Abstract
Background
Which are the consequences of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection in liver transplant (LT) recipients?
Methods
We attempted to address this question by reviewing our single‐center experience during the first 2 months of the pandemics at a high incidence area.
Results
Nineteen adult patients (5 females) were diagnosed by May 5, 2020. Median age was 58 (range 55‐72), and median follow‐up since transplantation was 83 (range 20‐183) months. Cough (84.2%), fever (57.9%), and dyspnea (47.4%) were the most common symptoms. Thirteen patients (68.4%) had pneumonia in x‐ray/CT scan. Hydroxychloroquine was administered in 11 patients, associated with lopinavir/ritonavir and interferon β in 2 cases each. Immunomodulatory therapy with tocilizumab was used in 2 patients. Immunosuppression (IS) was halted in one patient and modified in only other two due to potential drug interactions. Five (26.3%) patients were managed as outpatient. Two patients (10.5%) died, 10 (52.6%) were discharged home, and 2 (10.5%) were still hospitalized after a median follow‐up of 41 days from the onset of symptoms. Baseline IS regimen remained unchanged in all surviving recipients, with good liver function.
Conclusions
Our preliminary experience shows a broad spectrum of disease severity in LT patients with COVID‐19, with a favorable outcome in most of them without needing to modify baseline IS.
Keywords: COVID‐19, immunosuppression, liver transplant, prognosis, SARS‐CoV2
1. INTRODUCTION
The first case of SARS‐CoV‐2 (severe acute respiratory syndrome coronavirus 2) infection, now termed as coronavirus disease 2019 (COVID‐19), was diagnosed in Madrid on February 25, 2020. Within a few weeks, the infection spread extraordinarily. By the first week of March, some surgical procedures were already cancelled in our institution in anticipation of the lack of beds to admit patients with the infection. Actually, our last liver transplant (LT) procedure was performed on March 7th and the abdominal transplants program was closed shortly after. By the end of March, there were around 900 patients admitted with COVID‐19 in a hospital that usually has 1200 beds running. Some other transplant programs suffered similar circumstances, shortly before, as in Milano, 1 or afterward, as in New York, 2 the most punished city in United States till the end of March 2020.
Meanwhile, we started to diagnose SARS‐CoV‐2 infection in liver transplant recipients in our hospital. The first recipient was admitted to the hospital on March 15th and due to the scarce information available on the effects of this emerging infection in immunocompromised hosts, we started a prospective data collection. Information regarding the clinical presentation and outcome in liver transplant recipients is till the date, limited to small case series and preliminary data from registries. There is a report of a LT recipient with perioperative infection, 3 two cases during follow‐up in China, 4 , 5 3 cases from Lombardy, 6 6 cases from Madrid, 7 9 from an International Registry, 8 24 from a survey in Italy, 9 and 3 more in the United States. 10 , 11 , 12
Therefore, we aimed at presenting our preliminary experience involving COVID‐19 in the LT population during the first 2 months of this pandemic crisis in Madrid.
2. MATERIALS AND METHODS
We included adult LT recipients consecutively diagnosed with COVID‐19 at our tertiary‐care center between March 15 and May 5, 2020. Diagnosis was made by means of epidemiological, clinical, laboratory, and radiological data, and confirmed by RNA amplification.
In the case of a PCR negative but showing highly suggestive clinical symptoms, laboratory and radiologic data of COVID‐19, the final decision about diagnosis was made if all 3 expert infectious disease specialist (MFR, APJ, and RS) agree with the diagnosis. This observational study has been performed with the support of an “ad hoc” database set up since the first LT recipient was admitted, being completed with the information from the hospital electronic medical records (HCIS, DXC Technology). Demographic and clinical features, laboratory and radiology results, therapy, and recipient and graft outcomes were collected. The local Clinical Research Ethics Committee approved the study protocol (ref. no. 20/151) and granted a waiver of informed consent due to its retrospective observational design.
All immunocompromised patients admitted to the hospital were managed following a defined pathway designed by the Unit of Infectious Diseases, General Internal Medicine, and the different specialties involved in transplant patient care. In the case of LT patients, general surgeons evaluated these patients at the emergency room upon a suspicion of COVID‐19 diagnosis and located in a specific area with contact precautions, where they were tested for SARS‐CoV‐2 through real‐time reverse transcription polymerase chain reaction (rRT‐PCR). Depending on the clinical situation, they were admitted to the hospital, discharged home with prescriptions and isolation measures, or to a medicalized hotel if they cannot fulfill isolation measures at home.
