Coronavirus disease‐2019 (COVID‐19), caused by severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2), is known for being a life‐threatening respiratory illnesses, but there is increasing evidence of neurological manifestations. 1 Herein we report a patient with COVID‐19–associated inflammatory myopathy who presented with facial, bulbar, and proximal limb weakness.
A 58‐year‐old woman presented with cough, dyspnea, and myalgia. Vital signs were stable and her physical examination was unremarkable. Initial polymerase chain reaction (PCR) testing for SARS‐CoV‐2 was negative and the patient was discharged home. She returned 3 weeks later with more severe dyspnea, cough, dysarthria, dysphagia, odynophagia, and severe generalized weakness with inability to ambulate. She had no sensory symptoms or bowel or bladder dysfunction.
Physical examination was significant for tachycardia at 110 beats/min and oxygen saturation of 88% on room air. She had bilateral ptosis, facial weakness, hypernasal dysarthria, and profound symmetric proximal limb weakness. Reflexes were symmetrically diminished. Repeat SARS‐CoV‐2 PCR test was positive.
MRI of the entire neuroaxis showed no central or peripheral nervous system involvement, but it did demonstrate diffuse muscle edema and enhancement, with a region of myonecrosis (Figure 1A,B).
FIGURE 1.
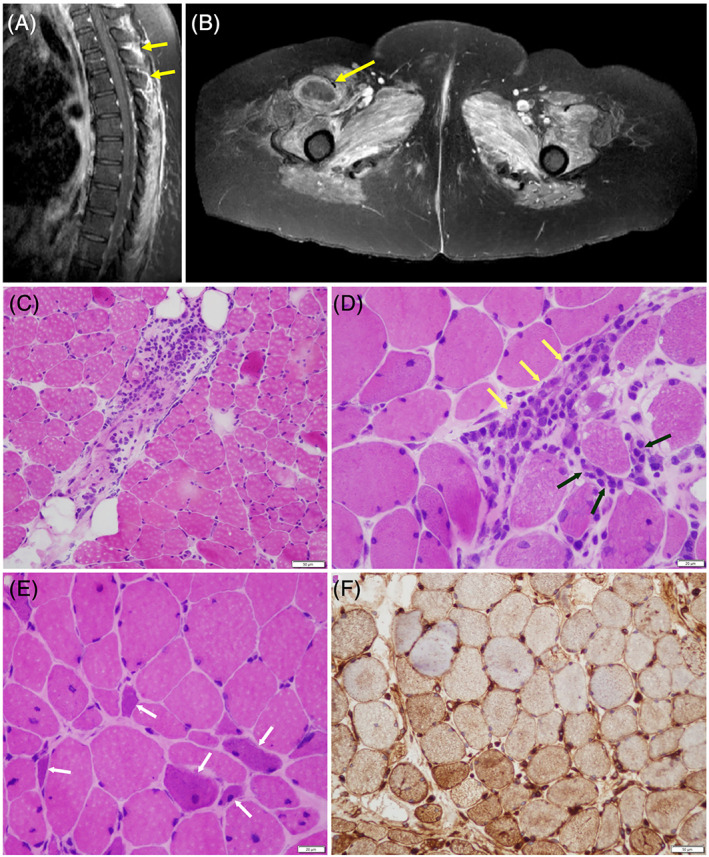
A and B, Sagittal T1WIs of contrast‐enhanced fat‐suppressed MRI of the paraspinal and thigh muscles demonstrate extensive edema and enhancement (yellow arrows in A) consistent with inflammatory myopathy; central nonenhancement in the vastus medialis (yellow arrow in B) is consistent with myonecrosis. C‐F, Biopsy of the left quadriceps muscle. Hematoxylin‐and‐eosin cryostat sections demonstrate multifocal, predominantly perimysial perivascular lymphocytic inflammation (C, and yellow arrows in D), with focal endomysial extension (black arrows in D). Multiple regenerating myofibers (white arrows in E) are recognized by their mild sarcoplasmic basophilia and enlargement of visible nuclei. There is upregulation of HLA class ABC on myofiber surfaces, and sarcoplasmic staining by immunohistochemistry can be identified by the brown staining of myofibers, most consistent with an inflammatory myopathy (F). Scale bar = 50 μm. HLA, human leukocyte antigen; T1WI, T1‐weighted image
Motor nerve conduction studies of the peroneal, tibial, ulnar, and median nerves were unremarkable, as were sensory studies of the ulnar and median nerves; no responses were elicited from the superficial peroneal and sural nerves. Three‐hertz repetitive stimulation of the ulnar nerve before and after 1‐minute exercise showed no change in compound muscle action potential amplitude recorded from the abductor digiti minimi muscle during a series of 10 stimuli. Needle electromyography of the right vastus lateralis, iliopsoas, and deltoid muscles revealed sparse fibrillation potentials; no motor units could be activated.
