antibody IgM positive or IgG positive result as the evidence for the diagnosis of SARS‐CoV‐2 infection. The dynamic changes of antibody detection will provide more reliable evidence for disease diagnosis and disease progression judgment. It shows that due to the difference in the outcome time and positive rate of IgM and IgG antibodies, they can form a certain cross complementary in the judgment of disease progression. The combined detection of IgM antibody and IgG antibody is more effective than the single detection of either.
In the process of antibody detection in the clinical laboratory, chemiluminescence and colloidal gold methods have their own characteristics. Although colloidal gold method is generally less sensitive than chemiluminescence, it is fast, simple, and requires no special detection equipment. Colloidal gold method can quickly provide results for clinical use, and can be used as a point‐of‐care testing method. Especially for sporadic cases and emergency, colloidal gold method can provide results quickly and conveniently. According to the comparison results of this study, especially in the course of 2 to 3 weeks, the clinical detection performance of the two methodological for IgM antibody is not significantly different, and can be chosen according to laboratory conditions. Chemiluminescence method has the characteristics of automation, sensitivity, and good repeatability. It is more suitable to choose Chemiluminescence method when requiring high sample detection throughput, and auxiliary diagnosis in the early stage of the disease, especially for suspected cases with negative nucleic acid results. Chemiluminescence method has higher sensitivity to help identify cases of infection. When dynamically observing changes in antibody titer, the use of chemiluminescence method is more advantageous.
Although it has been confirmed by preclinical and clinical studies that both colloidal gold method and chemiluminescence antibody detection reagents have relatively good specificity, it cannot completely avoid false positive results caused by cross‐reactions and interference factors. Therefore, the single antibody test results are not recommended to use as the only evidence for the diagnosis or elimination of SARS‐CoV‐2 infection. Multipletime‐point samples should be tested and continuous monitoring of changes for antibody level should be required.
CONFLICT OF INTERESTS
All the authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
YL had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. JH, PH, YG, SZ, and CX equally contributed to the manuscript and shared first authorship. Concept and design were contributed equally by JH, PH, YG, SZ, and CX. Data collection and drafting of the manuscript: RL, LF, RL, CH, JA, JD, GD, and LS.
ACKNOWLEDGMENTS
The authors would like to acknowledge Dr Jie Ma from Hubei Provincial Hospital of Integrated Chinese & Western Medicine, Prof Ailong Huang from Chongqing Medical University, Prof Beizhong Liu from Yongchuan Hospital of Chongqing Medical University, Prof Yaokai Chen from Chongqing Public Health Medical Center, Prof Pengcheng Shang from Public Health Clinical Center of Chengdu, Prof Huijun Li from Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology, Prof Ming Pan from Sichuan Center for Disease Control And Prevention, Prof Zejin Liu from Wuhan Asia General Hospital for their work and contribution in the clinical trials of the SARS‐CoV‐2 IgM/IgG products. Thank Innovita (Tangshan) Biological Technology Co, Ltd, Bioscience (Tianjin) Diagnostic technology Co, Ltd, Maccura Biotechnology Co, Ltd, and Zhuhai Livzon Diagnostics Inc for their authorization of the use of the clinical data of their IVD reagents.
He J, Hu P, Gao Y, et al. Comparison and application of different immunoassay methods for the detection of SARS‐CoV‐2. J Med Virol. 2020;92:2777–2784. 10.1002/jmv.26187
REFERENCES
- 1. WHO . Coronavirus disease (COVID‐2019) situation reports. Accessed May 6, 2020.
- 2. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization . (2020). Laboratory testing for coronavirus disease 2019 (COVID‐19) in suspected human cases: interim guidance. Accessed March 2, 2020.
