Abstract
The clinical course and outcomes of immunocompromised patients, such as transplant recipients, with COVID‐19 remain unclear. It has been postulated that a substantial portion of the disease burden seems to be mediated by the host immune activation to the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Herein, we present a simultaneous heart‐kidney transplant (SHKT) recipient who was hospitalized for the management of respiratory failure from volume overload complicated by failure to thrive, multiple opportunistic infections, and open non‐healing wounds in the setting of worsening renal dysfunction weeks prior to the first case of SARS‐CoV‐2 being detected in the state of Connecticut. After his third endotracheal intubation, routine nucleic acid testing (NAT) for SARS‐CoV‐2, in anticipation of a planned tracheostomy, was positive. His hemodynamics, respiratory status, and ventilator requirements remained stable without any worsening for 4 weeks until he had a negative NAT test. It is possible that the immunocompromised status of our patient may have prevented significant immune activation leading up to clinically significant cytokine storm that could have resulted in acute respiratory distress syndrome and multisystem organ failure.
Keywords: COVID‐19, immunosuppression, SARS‐CoV‐2, simultaneous heart‐kidney transplant
Abbreviations
- AKI
acute kidney injury
- ARDS
acute respiratory distress syndrome
- ATN
acute tubular necrosis
- CIT
cold ischemia time
- CNI
calcineurin inhibitor
- DGF
delayed graft function
- MMF
mycophenolate mofetil
- MRSA
methicillin‐resistant Staphylococcus aureus
- NAT
nucleic acid testing
- PNT
percutaneous nephrostomy tube
- POD
postoperative day
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- SHKT
simultaneous heart‐kidney transplant
- VRE
vancomycin‐resistant enterococcus
1. INTRODUCTION
The ongoing COVID‐19 pandemic resulting from the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is estimated to have infected over 4.8 million individuals and caused nearly 320 000 deaths in 188 countries as of May 20, 2020. 1 Although not well understood, it has been postulated that a substantial portion of the disease burden is mediated by an exaggerated immune activation in response to the virus. In some of the earliest studies out of China, Th17 helper T cells, stimulated by IL‐6 and IL‐23, were found to be a major player in the development of a diffuse alveolar injury, leading to acute respiratory distress syndrome (ARDS) and eventually multiorgan system failure. 2 In addition, circumstantial evidence has identified IL‐6 and IL‐17 as central components of the cytokine storm and putative therapeutic targets. 2 , 3
Transplant recipients with a baseline immunocompromised state present a unique cohort of individuals that may provide opportunity for delineating host‐virus interaction and the role of the immune response. To date, several single‐center series of solid organ recipients from Italy, Spain, and New York City have been reported. 4 , 5 , 6 , 7 The clinical phenotype of these cases has ranged from a mild flu‐like illness to severe ARDS and death, with a spectrum of immunosuppression modifications, including complete withdrawal to no alterations.
Herein, we present a simultaneous heart‐kidney transplant (SHKT) recipient who was hospitalized for ongoing respiratory failure due to volume overload, renal dysfunction, opportunistic infections and malnutrition weeks prior to the first case of SARS‐CoV‐2 reported in Connecticut and who incidentally had a positive nucleic acid test (NAT) for SARS‐CoV‐2. Importantly, this immunocompromised individual did not experience further clinical deterioration for 4 weeks until his NAT turned negative, raising the possibility of the host immune response as mediator of morbidity and mortality in COVID‐19 cases.
2. CASE REPORT
A 56‐year‐old African‐American man received a SHKT for underlying dilated cardiomyopathy from adriamycin treatment and chronic renal failure in January 2020 in our institution. He received his organs from a 52‐year‐old brain‐dead donor with a KDPI of 59% who died from an intracranial hemorrhage. The patient received immunosuppression as shown in Figure 1.
FIGURE 1.
