Abstract
Objective
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) emerged as a global pandemic in early 2020 with rapidly evolving approaches to diagnosing the clinical illness called coronavirus disease (COVID‐19). The primary objective of this scoping review is to synthesize current research of the diagnostic accuracy of history, physical examination, routine laboratory tests, real‐time reverse transcription–polymerase chain reaction (rRT‐PCR), immunology tests, and computed tomography (CT) for the emergency department (ED) diagnosis of COVID‐19. Secondary objectives included a synopsis of diagnostic biases likely with current COVID‐19 research as well as corresponding implications of false‐negative and false‐positive results for clinicians and investigators.
Methods
A Preferred Reporting Items for Systematic Reviews and Meta‐Analyses–Scoping Review (PRISMA‐ScR)–adherent synthesis of COVID‐19 diagnostic accuracy through May 5, 2020, was conducted. The search strategy was designed by a medical librarian and included studies indexed by PubMed and Embase since January 2020.
Results
A total of 1,907 citations were screened for relevance. Patients without COVID‐19 are rarely reported, so specificity and likelihood ratios were generally unavailable. Fever is the most common finding, while hyposmia and hypogeusia appear useful to rule in COVID‐19. Cough is not consistently present. Lymphopenia is the mostly commonly reported laboratory abnormality and occurs in over 50% of COVID‐19 patients. rRT‐PCR is currently considered the COVID‐19 criterion standard for most diagnostic studies, but a single test sensitivity ranges from 60% to 78%. Multiple reasons for false‐negatives rRT‐PCR exist, including sample site tested and disease stage during which sample was obtained. CT may increase COVID‐19 sensitivity in conjunction with rRT‐PCR, but guidelines for imaging patients most likely to benefit are emerging. IgM and IgG serology levels are undetectable in the first week of COVID‐19, but sensitivity (range = 82% to 100%) and specificity (range = 87% to 100%) are promising. Whether detectable COVID‐19 antibodies correspond to immunity remains unanswered. Current studies do not adhere to accepted diagnostic accuracy reporting standards and likely report significantly biased results if the same tests were to be applied to general ED populations with suspected COVID‐19.
Conclusions
With the exception of fever and disorders of smell/taste, history and physical examination findings are unhelpful to distinguish COVID‐19 from other infectious conditions that mimic SARS‐CoV‐2 like influenza. Routine laboratory tests are also nondiagnostic, although lymphopenia is a common finding and other abnormalities may predict severe disease. Although rRT‐PCR is the current criterion standard, more inclusive consensus‐based criteria will likely emerge because of the high false‐negative rate of PCR tests. The role of serology and CT in ED assessments remains undefined.
CME Information: Diagnosing COVID‐19 in the Emergency Department: A Scoping Review of Clinical Examinations, Laboratory Tests, Imaging Accuracy, and Biases
CME Editor: Corey Heitz, MD
Authors: Christopher R. Carpenter, MD, MSc , Philip A. Mudd, MD, PhD, Colin P. West, MD, PhD, Erin Wilber, and Scott T. Wilber, MD, MPH
If you wish to receive credit for this activity, please refer to the website: www.wileyhealthlearning.com/aem
Educational Objectives
After reading the article, participants should be able to discuss diagnostic accuracy of clinical findings and testing strategies for COVID‐19.
Activity Disclosures
This activity received no commercial support.
CME Editor Corey Heitz discloses no relevant financial relationships.
This activity underwent peer review in line with standards of editorial integrity and publication ethics. Conflicts of interest have been identified and resolved in accordance with John Wiley and Sons, Inc.'s Policy on Activity Disclosure and Conflict of Interest.
Accreditation
John Wiley and Sons, Inc. is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
John Wiley and Sons, Inc. designates this journal‐based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit ™. Physicians should only claim credit commensurate with the extent of their participation in the activity.
For information on applicability and acceptance of continuing medical education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within 1 hour. To successfully earn credit, participants must complete the activity during the valid credit period, which is up to two years from initial publication. Additionally, up to 3 attempts and a score of 70% or better is needed to pass the post test.