- 5. Yu F, Du L, Ojcius DM, Pan C, Jiang S. Measures for diagnosing and treating infections by a novel coronavirus responsible for a pneumonia outbreak originating in Wuhan, China. Microbes Infect. 2020;22:74‐79. 10.1016/j.micinf.2020.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yongqing T, Ming W, Wanzhou X, et al. Proposal for standardization of 2019 nCoV nucleic acid detection in clinical laboratories. Chin J Lab Med. 2020;43(3):209‐212. [Google Scholar]
- 7. Yating N, Xin H, Minya L, et al. Application of the technology of 2019 novel coronavirus specific antibody detection in serum. Med J Peking Union Med Coll Hospital. 2020;11:1‐9. [Google Scholar]
- 8. Ming G. Expert consensus on clinical application of coronavirus nucleic acid and antibody detection. Int J Lab Med. 2020:1‐10. http://kns.cnki.net/kcms/detail/50.1176.R.20200509.1104.002.html [Google Scholar]
- 9. Infantino M, Damiani A, Gobbi FL, et al. Serological assays for SARS‐CoV‐2 infectious disease: benefits, limitations and perspectives. Isr Med Assoc J. 2020;22:203‐210. [PubMed] [Google Scholar]
- 10. Okba NMA, Müller MA, Li W. SARS‐CoV‐2 specific antibody responses in COVID‐19 patients [published online ahead of print March 20, 2020]. medRxiv. 2020. 10.1101/2020.03.18.20038059 [DOI] [Google Scholar]
- 11. Xiaofei LI, Guiming LIU, Wanlan LI, et al. Application value of chemiluminescence immunoassay in the detection of 2019‐nCov antibodies. J Shandong Univ (Health Sci). 2020;58(5):46‐50. http://kns.cnki.net/kcms/detail/37.1390.r.20200423.1541.002.html [Google Scholar]
- 12. Key Points of Technical Review for Registration of SARS‐CoV‐2 Antigen/Antibody Detection Reagents (Trial version). https://www.cmde.org.cn/CL0004/20512.html
- 13. National Health Commission & National Administration of Traditional Chinese Medicine . Diagnosis and treatment protocol for novel coronavirus pneumonia (trial version 7). Chin Med J. 2020;133(09):1087‐1095. 10.1097/CM9.0000000000000819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Choe JY, Kim JW, Kwon HH, et al. Diagnostic performance of immunochromatography assay for rapid detection of IgM and IgG in coronavirus disease 2019 [published online ahead of print May 26, 2020]. J Med Virol. 2020. 10.1002/jmv.26060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Infantino M, Grossi V, Lari B, et al. Diagnostic accuracy of an automated chemiluminescent immunoassay for anti‐SARS‐CoV‐2 IgM and IgG antibodies: an Italian experience [published online ahead of print April 24, 2020]. J Med Virol. 2020. 10.1002/jmv.25932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cassaniti I, Novazzi F, Giardina F, et al. Performance of VivaDiag COVID‐19 IgM/IgG rapid test is inadequate for diagnosis of COVID‐19 in acute patients referring to emergency room department. Members of the San Matteo Pavia COVID‐19 Task Force [published online ahead of print March 30, 2020]. J Med Virol. 2020. 10.1002/jmv.25800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li Z, Yi Y, Luo X, et al. Development and clinical application of a rapid IgM‐IgG combined antibody test for SARS‐CoV‐2 infection diagnosis [published online ahead of print Febraury 27, 2020]. J Med Virol. 2020. 10.1002/jmv.25727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yong G, Yi Y, Tuantuan L, et al. Evaluation of the auxiliary diagnostic value of antibody assays for the detection of novel coronavirus (SARS‐CoV‐2) [published online ahead of print April 22, 2020]. J Med Virol. 2020. 10.1002/jmv.25919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jie B, Ling H, Jian Z, Hongbin Z, Quanhua Z. Comparative analysis of clinical application value of four different detection methods for infectious diseases. Label Immunoassays Clin Med. 2016;23(6):688‐690. [Google Scholar]
- 20. Xueqin LV, Yajing MA. Evaluation of the effect of electrochemiluminescence immunoassay, enzyme‐linked immunosorbent assay and colloidal gold on the determination of low concentration HBsAg. Int J Lab Med. 2013;34(09):1133‐1135. [Google Scholar]