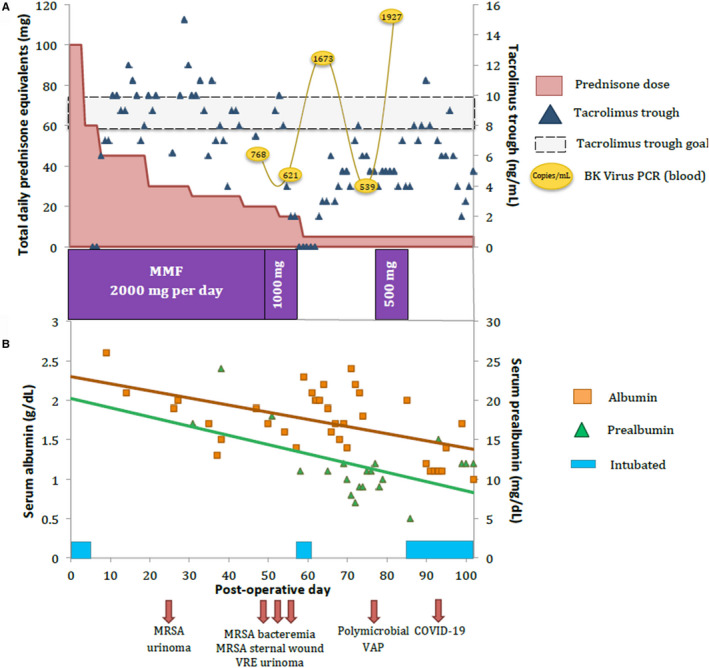
A, Dosage of prednisone equivalents (salmon), tacrolimus levels (blue diamond), and MMF (purple bars) dosage during the first 100 d after SHKT. BK viremia (yellow ovals) was detected on POD47 and has steadily increased. B, Albumin (orange squares) and pre‐albumin (green triangles) levels in the first 100 d after SHKT with the incidence of each infection detected, including COVID‐19 on POD88, and the periods requiring mechanical intubation (blue bars). MMF, mycophenolate mofetil; POD, postoperative day; SHKT, simultaneous heart‐kidney transplant
His immediate postoperative course was complicated by delayed graft function (DGF) of his kidney, and he received dialysis on postoperative day (POD) 3 for progressive hyperkalemia in the setting of oliguria. His sternal and kidney wounds became infected with methicillin‐resistant Staphylococcus aureus (MRSA) and Corynebacterium species for which he was treated with appropriate antibiotic coverage. He was discharged on POD 22, off hemodialysis with a creatinine of 2.4 mg/dL. His ureteral stent was removed in a routine office visit the following week.
He presented a couple days later with lower abdominal pain and found to have an elevated creatinine of 3.8 mg/dL from a urinoma. Following drainage, a percutaneous nephrostomy tube (PNT) was placed and a transplant kidney biopsy was performed for a persistently elevated creatinine, which showed acute tubular necrosis (ATN) without rejection. A week later (POD 50), he was admitted for a fever of 103.5°F and a leukocytosis of 14 600 cells/μL. A CT of the chest, abdomen, and pelvis revealed a left pleural effusion without any focal consolidation. The perinephric collection grew out vancomycin‐resistant Enterococcus (VRE) and blood cultures revealed MRSA bacteremia. In addition, he had developed a low‐grade BK Polyoma virus DNAemia (768 copies/mL). 8 Interestingly, his absolute lymphocyte count was measured to be 319 cells/μL (range: 850‐3900 cells/μL), which was higher than 3 weeks prior (0 cells/μL). He was initiated on antibiotic coverage and his immunosuppressive regimen was decreased by lowering his prednisone dose to 5 mg daily, his tacrolimus trough goal to 5‐8 μg/L, and discontinuation of MMF (Figure 1).