As there is not a specific and efficacious treatment of COVID‐19, the treatment protocol has changed during the study time. The first pneumonia cases were treated with hydroxychloroquine (HC) (400 mg/12 h during the first day, then 200 mg/12 h during 4‐9 days) and lopinavir/ritonavir (LPV/r; 200/100 mg twice a day for 14 days), after oral or written informed consent. Some patients also received subcutaneous β interferon (IFN‐ β) (250 μg every 48 hours). A single IV dose of tocilizumab (400 or 600 mg if body weight < 75 kg or ≥ 75 kg, respectively) was added in selected cases with excessive inflammatory response (C‐reactive protein [CRP] levels > 15 mg/dL and/or interleukin [IL]‐6 levels > 40 pg/mL) and worsening respiratory failures (bilateral lung infiltrates, oxygen saturation <92% at room air and/or partial pressure of arterial oxygen [PaO2]/FiO2 ratio < 300). Patients with mild infection could be discharged home with an outpatient HC prescription.
Immunosuppressive regimen was adjusted according to the severity of illness and the risk of drug‐drug interactions (ie, LPV/r).
Statistical analysis comprised mean ± standard deviation (SD) or the median with interquartile range (IQR) for quantitative data, and absolute and relative frequencies for qualitative variables.
3. RESULTS
Nineteen adult LT recipients with COVID19 were analyzed (one of them had undergone liver‐kidney transplantation). Only one had a suspected nosocomial SARS‐CoV‐2 infection, after being admitted for a liver biopsy and a percutaneous transhepatic cholangiography without any symptoms of COVID‐19. The first six patients of this series have been briefly reported in a previous study that also included kidney and heart transplant recipients diagnosed at our institution by March 23th. 7 Demographic and clinical features are depicted in Table 1. Five of them were female. Median age was 58 years (55‐72) but seven were older than 70 years. Most patients had comorbidities, and the median BMI was 30.1 (25.8‐32.4). Time from transplantation spanned from 2 to 314 months, with a median of 83 (20‐183) months. Five of the patients had been transplanted in the last 2 years. Eight patients were receiving tacrolimus‐based IS, 4 were on mTOr inhibitors (associated with mofetil mycophenolate and azathioprine in one case each), and 7 with mofetil mycophenolate or mycophenolic acid monotherapy.
TABLE 1.
Demographics, clinical characteristics, symptoms, and radiology in 19 liver transplant recipients diagnosed with COVID‐19
| Case | Gender/ age (years) |
BMI kg/m2 |
Type of SOT | Time since SOT (months) | Etiology | Comorbidities |
Maintenance IS |
Duration of symptoms a | Symptoms at presentation | Chest x‐ray | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DM | HT | LD | Cough | Fever | Dyspnea | Thoracic pain | |||||||||
| 1 | M/63 | 27.5 | Liver | 95 | HBV cirrhosis, HCC | + | + | − | EVE | 10 | + | + | − | − | Abnormal |
| 2 | M/72 | 27.6 | Liver | 65 | Cryptogenic cirrhosis | + | + | − | MMF, EVE | 4 | + | + | + | − | Abnormal |
| 3 | F/79 | 27.7 | Liver | 183 | HCV cirrhosis, HCC | + | + | − | AZA,EVE,PRED | 5 | + | + | + | − | Abnormal |
| 4 | M/73 | 30.5 | Liver | 194 | HBV cirrhosis | + | + | + | MMF | 21 | + | + | + | − | Abnormal |
| 5 | F/76 | 24.4 | Liver | 314 | HCV cirrhosis | − | + | + | TAC | 1 | + | + | + | + | Normal |
| 6 | F/46 | 31.2 | Liver | 76 | Acute liver failure | − | − | − | TAC | 1 | + | − | − | − | Normal |
| 7 | M/60 | 25.8 | Liver | 77 | HCV cirrhosis | − | − | + | MMF | 1 | − | + | − | − | Abnormal |
| 8 | M/55 | 30.1 | Liver | 193 | HCV cirrhosis | − | − | − | MPA | 7 | + | − | − | − | Abnormal |
| 9 | M/71 | 34.1 | Liver | 98 | HBV cirrhosis | + | + | + | MMF | 9 | + | − | + | − | Abnormal |
| 10 | M/52 | 27.6 | Liver + Kidney | 10 | Alcoholic cirrhosis | − | + | − | TAC, PRED | 6 | − | − | − | − | Normal |