In December 2019 a novel viral respiratory pathogen emerged in China, ultimately named severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) with the clinical illness dubbed coronavirus disease (COVID‐19). COVID‐19 became a global pandemic in early 2020 forcing governments worldwide to enact social isolation policies with dire economic ramifications. Emergency departments (ED) encountered decreased patient volumes before some in Seattle, New York City, New Orleans, and Detroit experienced waves of COVID‐19 patients mixed with asymptomatic patients or those concerned about potential exposures. Diagnosing COVID‐19 was hampered by inadequate supplies of reagents and kits, which was compounded by clinical and radiographic features that overlap with numerous seasonal viral respiratory infections. 1
The U.S. Food and Drug Administration (FDA) issued an emergency use authorization (EUA) on February 4, 2020, to enable Centers for Disease Control (CDC)–qualified laboratories to perform COVID‐19 testing. As of June 3, the FDA provided EUA for 85 commercial assays, including polymerase chain reaction (PCR) tests and immunoglobulin assays. 2 Early real‐time reverse transcription PCR (rRT‐PCR) tests had false‐negative (1 – sensitivity) rates as high as 40%. 3 As waves of COVID‐19 patients present to EDs in the coming months with symptoms or potential exposures, understanding the diagnostic accuracy and reliability of history, physical examination, routine laboratory tests, advanced imaging, and an evolving array of COVID‐19 diagnostics will be essential knowledge to inform the timing of testing, optimal specimen and test selection, shared decision making, and ultimately derivation of clinical instruments to guide disposition, follow‐up, and shared decision‐making choices (Figure 1). 4 This review provides a narrative overview of published research with the primary objective to describe the frequency, causes, and implications of false‐negative rRT‐PCR for diagnosis and surveillance. Secondary objectives include describing potential diagnostic biases in current rapid‐cycle COVID‐19 diagnostic research reports, while providing recommendations for clinicians for interpreting results with knowledge of these design and reporting limitations. A final objective is to add context to rRT‐PCR ordering and interpretation by understanding the diagnostic accuracy and additive value of history, physical examination, routine hematology and chemistry tests, computed tomography (CT), and serology for COVID‐19 immunoglobulins.
Figure 1.
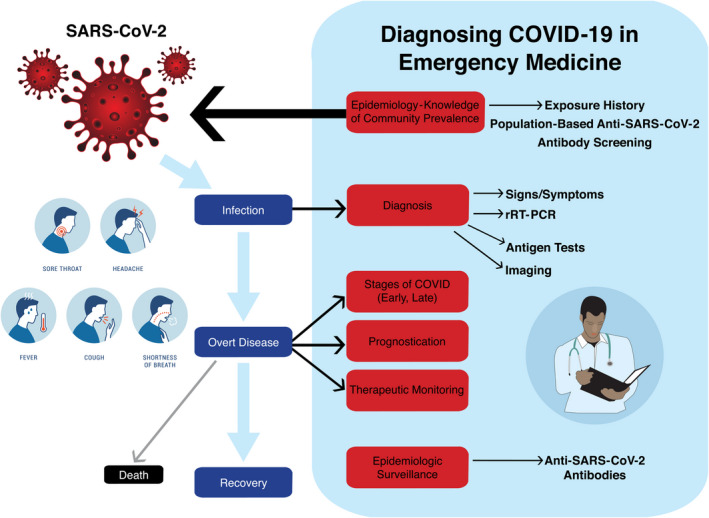
Diagnosing COVID‐19 in the ED requires assessment of exposure history and presenting signs and symptoms, interpretation of routine laboratory tests and imaging, and rRT‐PCR testing. Repeat rRT‐PCR testing is sometimes required to rule out the diagnosis. In some cases, CT and antigen testing may enhance diagnostic accuracy. The role of antibody testing for surveillance or ED decision making remains undefined. Image created by Kai Choummanivong. rRT‐PCR = real‐time reverse transcription–polymerase chain reaction.
Methods
Search Strategy
This is a scoping review that adheres to PRISMA‐ScR reporting recommendations. 5 The published literature was searched using strategies created by a medical librarian for COVID‐19 and diagnostic accuracy. The search was implemented in PubMed 1946– and Embase 1947– with a date limit from January 1, 2020, until present with an English language limit. The search strategy used a combination of standardized terms and keywords, including but not limited to (Covid‐19 or Novel Coronavirus or SARS‐COV‐2) and (diagnosis or PCR or serology or CRISPR‐CAS or sensitivity/specificity; Data Supplement S1, available as supporting information in the online version of this paper, which is available at http://onlinelibrary.wiley.com/doi/10.1111/acem.14048/full). The testing search was based on Cheng et al., 6 adding to this prior publication by incorporating clinical examination, imaging, and serology into the synthesis of current diagnostic research. The searches were performed on April 23 and May 5, 2020.