During this time, worsening fluid overload and diuretic resistance prompted initiation of continuous venovenous hemodialysis. By POD 56, he required emergent endotracheal intubation for a witnessed aspiration event. He was successfully weaned from ventilatory support on POD 60, which was the same day Connecticut detected its first COVID‐19 case, approximately 68 miles south of our institution. Subsequently, work up for a worsening productive cough revealed Klebsiella pneumonia, Morganella morganii, and Stenotrophomonas maltophilia in his sputum; he was started on treatment for a presumed left lower lobe ventilator‐associated pneumonia. His low‐grade BK Polyoma virus DNAemia worsened (1927 copies/mL; Figure 1). He suffered ongoing respiratory distress in the setting of a weak respiratory drive and failure to thrive from worsening malnutrition (albumin 1 g/dL; pre‐albumin, 15 mg/dL; Figure 1), resulting in reintubation the following week. Interestingly, his initial ventilator settings were weaned down rapidly to minimal support (pressure support of 5 mm Hg, positive end‐expiratory pressure of 5 mm Hg, and an FiO2 of 40%). Given his weak respiratory drive, recurrent aspirations, persistent cough, and malnutrition, an elective tracheostomy was planned to assist with rehabilitation. Due to rapidly rising COVID‐19 cases in Connecticut and to minimize transmission, we began screening patients scheduled for surgical intervention. Accordingly, a routine pre‐tracheostomy SARS‐CoV‐2 NAT test was performed and was positive. He remained afebrile, on minimal ventilator settings, and with an improving chest X‐ray (Figure 2). He was started on hydroxychloroquine 200 mg twice a day along with azithromycin 250 mg daily for 3 days per our guidelines. Inflammatory markers procalcitonin (17.2 ng/mL immediately after NAT test), ferritin (442 μg/L), lactate dehydrogenase (LDH; 296 U/L), and C‐reactive protein (CRP; 13.9 mg/dL) were stable in the first 2 weeks after his COVID‐19 diagnosis (procalcitonin 1.0 ng/mL; ferritin 412 μg/L; LDH 206 U/L; CRP 8.9 mg/dL). The tracheostomy was performed 18 days after testing positive. After weekly SARS‐CoV‐2 NAT testing, the virus was undetectable at Day 26.
FIGURE 2.
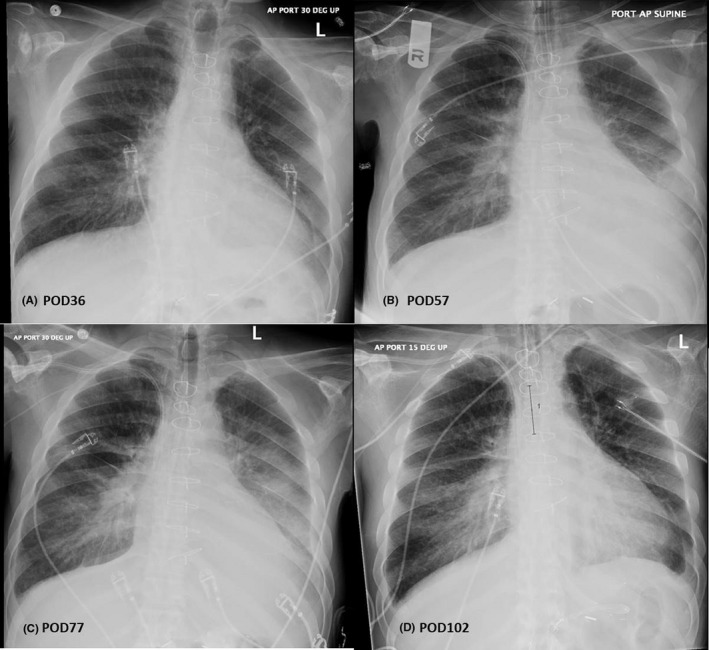
A, Chest X‐ray on POD36 after patient was readmitted for the first time for a fever work‐up demonstrating some mild central vascular prominence, a left pleural effusion, but no consolidation; B, POD57, after a witnessed aspiration even demonstrating interval progression of interstitial pulmonary edema with increasing left small pleural effusion. C, POD77, after which he was started on ventilator‐associated pneumonia treatment with a left lower lobe consolidation and a sputum specimen grew out Klebsiella pneumonia, Morganella morganii, and Stenotrophomonas maltophilia. D, POD102, 13 d after testing positive for COVID‐19, demonstrating pulmonary vascular congestion, a small bilateral pleural effusions and stable bibasilar opacities. POD, postoperative day
3. DISCUSSION
Simultaneous heart‐kidney transplant recipients are known to have lower rates of acute rejection and cardiac allograft vasculopathy compared to solitary heart transplant recipients, presumably because of increased immunosuppression and heightened surveillance. 9 Expectedly, increased immunosuppression raises the risk of infection and associated complications. As presented, the clinical course of our patient demonstrated recurrent infections and debility, which were significantly impacted by his immunosuppression and malnutrition. While COVID‐19 could unleash a cytokine storm and clinical devastation in an immunocompetent individual, in this index case, an immunocompromised state may have been paradoxically protective, explaining a paucisymptomatic clinical course.