| 11 | M/77 | 22.4 | Liver | 164 | HCV cirrhosis | − | − | − | MMF | 1 | + | + | + | + | Normal |
| 12 | F/53 | 33.6 | Liver | 2 | Alcoholic cirrhosis | − | + | − | TAC,MMF,PRED | 10 | + | − | + | + | Normal |
| 13 | M/53 | 32.5 | Liver | 2 | Alcoholic cirrhosis, HCC | − | − | − | TAC | — | − | − | − | − | Abnormal |
| 14 | F/56 | 24.0 | Liver | 20 | Acute liver failure | − | − | − | TAC | 5 | + | + | + | − | Abnormal |
| 15 | M/58 | 30.2 | Liver | 76 | HCV cirrhosis | − | − | − | MMF | 3 | + | − | − | − | Abnormal |
| 16 | M/56 | 33.8 | Liver | 10 | HCV cirrhosis, HCC | − | − | − | TAC, MMF | 12 | + | + | − | − | Normal |
| 17 | M/71 | 25.5 | Liver | 212 | HBV cirrhosis, HCC | − | − | − | EVE | 5 | + | + | − | − | Abnormal |
| 18 | M/57 | 32.4 | Liver | 113 | HCV cirrhosis, HCC | − | + | − | MMF | 5 | + | + | − | − | Abnormal |
| 19 | M/57 | 31.6 | Liver | 83 | HCV cirrhosis, HCC | + | + | − | TAC | 4 | + | − | + | + | Abnormal |
Abbreviations: AZA, azathioprine; DM, diabetes mellitus; EVE, everolimus; F, female; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HT, hypertension; IS, immunosuppression; LD, lung disease; M, male; MMF, mycophenolate mofetil; MPA, mycophenolic acid; SOT, solid organ transplantation; TAC, tacrolimus.
Before diagnosis, in days.
All patients but 3 presented with cough (84.2%), but only 8 (44.4%) had a temperature higher than 38°C, and 5 showed thoracic pain. Six patients had diarrhea and 3 nausea and vomiting. Only two patients complained of olfactory or taste disorders.
Lung infiltrates were present on thoracic x‐ray and/or CT scan at presentation in thirteen patients (68.4%) which were bilateral in 12 of them (63.1%).
Two patients had unusual thrombotic complications. Patient #13 showed a right hepatic vein branch thrombus and a second degree right portal vein branch thrombus in a Liver CT, probably related to a percutaneous transhepatic cholangiography procedure. Patient #12 had an intermittent right upper quadrant pain for a week, when admitted, and at abdominal CT scan showed an acute thrombus partially occupying the inferior vena cava from the hepatic veins‐caval anastomosis area to the left renal vein.
All had positive rRT‐PCR for SARS‐CoV‐2 except patient #8, and #18. Patient #8 had a typical COVID‐19 presentation (olfactory and taste impairment, diarrhea, and bilateral consolidations at chest x‐ray [Figure 1]). The test was done twice, one at admission and the other 5 days later. His symptoms started 1 week before being evaluated and most probably he became then rRT‐PCR negative or the result was related to swab sampling or less probably to test sensitivity. 13 His spouse had also typical symptoms, 1 week before the patient. Patient #18 was admitted in March due to cough and diarrhea, with related renal failure. He had a negative PCR result for SARS‐CoV‐2. Hepatitis A, CMV and C. difficile infection were ruled out. He improved and was discharged after 4 days, but he had shared room with another patient that later had a positive PCR. He has been admitted after 18 days with cough, fever and bilateral pneumonia, highly suspicious of COVID‐19, again with negative PCR.
FIGURE 1.

Chest x‐ray of patient ♯9. Left, day of admission. Parenchymal opacity in apical segment of lower left lung. Center, 5 d later, marked radiologic worsening with patchy consolidations peripherally distributed in middle and lower pulmonary fields. Right, a week from the previous study: worsening radiologic pattern with bilateral and peripheral consolidations compatible with severe involvement by COVID‐19. Due to the increase of acute phase reactants (APR), he received tocilizumab on the 6th day of admission, with improvement, being discharged 3 d after the x‐ray on the right
Lowest lymphocyte count during admission ranged from 0.2 × 109/L to 1.2 × 109/L. CRP values ranged from 1.47 to 216.1 mg/dL (Table 2 shows laboratory results, treatment and outcome).
TABLE 2.