Study Selection
One author (CRC) reviewed the title and abstracts for all identified citations. Other authors reviewed the manuscripts identified and added pertinent references. Original research studies describing the frequency of history/physical examination findings or diagnostic accuracy (sensitivity, specificity, likelihood ratios) of history/physical examination, laboratory tests, or imaging for COVID‐19 were included. Exclusion criteria included non‐English, animal research, study protocols, prevention, pathophysiology, laboratory processing, or policy manuscripts. Two authors (CRC, SW) abstracted diagnostic accuracy data and reported adherence to the Standards for Reporting of Diagnostic Accuracy (STARD) guidelines. 7 Compliance with STARD was used as a measure of research quality. The same two authors synthesized the results into summary conclusions.
Results
A total of 1,907 citations were screened (Figure 2). None of the studies cite or adhere completely to either the STARD 7 or the updated reporting framework for history and physical examination. 8 Many of these early publications are letters or case reports with uncertain editorial rigor judging by the turnaround time from initial submission to publication. Many studies rely on rRT‐PCR as the criterion standard for COVID‐19, but few contemplate the possibility or likelihood of false‐negative or false‐positive rRT‐PCR results. None of the studies discuss the possibility of various diagnostic biases (spectrum, incorporation, partial verification, differential verification, or imperfect criterion standard), nor the potential skew of these biases in observed estimates of sensitivity or specificity. 9
Figure 2.
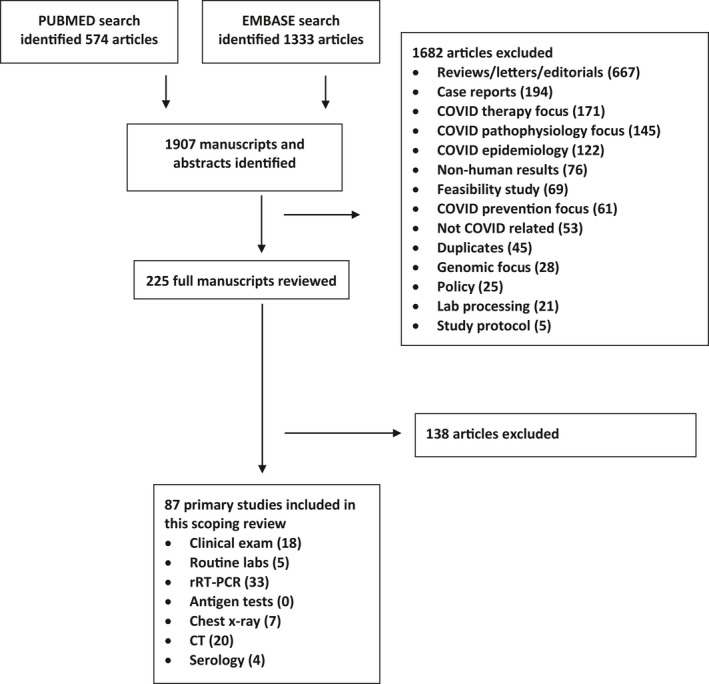
Study selection process. rRT‐PCR = real‐time reverse transcription–polymerase chain reaction.
Clinical Examination
Fever is the most commonly reported finding in 84% to 87% of COVID‐19 cases, 10 , 11 , 12 but fever alone does not distinguish this virus from other infections. 13 Therefore, absence of fever is inadequate for travel screening and likely for other decision thresholds such as whether ED staff can work shifts. 14 Hyposmia (diminished sense of smell) and hypogeusia (diminished taste) have also emerged as COVID‐19 symptoms. Both hyposmia (positive likelihood ratio [LR+] = 5.3, negative likelihood ratio [LR–] = 0.61) and hypogeusia (LR+ = 7.1, LR– = 0.38) are better to rule in than to rule out COVID‐19, but neither may be fully adequate for either purpose. 15 Although multiple COVID‐19 studies report acute smell or taste disorders as a distinguishing symptom, no other studies report diagnostic accuracy or sufficient details to compute likelihood ratios for hyposmia or hypogeusia. 16 , 17 , 18 , 19 , 20 , 21 Loss of smell is not necessarily associated with nasal obstruction or rhinorrhea. 22 In one case–control study, new‐onset smell and taste disorders are more common with COVID‐19 than with influenza (39% vs. 13%). 16 Consequently, influenza decision aids or diagnostic algorithms do not incorporate hyposmia or hypogeusia. 23 , 24 Anosmia, which may be the only complaint in some COVID patients, 25 is noted by 47% to 73% of COVID‐19 patients and is the initial symptom in 27%. 18 , 19 , 20 Additionally, 71% recall an acute onset of symptoms associated with taste and smell. 16 Anosmia is more common in women and may persist for 2 weeks. 17 Predictive models incorporating a change in taste or smell to distinguish COVID‐19 from viral mimics appear most sensitive. 26 Cough is only present in 58% patients. 10 , 12 Neither cough, dyspnea, sore throat, nor fatigue distinguish COVID‐19 from other illnesses, 13 but current studies do not quantify accuracy. 12 , 27
Routine Laboratory Tests
Lymphopenia occurs in over 50% of COVID‐19 patients. 10 , 11 , 28 Neutrophil‐to‐lymphocyte and platelet‐to‐lymphocyte ratios do not distinguish COVID‐19. 29 Elevated lactate dehydrogenase (LDH) is also frequently described. 10 , 28 , 30 , 31 None of these laboratory findings are commonly utilized in the diagnosis of influenza, but their prevalence and accuracy to distinguish COVID‐19 from other viral mimics merit further evaluation. 23 , 24 Elevated prothrombin time (PT), ferritin, D‐dimer, or IL‐6 are associated with severe COVID‐19. 31 , 32 , 33 Existing studies do not report sensitivity or specificity of these laboratory tests.