This report highlights interesting observations. First, an intact immune system appears to be necessary for clinical deterioration from COVID‐19, and by extension, immunocompromised patients might remain asymptomatic despite viral infection. In one of the largest studies to date on the immune dysregulation in COVID‐19 patients, Qin et al 10 report on 452 patients with laboratory‐confirmed COVID‐19 from Wuhan, China. Their analysis revealed an increase of the neutrophil:lymphocyte ratio and a T‐cell lymphopenia—especially a decrease of CD4+ T cells—among patients with the most severe manifestations of COVID‐19. Based on these data, it is theorized that SARS‐CoV‐2 damages lymphocytes, especially regulatory T lymphocytes, leading to a loss of crucial inhibitory signals involved with the maintenance of self‐tolerance and immune homeostasis. Furthermore, COVID‐19 human postmortem blood sample analysis identifies high concentrations of the proinflammatory CCR6+ Th17 in CD4+ T cells, implicating Th17 responses with vascular permeability and leakage in the pulmonary vasculature. 2
Thus, it is conceivable that in transplant recipients whose immunosuppression regimen typically includes a calcineurin inhibitor (CNI) and an antimetabolite, the immune response to SARS‐CoV‐2 may be somewhat impaired. More specifically, with their inhibitory effect on IL‐2 production, CNIs diminish the proliferative response of T cells; and MMF has been shown to have a potent direct inhibitory effect on IL‐17, a known stimulus of the Th17 response. 11
Interestingly, a case of an SHKT from Los Angeles was recently published with some similarities. 12 The patient presented with leukopenia, which prompted the withdrawal of MMF. Although the patient was admitted for oxygen supplementation, he never required intubation, prompting the authors to suggest that his immunosuppressed state led to a mild form of COVID‐19. 12 To our knowledge, ours is only the 5th SHKT reported in the literature. Pereira et al 5 reported three SHKT, two of whom had mild COVID‐19 manifestations and none of whom died.
Second, while malnutrition portends a poor prognosis with COVID‐19 in immunocompetent individuals, 13 the clinical manifestations to COVID‐19 in malnourished and immunosuppressed individuals are unknown. Our patient developed multiple sequelae of severe protein‐calorie malnutrition, namely multiple non‐healing surgical wounds, a significant sacral decubitus ulcer, a ureteral anastomotic leak, respiratory failure, and failure to thrive. Certainly, malnutrition, micronutrient abnormalities, and concomitant infections are thought to contribute to a state of anergy in the differentiation of the T helper cell response to viral stimuli, 14 which could conceivably lead to a more favorable outcome in states of unregulated inhibitory signals mediated by T helper cells.
In summary, this report highlights the first case of an asymptomatic incidental COVID‐19 in an unlikely individual—a dual organ transplant recipient who was immunosuppressed and malnourished with recurrent infections and non‐healing wounds. Although we acknowledge that this is a single case report, it provides a clinical observation on the need of an intact immune system for the clinical manifestations of COVID‐19. Further research to test this hypothesis is necessary.