Laboratory results, therapeutic approaches, management of immunosuppression, and outcomes
| Case |
WBC a (x109/L) |
Lympho (x109/L) |
CRP (mg/dL) |
AST (U/L) |
ALT (U/L) |
GGT (U/L) |
ALP (U/L) |
Antiviral therapy | Respiratory support | Change in IS regimen | Current status |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4.5 | 0.7 | 6 | 42 | 30 | 29 | 66 | HCQ, LPV/r | NC | EVE→TAC & MMF | A |
| 2 | 11.4 | 0.8 | 19 | 170 | 107 | 127 | 136 | HCQ,LPV/r,IFN‐β | HFOT | MMF & EVE→TAC | D |
| 3 | 3.5 | 0.5 | 9.7 | 51 | 31 | 927 | 301 | HCQ, IFN‐β | NC | None | A |
| 4 | 15.5 | 1.2 | 22 | 770 | 307 | 47 | 71 | None | IMC | D/C MMF | D |
| 5 | 2.0 | 0.6 | 1 | 23 | 13 | 20 | 78 | HCQ | None | None | A |
| 6 | 5.7 | 2.5 | 1 | 23 | 23 | 54 | 75 | None | None | None | A |
| 7 | 4.3 | 0.8 | — | 20 | 17 | 24 | 51 | HCQ | None | None | A |
| 8 | 5.9 | 0.2 | 24 | 76 | 126 | 87 | 83 | HCQ, TCZ | NC | None | A |
| 9 | 6.9 | 0.5 | 16 | 18 | 22 | 51 | 70 | TCZ | NC | None | A |
| 10 | 3.2 | 0.2 | 2 | 19 | 8 | 65 | 63 | None | NC | None | A |
| 11 | 5.9 | 0.5 | 2 | 19 | 17 | 28 | 62 | HCQ | None | None | A |
| 12 | 5.0 | 0.7 | 1 | 114 | 142 | 89 | 95 | HCQ | None | None | A |
| 13 | 2.0 | 0.4 | — | 18 | 23 | — | — | None | None | None | A |
| 14 | 1.2 | 0.2 | 5 | 21 | 15 | 109 | 91 | HCQ | None | None | A |
| 15 | 6.0 | 1.6 | — | 17 | 10 | 40 | 74 | None | None | None | A |
| 16 | 3.3 | 1.0 | — | 20 | 21 | 28 | 80 | None | None | None | A |
| 17 | 5.3 | 1.1 | 8 | 68 | 66 | — | 43 | HCQ | None | None | A |
| 18 | 6.7 | 1.6 | 19 | 22 | 18 | 32 | 41 | None | NC | None | A |
| 19 | 3.3 | 0.5 | 10 | 41 | 42 | — | 81 | HCQ | NC | None | A |
Abbreviations: A, alive; ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase; CRP, C‐reactive protein; D, dead; EVE, everolimus; GGT, gamma‐glutamyl transferase; HCQ, hydroxychloroquine; HFOT, high‐flow oxygen therapy (ie, non‐rebreather oxygen mask with reservoir bag); IFN‐β, interferon‐β; IMV, invasive mechanical ventilation; LPV/r, lopinavir/ritonavir; MMF,mycophenolate mofetil; MPA, mycophenolic acid; NC, nasal cannula; TAC, tacrolimus; TCZ, tocilizumab; WBC, white blood count.
Laboratory results shown corresponding to peak.
Five patients received outpatient care. HC was prescribed in two of them. One (case #7), asymptomatic when a follow‐up CT (ordered as a scheduled follow‐up of an incidental hepatocellular carcinoma found in the resected specimen) showed bilateral ground glass infiltrates (Figure 2). He had mild symptoms afterward: some weakness, dysthermic feeling without fever and some head pressure without headache. Patient #5 had fever, cough, thoracic pain and dyspnea when seen at the Emergency Department (ED), with a normal chest x‐ray. The third patient (#6) lost her mother due to COVID‐19, but she only showed transient nausea and vomiting, with weakness and myalgias. Other two patients (#15, #16) were managed by their primary care physicians. All five of them remained at home in isolation.
FIGURE 2.