rRT‐PCR
Most studies use rRT‐PCR as the criterion standard for diagnosing COVID‐19. This test as used in current assays provides a qualitative detection of nucleic acid from the SARS‐CoV‐2 virus. Current research describes a variety of reagents and target RNA sequences with no accepted standard assay format. The U.S. CDC‐developed rRT‐PCR test detects two separate regions of the SARS‐CoV‐2 nucleocapsid gene (N1 and N2). Test results are considered positive when amplification of both the N1 and the N2 regions of the virus are detected and negative when both N1 and N2 are not detected. Detection of only one of the two amplified regions of the nucleocapsid gene results in an inconclusive test that must be repeated. This test can be performed on multiple diagnostic testing platforms and validated by laboratories under the U.S. FDA EUA. 2 Inadequate supplies of reagents have restricted testing capacity and time to diagnosis in many settings, 34 , 35 prompting laboratory researchers to explore the concept of specimen pooling in which multiple patients’ samples are tested simultaneously with further individual testing only if the pooled specimen is positive. 36 The optimal pool specimen when COVID‐19 community prevalence is less than 10% is four patients, which improves testing efficiency by 69%. 37
There is limited information on the diagnostic accuracy of the rRT‐PCR test. Although an increasing number of studies provide head‐to‐head comparisons, 38 , 39 , 40 systematic reviews provide little quantitative accuracy data and no meta‐analysis or assessment of individual study quality. 41 No rRT‐PCR test is clearly superior to others in terms of diagnostic accuracy, but some provide faster results and commercial tests may be less sensitive than hospital‐developed tests. 40 , 42 It is known, however, that false negatives are frequent, so current recommendations advise incorporating patient’s exposure risk, clinical signs and symptoms, routine laboratory and imaging findings, serology, and (when available) CT results into real‐time determination of COVID‐19 status. Repeat or even serial rRT‐PCR testing is required to confidently exclude COVID‐19. Multiple studies report initially negative rRT‐PCR results becoming positive with subsequent rRT‐PCR tests in the following days or weeks. 43 , 44 Others report hospitalized COVID‐19 patients with initially positive rRT‐PCR tests becoming negative prior to discharge with subsequent readmission for positive tests in the ensuing days. 45 Ren et al. 46 noted rRT‐PCR sensitivity with one test was 78% and increased to 86% with a second test. A strategy of three negative rRT‐PCR results is superior to two negative rRT‐PCR followed by bronchoalveolar lavage. 47 Repeating initially negative rRT‐PCR up to five times increases sensitivity to 98%. 48 COVID‐19 patients identified on first rRT‐PCR often have more severe disease associated with higher mortality, likely due to higher quantities of virus in those individuals. 48 Older patients are more likely to remain rRT‐PCR positive for an extended period, but whether this means they are contagious has yet to be determined. 44
Potential reasons for false‐negative rRT‐PCR results are summarized in Table 1. 49 , 50 , 51 Emergency physicians will rely on the rRT‐PCR assay selected by their hospital laboratory, which may balance the limit of detection and sensitivity against turnaround time, complexity, cost, workflow, availability of reagents and kits, specimen type, and laboratory personnel risk handling those specimens. 52 Patients under investigation for COVID‐19 who ultimately rule out are rarely reported in currently available studies, so specificity and false positives are generally not reported in the literature. However, false positives appear rare. 53 In CDC testing, there was no significant cross‐reactivity with other common respiratory viruses or seasonal coronaviruses. 54 Contamination of the specimen or reagents used in the rRT‐PCR is therefore the main mechanism for false‐positive results. The CDC recommends protocols to prevent and detect potential contamination to minimize false‐positive results. 49 , 54
Table 1.