On admission, creatine kinase (CK) was elevated to 700 U/L. Anti–Sjögren‐syndrome–related antigen (anti‐SSA) and anti–small ubiquitinlike modifier‐1–activating enzyme (anti–SAE 1) antibodies were both strongly positive and Ku antibody was weakly positive. Acetylcholine receptor (AChR) binding, modulating, and muscle‐specific kinase (MuSK) antibodies were all negative (Table 1).
TABLE 1.
Laboratory results
| Complete blood count | Normal |
| Comprehensive metabolic panel | Normal |
| Coagulation panel | Normal |
| Troponin I (ng/mL) | 0.128‐0.045 (≤0.012) |
| Interleukin‐6 | 153 (0‐15.5) |
| D‐dimer (ng/mL) | 1532 (0‐243) |
| Serum ferritin (ng/mL) | 1353 (13‐150) |
| High‐sensitivity C‐reactive protein (mg/dL) | 110 (1‐4) |
| Erythrocyte sedimentation rate (mm/h) | 94 (0‐30) |
| Urine myoglobin (μg/L) | 705 (≤21) |
| Thyroid‐stimulating hormone (mIU/mL) | 2.12 (0.27‐4.2) |
| Aldolase | Negative |
| Myomarker panel a | Positive for Ku, anti–SAE 1 IgG, and anti–SS‐A |
| Gangliosides GQ1b IgG antibody | Negative |
| Antinuclear antibody titer | 1:1280 (<1/80) |
| Lupus anticoagulant | Positive |
| Chromatin antibody | Positive |
| β2‐glycoprotein (IgG, IgA, IgM) | Negative |
| Anti–double‐stranded DNA antibody (IU/mL) | Negative |
| Cytoplasmic antineutrophil cytoplasmic antibody | Negative |
| Anti–Smith antibody | Negative |
| Anti–ribonuclear protein | Negative |
| Rheumatic factor | Negative |
| HIV‐1 and ‐2 antigen/antibody | Negative |
| Hepatitis panel | Negative |
Abbreviation: Ig, immunoglobulin.
Anti‐Jo1Ab, PL‐7, PL‐12, EJ, OJ, SRP, MI‐2, fibrillarin, anti–melanoma differentiation–associated gene 5, nuclear matrix protein, transcriptional intermediary factor‐γ, anti–PM Scl‐100, U2 small nuclear ribonucleoprotein, Ku, anti–Sjögren‐syndrome–related antigen A, and anti–small ubiquitinlike modifier‐1–activating enzyme.
Muscle biopsy showed perivascular inflammatory infiltration with endomysial extension, regenerating fibers, and upregulation of human leukocyte antigen class ABC expression on non‐necrotic fibers. Cytochrome oxidase/succinic dehydrogenase enzyme histochemistry was unremarkable. Vacuolar change and curvilinear bodies (on electron microscopy) suggestive of hydroxychloroquine myopathy were absent (Figure 1C‐F).