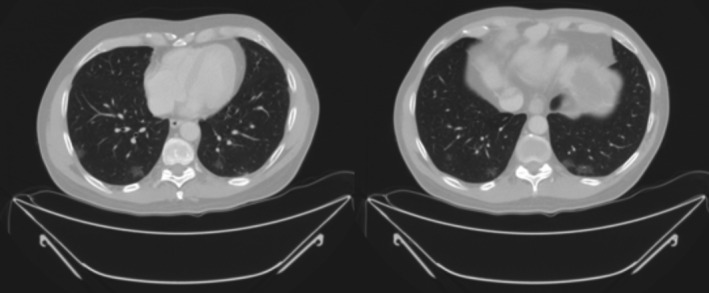
Chest CT scan from patient 7. Multiple ground glass infiltrates, predominantly peripheral, in both lungs, highly suspicious of COVID‐19
Hydroxychloroquine was used in 9 of the admitted patients. In 2 with LPV/r, and in one of these with IFN‐β, another patient received HC plus IFN‐β, without LPV/r. Two more patients were treated with tocilizumab, one in addition to HC. Both had bilateral pneumonia and high PCR, LDH, ferritin, and D‐dimer levels (data not shown). None of them showed infectious complications, being discharged after 2 weeks of admission. Only two patients received high doses of steroids (250 methylprednisolone for 3 days, and 60 mg/12 h, then tapered), one of them treated with tocilizumab. Both patients were discharged in less than 2 weeks.
Patient #4 arrived to a nearby district hospital in a very severe condition, after more than 2 weeks of symptoms and two previous visits to the ED where he was diagnosed of possible viral pneumonia. He began to show symptoms while he was vacationing at the seaside in other region, before the health personnel were fully aware of the pandemics in our country. He was admitted to the ICU, and rapidly deteriorated, without receiving any specific antiviral treatment.
As only five patients were receiving their graft in the previous 2 years, most of our patients were receiving a mild immunosuppressive regimen (drug levels nor shown). Fifteen patients were on IS monotherapy, four of them with mTOR inhibitors and seven with MMF/MPA. IS was halted in patient #4 when admitted in the ICU. Two patients (# 1,2) treated with LPV/r were under everolimus before admission. They were shifted to prolonged‐release tacrolimus, in very low dose (0.5 mg/wk) due to the CYP3A4 inhibition by ritonavir. 14 No other patient required changes in the IS treatment. Excluding the two patients that died, only four patients had mild to moderate liver enzymes deterioration during admission. Patient #3 had an increase of cholestatic enzymes. She had been treated with IF‐β for 6 days. As she progressively improved, a liver biopsy was not performed. Patients #8, 12, and 17 had transient transaminases elevation.
Two patients (10.5%) died before the end of 3 weeks of symptom onset, both due to respiratory failure, though the addition of pulmonary embolism was suspected in patient #4. As of May 7, 15 patients are at home in good condition. Discharged patients stayed from 5 to 27 days, with a mean of 11.4 days. The liver‐kidney transplant recipient had the longest admission. He has an ileostomy due to post‐transplant complications, and he began with olfactory and taste disorders and diarrhea, without respiratory symptoms and had a normal chest x‐ray. He stayed without oxygen support, low CRP levels (1.6 mg/dL), with slow improvement of diarrhea and hyponatremia.
Only two patients (#13, #18) remained at the hospital at the time of this writing. Patient #13 is the one with hospital‐acquired infection and is recovering from a severe cholangitis and without showing any symptoms of COVID‐19 and with a SARS‐CoV‐2 PCR negative. He is waiting to be transferred to a nearby chronic care hospital. Patient # 18 had been recently admitted, and he is now needing nasal cannula oxygen support, correctly evolving, with good prognosis.
None of the patients was readmitted excepting the one previously admitted for diarrhea. There is no case of recurrence.
All living patients are with the same immunosuppression treatment that had before the infection, with good liver function.
4. DISCUSSION
Little is known yet about COVID‐19 infection in LT recipients. Transplant patients are considered at risk for any kind of infection, and this risk is a function of two factors: epidemiological exposures and the net state of IS. 15 The exposure to SARS‐CoV‐2 has been very high in our area during the recent weeks, with more than 61 500 cases confirmed by PCR in the region of Madrid, 16 out of a population of 6.6 million (0.95%). 17 Thus, the rate of infection in our liver recipients is quite similar to the general population, 19 out of 1200 living patients. In our series, factors contributing to the net state of IS are immunosuppresive therapy, that was relatively low in the majority of our patients due to the long median follow‐up since transplantation. On the other hand, comorbidities were present in many of them, mainly diabetes, and advanced age which have been associated with an increasing risk of severe disease in SARS‐CoV‐2 infection. 18
A recent report from China 19 on two patients, one with a kidney transplant (51 years old) and the other with a bone marrow transplant (58 years), is quite discouraging. They were severe cases, with respiratory failure and needing mechanical ventilation, and both died. Maintenance IS (mycophenolate mofetil and steroids, and cyclosporine A) was discontinued, and methylprednisolone and prophylactic antibiotics were initiated, but still both developed nosocomial bacterial infection.