Common Causes of False‐negative rRT‐PCR
|
Laboratory handling (heat inactivation) Limit of detection (RNA particle detection) Mutations in the probe target Sampling procedure (training, fidelity, patient cooperation) Selective virus replication (patient variability, disease severity variability) Specimen sampled (NP, OP, saliva, sputum, BAL, stool) Test kit quality Timing of sampling in course of disease |
BAL = bronchoalveolar lavage; NP = nasopharyngeal; OP = oropharyngeal; rRT‐PCR = real‐time reverse transcription–polymerase chain reaction.
Nasopharyngeal (NP) samples are most commonly obtained and studied, but oropharyngeal (OP), saliva, sputum, stool, blood, and/or urine specimens can also be evaluated. Obtaining NP samples requires time and appropriate training, increases exposure to staff secondary to coughing and gagging, and is uncomfortable for patients. Methodologically, few rRT‐PCR accuracy studies describe how research or clinical staff were trained to collect NP specimens, so fidelity and reproducibility remain in question. 55 Wang et al. 56 provide videos describing NP and OP collection methods and note poor agreement between the two sampling methods (kappa = 0.31) and a higher yield with NP. Saliva can be collected outside the hospital without training, perhaps as part of a telemedicine evaluation for COVID‐19. 57 , 58 One small Italian study indicated that saliva specimens demonstrate detectable SARS‐CoV‐2 and the limit of detection is not affected by patient age. 59 Sputum samples exhibit higher viral load than OP sites. 60 , 61 , 62 However, as already noted many patients under investigation lack a cough and fewer still have sputum production. Expectoration of sputum may also expose health care workers collecting the sample to aerosols that would not have been generated without a sample collection attempt. Furthermore, a Bayesian analysis of prevalence‐dependent positive and negative predictive values by Ghosal and Sinha 63 demonstrates that even when the COVID‐19 prevalence is high (54%), the positive predictive value (PPV) of sputum rRT‐PCR is only 95.7%, and the negative predictive value (NPV) is 52%. PPV and NPV vary with disease prevalence. Specifically, PPV increases with higher disease prevalence and NPV increases with lower disease prevalence making extrapolation to clinical populations challenging if the study prevalence does not match the patients to whom the test is applied. 64 For this reason, diagnosticians prefer likelihood ratios. 65 Blood and urine are inadequate specimens for rRT‐PCR because most patients do not exhibit virus in these body fluid compartments. 66
In addition to the CDC‐developed rRT‐PCR test, manufacturers have developed molecular tests that target different portions of the SARS‐CoV‐2 viral genome and are performed on rapid testing platforms. For example, reverse transcription loop‐mediated isothermal amplification can detect SARS‐CoV‐2 within 30 minutes. 67 , 68 , 69 Other laboratories are exploring high‐throughput sequencing for inconclusive fluorescence quantitative PCR specimens as a rapid mediator for the presence or absence of SARS‐CoV‐2. 70 The diagnostic accuracy of these tests is similarly not reported, but these tests have not shown cross‐reactivity to other respiratory viruses and bacteria.