Our presumptive diagnosis was COVID‐19–associated myositis and a 5‐day course of 1000 mg intravenous methylprednisolone was administered. Elevated interleukin‐6 was found and tocilizumab was given. Anticoagulation was given for left brachial vein thrombosis and a 5‐day course of hydroxychloroquine and azithromycin was also administered. Over 2 weeks, her CK levels normalized and she recovered the ability to raise her arms and legs from the bed. She showed slow improvement in bulbar function and her speech became more intelligible. She did not require mechanical ventilation; however, a percutaneous endoscopic gastrostomy (PEG) tube was inserted to allow for sufficient nutrition.
Viral infection is a well‐known cause of myositis in which influenza A and B are the most common causes in the United States. 2 Myopathy with elevated CK levels has been reported in patients with severe acute respiratory syndrome (SARS). 3 Limited pathological studies suggested vasculitis or immune‐mediated mechanism as the potential cause for myopathy, without evidence of direct viral invasion. 4 , 5
Myalgias have been reported in up to a half of the patients with SARS‐CoV‐2 infection. Serum CK levels elevations depend on the severity of disease, ranging from mild to frank rhabdomyolysis. 1 , 6 , 7 , 8 A recent report described a COVID‐19 patient with myalgia, proximal weakness, elevated CK level, and muscle edema on MRI. 9
Herein we have reported a COVID‐19 patient with associated myositis involving the proximal limb, bulbar, and facial muscles. The severe immune activation known to occur in COVID‐19 patients likely plays a major pathophysiological role. Myositis and myasthenia gravis (MG) may coexist and, although specific antibodies were negative and ulnar repetitive nerve stimulation was normal, the severe bulbar involvement raises the possibility of superimposed MG. This is especially of interest in light of a recent report of patients with MG and COVID‐19, one of whom was successfully extubated after treatment with tocilizumab. 10 Direct viral invasion was not detected by electron microscopy. The finding of multiple serologic autoimmune antibodies is intriguing, suggesting an epiphenomenon rather than activation or unmasking of a specific immune response directed to the muscles. Further studies are needed to elucidate the mechanisms, appropriate treatment, and long‐term clinical outcomes of muscular manifestations associated with COVID‐19.
CONFLICT OF INTEREST
The authors declare no potential conflicts of interest.
ETHICAL PUBLICATION STATEMENT
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
REFERENCES
- 1. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):e201127. 10.1001/jamaneurol.2020.1127. [published online ahead of print, 2020 Apr 10]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Crum‐Cianflone NF. Bacterial, fungal, parasitic, and viral myositis. Clin Microbiol Rev. 2008;21:473‐494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tsai LK, Hsieh ST, Chang YC. Neurological manifestations in severe acute respiratory syndrome. Acta Neurol Taiwan. 2005;14:113‐119. [PubMed] [Google Scholar]
- 4. Ding Y, Wang H, Shen H, et al. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J Pathol. 2003;200:282‐289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Leung TW, Wong KS, Hui AC, et al. Myopathic changes associated with severe acute respiratory syndrome: a postmortem case series. Arch Neurol. 2005;62:1113‐1117. [DOI] [PubMed] [Google Scholar]
- 6. Berger JR. COVID‐19 and the nervous system. J Neurovirol. 2020;26:143‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chan KH, Farouji I, Abu Hanoud A, Slim J. Weakness and elevated creatinine kinase as the initial presentation of coronavirus disease 2019 (COVID‐19). Am J Emerg Med. 2020;S0735‐6757(20):30353‐3. 10.1016/j.ajem.2020.05.015. [published online ahead of print, 2020 May 11]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Suwanwongse K, Shabarek N. Rhabdomyolysis as a presentation of 2019 novel coronavirus disease. Cureus. 2020;12:e7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beydon M, Chevalier K, Al Tabaa O, et al. Myositis as a manifestation of SARS‐CoV‐2. Ann Rheum Dis. 2020. 10.1136/annrheumdis-2020-217573. [published online ahead of print, 2020 Apr 23]. [DOI] [PubMed] [Google Scholar]
- 10. Anand P, Slama MCC, Kaku M, et al. COVID‐19 in patients with myasthenia gravis. Muscle Nerve. 2020. 10.1002/mus.26918. [published online ahead of print, 2020 May 11]. [DOI] [Google Scholar]