Initial experience with COVID‐19 in liver transplant patients is shown in Table 3. Bin et al 4 described a LT patient that developed a severe COVID‐19 pneumonia and survived. IS (tacrolimus) was temporarily withdrawn and treated with low‐dose steroids. He also received umifenovir, LPV/r, intravenous immunoglobulin, IFN‐α, prophylactic cefoperazone, and nutrition support. The authors suggested that reduction or temporary withdrawal of IS may be beneficial for rehabilitation of immunity.
TABLE 3.
Initial world experience with COVID‐19 in liver transplant recipients
| Author | Reference | Site | Patients (n) | IS | Time after transplantation | Hospitalization | Outcome Alive/dead |
|---|---|---|---|---|---|---|---|
| Qin et al | 3 | China | 1 | TAC + st titrated to lower doses | Perioperative | 1/1 | 1/0 |
| Bin et al | 4 | China | 1 | TAC withdrawn + st | 30 mo | 1/1 | 1/0 |
| Huang et al | 5 | China | 1 | TAC + MMF dose halved | 32 mo | 1/1 | 0/1 |
| Bhoori et al | 6 | Lombardy, Italy | 6 | Minimal (long‐term patients) |
3 > 2 y 3 < 2 y |
3/6 | 3/3 |
| Fernández et al | 7 | Madrid, Spain | 6 | Low, 4/6 monotherapy | >5 y | 5/6 | 4/2 |
| Webb et al | 8 | International registries | 39 | Diverse | 23 | NR | 30/9 |
| Agnes et al | 9 | North‐Central Italy | 24 | NR | 5 (21%) LT in 2020 | 17/24 | 19/5 |
| Kates et al | 10 | USA | 1 | CsA, maintained | 19 y | 1/1 | 1/0 |
| Hammami et al | 11 | USA | 1 | TAC, maintained | 10 y | 1/1 | 1/0 |
| Lagana et al | 12 | USA | 1 |
TAC, MMF, st (st taper, MMF d/c) |
Postoperative | 1/1 | 1/0 |
Abbreviations: CsA, cyclosporine; d/c, discontinuation; IS, immunosuppression; LT, liver transplant; MMF, mycophenolate mofetil; NR, not reported; St, steroids.
Another recent case from China 5 showed a rapid progression of respiratory insufficiency, several nosocomial infections, and multi‐organ failure, dying 1 month and a half after admission. A 37‐year‐old LT recipient had a perioperative SARS‐CoV‐2 infection, 3 starting with persistent fever on transplant postoperative day 9. He was treated with oseltamivir, recombinant human granulocyte colony‐stimulating factor (rh‐GCSF), and intravenous immunoglobulin. Tacrolimus and steroids were maintained though titrated to lower doses, and he received oxygen through high‐flow nasal cannula, maintaining a saturation between 95% and 99%. He was discharged 51 days after transplantation.
There were 3 deaths in long‐term (>10 years) LT recipients at the Istituto Nazionale Tumori in Milano. 6 They were receiving low‐dose IS, had community‐acquired pneumonia, needed oxygen at admission and rapidly developed severe respiratory distress syndrome requiring mechanical ventilation. All died between 3 and 12 days after the onset of pneumonia and tested positive for SARS‐CoV‐2 by PCR. They were male, older than 65 years, overweight, diabetic, and had hypertension. However, three other patients with a follow‐up of less than 2 years since LT that tested SARS‐CoV‐2 positive had an uneventful disease. In contrast, data on 39 cases collected in two registries 8 did not show significant differences in comorbidity among those patients that died, nine, and those who survived. A recent survey from Italy collected 24 cases in the Northern‐Central regions. 9 Seven did not require hospitalization, and mortality affected 21% of the patients. There are 3 recent cases reported from the United States, 10 , 11 , 12 with good outcome.
Lagana et al 12 describe the pathology of a postoperative liver biopsy from an infant whose donor tested positive for COVID‐19, what could be the first case of SARS‐CoV‐2 transmission by a transplant. The biopsy showed moderate acute hepatitis with prominent clusters of apoptotic hepatocytes and associated cellular debris. Lobular lymphohistiocytic inflammation and typical portal features of mild to moderate acute cellular rejection were noted.
All but one of our cases have been community‐acquired. The patient with a possible hospital‐acquired SARS‐CoV‐2 did not show virus‐related symptoms so far.