Antigen Tests
On May 8, 2020, the U.S. FDA issued an EUA for a SARS‐CoV‐2 antigen test. 71 This test detects SARS‐Co‐V or SAVS‐CoV‐2 nucleocapsid protein antigens in NP or nasal specimens using a lateral‐flow immunofluorescent sandwich assay. 71 This assay is performed on a point‐of‐care device in laboratories that are able to perform high, moderate, or waived complexity tests and can provide results within minutes. 72 While the diagnostic accuracy of this test is not available at this time, the FDA reports high specificity but sensitivity that is less than that of rRT‐PCR. The FDA and the manufacturer recommend that negative results “be treated as presumptive and confirmed with a molecular assay, if necessary for patient management.” 73
Chest X‐ray
Chest X‐ray is essential to evaluate for COVID‐19 mimics such as pneumonia, pleural effusion, or pulmonary edema. Typical COVID‐19 findings include hazy opacities that are often bilateral and peripheral. 74 With the exception of one outlier, the reported sensitivity of single‐view chest X‐ray for COVID‐19 ranges from 33% to 60%. 75 , 76 , 77 , 78 , 79 Chen et al. 80 represent the outlier reporting 100% accuracy of chest X‐ray early in the COVID‐19 pandemic. Currently available COVID‐19 chest X‐ray studies do not report specificity or reliability. 75
CT of the Chest
Computed tomography findings suggesting COVID‐19 include ground glass opacity (often bilateral) and peripheral predominant lesions without mediastinal adenopathy or pleural effusions, although these findings represent nonspecific manifestations of acute lung injury associated with numerous infectious and noninfectious etiologies. 81 , 82 Incidental findings consistent with COVID‐19 are observed on CT of the chest in patients without respiratory symptoms. 83
Multiple studies report COVID‐19 identified by CT after one or more negative rRT‐PCR tests. 84 , 85 , 86 , 87 When the COVID‐19 epidemic erupted in China, clinicians lacked access to rRT‐PCR kits and then as rRT‐PCR became available, low rRT‐PCR sensitivity reinforced belief in the additive value of CT for many. 88 These observations and scenarios prompted some to advocate for CT as a first‐line supplement to the diagnostic evaluation for COVID‐19, combining rRT‐PCR with CT. 89 , 90 If CT alone or in combination with rRT‐PCR reduced false‐negative rates, the positive public health implications for case identification and control of disease transmission could be substantial. However, these benefits must be balanced against the cost of CT, medical radiation dangers, or practical limitations in busy hospitals with hourly trauma and stroke arrivals and potentially time‐dependent emergencies juxtaposed against advised CT shutdowns for COVID‐19 cleaning requiring 30 or more minutes. 91 , 92 This cleaning time would also delay access to CT for every patient in the ED, thereby prolonging potential exposure to those in the ED to other patients with COVID‐19. 92 Some propose that COVID‐19 patients wear N95 masks and plastic bags over their heads to eliminate or reduce these cleaning times. 27 Pragmatically, among those detected by CT no defined benefit, such as reduced mortality or faster resolution of COVID‐19 symptoms has been described. 93 In addition, radiologists’ sensitivity for diagnosing COVID‐19 by CT findings ranges from 72% to 94% with specificity from 24% to 100%. 94 Preliminary artificial intelligence studies report radiologists’ sensitivity improves from 79% to 88% and specificity from 88% to 91% with this artificial intelligence image augmentation, 95 while others hypothesize that the most valuable role for this technology may quantify the proportion of lung affected by COVID‐19. 96 Despite these issues, multiple studies highlight the fact that the sensitivity of CT is substantially higher than that of the first rRT‐PCR, while combining CT and rRT‐PCR provides maximal sensitivity (~97%). 89 , 97 , 98 Theoretically, the sensitivity of CT would decline when testing populations outside of an epidemic (low prevalence rates), while specificity would be reduced when mimics like influenza are more common. 9 , 27 The British Society of Thoracic Imaging recommends against CT when rRT‐PCR is positive, but to consider CT when the initial rRT‐PCR is negative to identify coexisting disease or potential COVID‐19 complications such as pulmonary embolism. 99 Tavare et al. 100 developed a single‐center protocol to prioritize inpatient CT decision making for initial COVID‐19–negative rRT‐PCR patients based on initial clinical suspicion and chest X‐ray findings.
Serology
The U.S. FDA has also issued an EUA for the development of SARS‐CoV‐2 antibody tests. These antibody tests detect circulating IgM, IgG, or both that are reactive against SARS‐CoV‐2 virus using lateral‐flow assays (LFAs) or enzyme‐linked immunosorbent assay (ELISA). 2 However, unlike rRT‐PCR tests, there are data regarding the diagnostic accuracy of these serologic tests. Whitman and colleagues 101 evaluated 10 LFA and two ELISA tests for SARS‐CoV‐2 antibodies. They used plasma or serum samples from patients with symptomatic, rRT‐PCR confirmed‐positive patients as the criterion standard for disease, and pre–COVID‐19 specimens from the American Red Cross as negative controls. Sensitivity of both IgM and IgG varied by days since symptoms onset, with sensitivities for either IgM or IgG at >20 days ranging from 82% to 100%. Specificity for either IgM or IgG also varied by test, ranging from 87% to 100%. 101 Similarly, Bendavid and colleagues 102 used a commercially available LFA test to perform a seroprevalence study in Santa Clara county, California, and reported a sensitivity of 80% and a specificity of 99.5%.
True‐positive serologic tests for SARS‐CoV‐2 antibodies indicate prior infection with SARS‐CoV‐2 and the development of an immune response. This may be helpful in identifying those who were asymptomatic or minimally symptomatic at the time of infection as well as those who were unable to receive a molecular test when symptomatic. While some experts believe that the presence of IgM or IgG reactive against SARS‐CoV‐2 will confer immunity, 103 this has not yet been shown. 104 If the presence of antibodies on a true‐positive serologic test does confer immunity, the titer of antibodies required to confer immunity remains unknown, as does the duration of that immunity.