CONFLICT OF INTEREST
The authors of this manuscript have no conflicts of interest to disclose as described by Transplant Infectious Disease.
AUTHOR CONTRIBUTIONS
Serrano involved in study conception and design. Serrano and Kutzler involved in acquisition of data. Serrano, Kutzler, and Jaiswal involved in analysis and interpretation of data. Serrano, Kutzler, and Jaiswal involved in drafting of manuscript. Serrano, Kutzler, Rochon, Radojevic, Lawlor, Hammond, Gluck, Feingold, and Jaiswal involved in critical revision.
ACKNOWLEDGEMENTS
The authors would like to acknowledge all of the healthcare workers of Hartford HealthCare for their selfless dedication, tireless work, and unparalleled compassion in caring for the patients of Connecticut during the COVID‐19 pandemic.
Serrano OK, Kutzler HL, Rochon C, et al. Incidental COVID‐19 in a heart‐kidney transplant recipient with malnutrition and recurrent infections: Implications for the SARS‐CoV‐2 immune response. Transpl Infect Dis. 2020;22:e13367. 10.1111/tid.13367
REFERENCES
- 1. Dong E, Du H, Gardner L. An interactive web‐based dashboard to track COVID‐19 in real time. Lancet Infect Dis. 2020;20(5):533‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang C, Wu Z, Li JW, et al. The cytokine release syndrome (CRS) of severe COVID‐19 and Interleukin‐6 receptor (IL‐6R) antagonist Tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020;29:105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Akalin E, Azzi Y, Bartash R, et al. Covid‐19 and kidney transplantation. N Engl J Med. 2020;382(25):2475–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pereira MR, Mohan S, Cohen DJ, et al. COVID‐19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant. 2020;1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alberici F, Delbarba E, Manenti C, et al. A single center observational study of the clinical characteristics and short‐term outcome of 20 kidney transplant patients admitted for SARS‐CoV2 pneumonia. Kidney Int. 2020;97(6):1083‐1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fernández‐Ruiz M, Andrés A, Loinaz C, et al. COVID‐19 in solid organ transplant recipients: a single‐center case series from Spain. Am J Transplant. 2020. 10.1111/ajt.15929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hirsch HH, Randhawa PS, AST Infectious Diseases Community of Practice . BK polyomavirus in solid organ transplantation‐guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33(9):e13528. [DOI] [PubMed] [Google Scholar]
- 9. Raichlin E, Kushwaha SS, Daly RC, et al. Combined heart and kidney transplantation provides an excellent survival and decreases risk of cardiac cellular rejection and coronary allograft vasculopathy. Transplant Proc. 2011;43(5):1871‐1876. [DOI] [PubMed] [Google Scholar]
- 10. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID‐19 in Wuhan, China. Clin Infect Dis. 2020;ciaa248. 10.1093/cid/ciaa248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abadja F, Atemkeng S, Alamartine E, et al. Impact of mycophenolic acid and tacrolimus on Th17‐related immune response. Transplantation. 2011;92(4):396‐403. [DOI] [PubMed] [Google Scholar]
- 12. Hsu JJ, Gaynor P, Kamath M, et al. COVID‐19 in a high‐risk dual heart and kidney transplant recipient. Am J Transplant. 2020. 10.1111/ajt.15936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li X, Wang L, Yan S, et al. Clinical characteristics of 25 death cases with COVID‐ 19: a retrospective review of medical records in a single medical center, Wuhan, China. Int J Infect Dis. 2020;9712(20):128‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu CH, Huang TC, Lin BF. Folate deficiency affects dendritic cell function and subsequent T helper cell differentiation. J Nutr Biochem. 2017;41:65‐72. [DOI] [PubMed] [Google Scholar]