Apart from the two patients that did not survive, other four showed liver profile deterioration. There was not any case of biopsy proven acute rejection as they spontaneously improved (one after IF‐β withdrawal). The balance between IS and infection progression is very difficult to establish in these patients especially when we do not know much about the pathophysiology of the disease.
The efficacy of the current antivirals used in the COVID‐19 therapy is not clear, but seems low in any case. A recent publication of a trial with LPV/r failed to demonstrate a clear benefit in patients with severe COVID‐19. 20 Such results, the occurrence of drug shortages at our center and the risk of interactions with IS agent, lead us to reconsider the role of LPV/r for treating LT recipients. Considering that the first report of pathological characteristics of the patient who died from severe infection with SARS‐CoV‐2 showed a very high concentration of proinflammatory cytokines, 21 the use of the anti‐IL‐6‐receptor tocilizumab in moderate to severe cases with markers (CRP, and/or IL6 levels) of intense inflammatory response has been promoted in our institution and elsewhere. 11 , 22 , 23 For this reason, in our institution, those patients with elevated acute phase reactants and moderate/severe disease are treated with tocilizumab.
It is interesting to note that two of our patients had thrombotic events that could be related to the infection. Recent literature supports the fact that SARS‐CoV‐2 infection could predispose to thrombotic disease. 24 , 25 This is the reason why prophylactic antithrombotic therapy is now widely used in these patients.
In our experience with COVID‐19 in LT recipients, we have seen a broad spectrum of severity. Two patients died of respiratory failure at the beginning of our experience. Both were male in the 8th decade of life, diabetic and had hypertension. Out of the remaining 17 patients, 5 could be managed as outpatient with close communication with the hospital staff, and the highest oxygen concentration administered was 40% in 2 of the admitted patients that survived.
Regarding immunosuppressive drug changes, we stopped mTOR inhibitors in two of our first patients on LPV/r and shifted to tacrolimus in low dose, and MMF was halted in patient #4 shortly before his fatal outcome. All the other patients maintained their IS treatment, and only 2 of them had a transient deterioration of their liver profile that is difficult to know if it is due to drug toxicity or the disease itself, as the IS drug levels were closely monitored (data not shown). The majority of our patients were transplanted long time before the infection, allowing a mild IS. But, based on our experience with the first cases, we maintained the IS even in those 5 patients with a transplant time of less than 2 years, with a good outcome.
Our preliminary experience does not permit to know which is the best treatment for LT patients with COVID‐19 disease.
Our experience with LT recipients and SARS‐CoV‐2 infection shows a varied picture in terms of clinical presentation and severity. Though 7 of our patients were older than seventy, there were only 2 deaths. IS dose adjustment does not seem needed in long‐term survivors that usually have low maintenance drug levels.
AUTHOR CONTRIBUTIONS
All authors contributed to the concept, data collection, analysis, drafting, and final approval of the manuscript.
ACKNOWLEDGEMENTS
MFR holds a research contract "Miguel Servet" (CP 18/00073) from the Spanish Ministry of Science and Innovation, Instituto de Salud Carlos III.
Loinaz C, Marcacuzco A, Fernández‐Ruiz M, et al. Varied clinical presentation and outcome of SARS‐CoV‐2 infection in liver transplant recipients: Initial experience at a single center in Madrid, Spain. Transpl Infect Dis. 2020;22:e13372. 10.1111/tid.13372
REFERENCES
- 1. Gori A, Dondossola D, Barbara A, et al. Coronavirus disease 2019 and transplantation: a view from the inside [published online ahead of print, 2020 Mar 17]. Am J Transplant. 2020:e15853. 10.1111/ajt.15853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Halazun KJ, Rosenblatt R. Lest we forget [published online ahead of print, 2020 Mar 31]. Am J Transplant. 2020:e15888. 10.1111/ajt.15888 [DOI] [Google Scholar]
- 3. Qin J, Wang H, Qin X, et al. Perioperative presentation of COVID‐19 disease in a liver transplant recipient [published online ahead of print, 2020 Mar 27]. Hepatology. 2020:e31257. 10.1002/hep.31257 [DOI] [PubMed] [Google Scholar]