False‐positive results may be due to cross‐reactivity with other coronavirus strains that cause the common cold. The FDA recommends the following information be included in the instructions for use and patient test reports:
Positive results may be due to past or present infection with non–SARS‐CoV‐2 coronavirus strains, such as coronavirus HKU1, NL63, OC43, or 229E. 105
Discussion
Knowledge of the diagnostic characteristics, including sensitivity, specificity, and likelihood ratios of tests for SARS‐CoV‐2, the virus that causes COVID‐19, is important to understand how to best apply these tests for patient care and disease surveillance. Because this novel virus emerged as a significant pathogen in humans only a few months ago, diagnostic tests have been developed rapidly under FDA EUAs in the United States. Consequently, we have less information about the diagnostic accuracy of these tests than we would under normal circumstances, but we do know that both false negatives and false positives may occur. An illustration of the false‐positive and false‐negative rate as a function of prevalence for two serologic tests for SARS‐CoV‐2 is provided in Figure 3.
Figure 3.
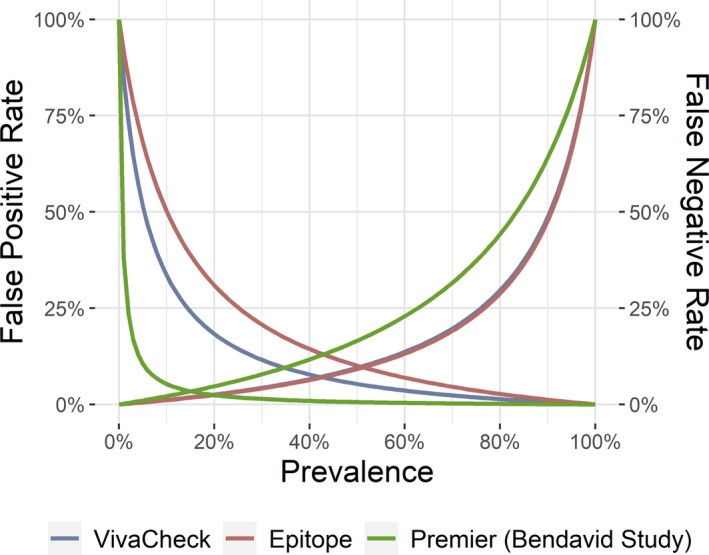
False‐positive and false‐negative rates as a function of pretest probability (or prevalence for surveillance studies) for serologic tests for SARS‐CoV‐2 antibodies. The left side of the graph illustrates the false‐positive rate, and the right illustrates the false‐negative rate.
False‐negative Test Implications
False‐negative tests commonly occur with rRT‐PCR tests for several reasons (Table 1). There are a number of potential implications of a false‐negative rRT‐PCR test for SARS‐CoV‐2. From the patient’s standpoint, a patient with a negative test may lead to an assumption that they are not infected and subsequently diminished adherence to instructions to isolate and take other infection control measures, increasing the risk of infecting others. In the hospital setting, precautions may be relaxed in the presence of a negative test, increasing the risk of transmission to health care workers and other patients. In patients with moderate or high pretest probability of disease, a negative test may not reduce the posttest probability of disease below a level where precautions to prevent spread of disease become less necessary. In patients with a low pretest probability of disease, the likelihood of disease given a negative test will be low. However, even low individual likelihoods of disease can cumulatively contribute to substantial risk of outbreaks across larger groups for more contagious infectious diseases, such as COVID‐19.