- 4. Liu B, Wang Y, Zhao Y, Shi H, Zheng F, Chen Z. Successful treatment of severe COVID‐19 pneumonia in a liver transplant recipient [published online ahead of print, 2020 Apr 3]. Am J Transplant. 2020:e15901. 10.1111/ajt.15901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huang J‐F, Zheng KI, George J, et al. Fatal outcome in a liver transplant recipient with COVID‐19 [published online ahead of print, 2020 Apr 10]. Am J Transplant. 2020:e15909. 10.1111/ajt.15909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bhoori S, Rossi RE, Citterio D, Mazzaferro V. COVID‐19 in long‐term liver transplant patients: preliminary experience from an Italian transplant centre in Lombardy. Lancet Gastroenterol Hepatol. 2020;5(6):532‐533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fernández‐Ruiz M, Andrés A, Loinaz C, et al. COVID‐19 in solid organ transplant recipients: a single‐center case series from [published online ahead of print, 2020 Apr 16]. Spain. Am J Transplant. 2020:e15929. 10.1111/ajt.15929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Webb GJ, Moon AM, Barnes BAS, Marjot T. Determining risk factors for mortality in liver transplant patients with COVID‐19. Lancet Gastroenterol Hepatol. 2020;5(7):643‐644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Agnes S, Andorno E, Avolio AW, et al. Preliminary analysis of the impact of COVID‐19 outbreak on Italian liver transplant programs [published online ahead of print, 2020 May 6]. Liver Transpl. 2020:e25790. 10.1002/lt.25790 [DOI] [Google Scholar]
- 10. Kates OS, Fisher CE, Stankiewicz‐Karita HC, et al. (COVID‐19) identified in solid organ transplant recipients in the United States [published online ahead of print, 2020 Apr 24]. Am J Transplant. 2020:e15944. 10.1111/ajt.15944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hammami HB, Garibaldi B, Shah P, et al. Clinical course of COVID‐19 in liver transplant recipient on hemodialysis and response to Tocilizumab therapy: a case report [published online ahead of print, 2020 May 2]. Am J Transplant. 2020:e15985. 10.1111/ajt.15985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lagana SM, De Michele S, Lee MJ, et al. COVID‐19 associated hepatitis complicating recent living donor liver transplantation [published online ahead of print, 2020 Apr 17]. Arch Pathol Lab Med. 2020. 10.5858/arpa.2020-0186-SA [DOI] [PubMed] [Google Scholar]
- 13. Ai T, Yang Z, Hou HH, et al. Correlation of chest CT and RT‐PCR testing in coronavirus disease 2019 (COVID‐19) in China: a report of 1014 cases [published online ahead of print, 2020 Feb 26]. Radiology. 2020;e200642. 10.1148/radiol.2020200642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li F, Lu J, Ma X. CYP3A4‐mediated lopinavir bioactivation and its inhibition by ritonavir. Drug Metab Dispos. 2012;40:18‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fishman JA. Infection in organ transplantation. Am J Transplant. 2017;17(4):856‐879. [DOI] [PubMed] [Google Scholar]
- 16. Instituto de Salud Carlos III , Centro Nacional de Epidemiología . Informesobre la situación de COVID‐19 en España nº 28, 4 de mayo de 2020. Available at https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Documents/INFORMES/Informes%20COVID‐19/Informe%20n%C2%BA%2028.%20Situaci%C3%B3n%20de%20COVID‐19%20en%20Espa%C3%B1a%20a%2004%20de%20mayo%20de%202020.pdf. Accessed May 6, 2020.
- 17. Instituto Nacional de Estadística . https://www.ine.es/jaxiT3/Datos.htm?t=2881#!tabs‐tabla. Accessed April 15, 2020.
- 18. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huang J, Lin H, Wu Y, et al. COVID‐19 in post‐transplantation patients‐ report of two cases [published online ahead of print, 2020 Apr 3]. Am J Transplant. 2020:e15896. 10.1111/ajt.15896 [DOI] [Google Scholar]
- 20. Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe COVID‐19. N Engl J Med. 2020;382(19):1787‐1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang X, Song K, Tong F, et al. First case of COVID‐19 in a patient with multiple myeloma successfully treated with tocilizumab. Blood Adv. 2020;4:1307‐1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID‐ 19: a single center experience. J Med Virol. 2020;92(7):814‐818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang Y, Cao W, Xiao M, et al. Clinical and coagulation characteristics of 7 patients with critical COVID‐2019 penumonia and acro‐ischemia. Zhongua Xue Ye Xue Za Zhi. 2020;41:E006. [DOI] [PubMed] [Google Scholar]
- 25. Bikdeli B, Madhavan MV, Jimenez D. COVID‐19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow‐up. J Am Coll Cardiol. 2020;75(23):2950‐2973. [DOI] [PMC free article] [PubMed] [Google Scholar]