False‐negative tests are also a consideration with serologic testing. However, since these tests should generally not be used to assess an active infection, the risks of a false negative are less significant for disease transmission. A false‐negative serologic test would incorrectly classify a person as not having an immune response to SARS‐CoV‐2. If “immunity passports” became a reality, this could incorrectly and adversely affect a person’s ability to travel or work. 104
CT as Diagnostic Adjunct to Reduce False negatives
The increased sensitivity of CT for COVID‐19 might provide a net public health benefit if false‐negative rRT‐PCR patients with higher clinical suspicion were accurately identified during the initial ED evaluation. Mathematical models provide a theoretical basis for the concept that increasing diagnostic efficiencies (for example, by improving sensitivity with addition of CT) will decrease the risk of COVID‐19 transmission. 106 Pending the availability of rapid, reliable, and sufficiently accurate COVID‐19 tests in ED settings, the identify–isolate–inform (3I) approach to decrease spread might be improved with more liberal CT use. 107 One Italian hospital reported liberal CT screening for respiratory patients with possible false‐negative chest X‐ray results, but thus far has not reported on the positive or negative impact of that approach on individual patient care or public health. 108 In the early stages of COVID‐19, as many as 50% of patients with respiratory symptoms may have normal imaging. 96 Radiologists have also noted that the quality of early CT accuracy studies is questionable because the rRT‐PCR assay used as either the criterion standard is not described or the accuracy of that standard is undefined. 82 In addition, CT findings are not pathognomonic for COVID‐19 as influenza, cytomegalovirus, and atypical pneumonia have similar findings. 96 , 109 As a consequence of these CT limitations in addition to the costs, radiation exposure, and downstream effect on other patients in terms of diagnostic delays and cross‐contamination, multiple groups, including the Fleischner Society and the British Society of Thoracic Imaging, discourage CT as a routine screening approach. 99 , 110 Nonetheless, CT likely plays a role when rRT‐PCR tests are either too inaccurate or unavailable or suffer unacceptably slow turnaround times in patients with higher levels of COVID‐19 concern based on exposure history or other clinical findings. 74 The public health benefits of a more liberal CT screening approach from the ED merit additional research.
False‐positive Test Implications
False‐positive tests associated with rRT‐PCR for SARS‐CoV‐2 are believed to be rare and would most commonly occur due to contamination. False‐positive tests may occur more commonly with serologic tests, which have reported specificities ranging from 87% to 100%. 101 The PPV is a function of both test sensitivity and specificity and the pretest probability of disease. This implies that positive test results are more likely to represent false‐positive results when the pretest probability of disease is low. 111 The instability of PPV is especially important as we apply imperfect diagnostic tests to low‐risk patient populations, such as asymptomatic patients in low‐prevalence communities. As an example, the antibody test used in a California community study has a reported sensitivity of 80.3% and a specificity of 99.5% (LR+ = 161, LR– = 0.20). 102 Given the high specificity, clinicians expect a low number of false‐positive tests. However, in a community with a low prevalence, for example, 1.25% (similar to the corrected raw prevalence in the California study 102 ), the posttest probability of COVID‐19 given a positive rRT‐PCR is only 67%, with 33% resulting in false positives.
For diagnostic tests, a manipulation of Bayes’ theorem can illustrate the effect of pretest probability of disease on false positives by determining the pretest probability at which false positives are equal to true positives. Above this probability, true positives exceed false positives, while below this probability, false positives exceed true positives. The equation is
For the data in the study by Bendavid et al., 102 the pretest probability at which true‐positive and false‐positive results are equally likely is 0.62%. However, for the Epitope ELISA results provided in the study by Whitman et al., 101 with a sensitivity of 90.91% and a specificity of 81.82%, the probability at which true‐positive and false‐positive results are equally likely is 10%. Thus, if a patient with a <10% pretest probability of disease received a positive test result using this assay, it would be more likely to be a false positive than a true positive.
There are a number of potential adverse consequences of a false‐positive rRT‐PCR test for SARS‐CoV‐2. The CDC has published a Fact Sheet for Healthcare Providers that states that:
The CDC 2019‐nCoV Real‐Time RT‐PCR Diagnostic Panel has been designed to minimize the likelihood of false positive test results. However, in the event of a false positive result, risks to patients could include the following: a recommendation for isolation of the patient, monitoring of household or other close contacts for symptoms, patient isolation that might limit contact with family or friends and may increase contact with other potentially COVID‐19 patients, limits in the ability to work, the delayed diagnosis and treatment for the true infection causing the symptoms, unnecessary prescription of a treatment or therapy, or other unintended adverse effects. 111
For serology tests, the above risks of false positives could also exist if the test was thought to represent an active infection. Conversely, there are additional potential risks to patients and society with a false‐positive serologic test for SARS‐CoV‐2 antibodies. Patients may assume that they have developed immunity to COVID‐19, leading to a reduction in risk‐mitigating activities such as physical distancing. Health care workers with false‐positive tests may similarly reduce their vigilance and use of precautions due to an incorrect assumption that they have immunity. This could place these individuals and their close contacts at increased risk of contracting COVID‐19.
Potential Diagnostic Biases Skew Observed Accuracy
Multiple forms of diagnostic bias exist and each skew measured estimates of sensitivity and specificity in different directions. 9 Incorporation bias is possible when the criterion standard includes the index test (for example, rRT‐PCR) in ultimately determining whether the disease is present or absent. Incorporation bias increases measured sensitivity and specificity. This is pertinent to COVID‐19 because most early studies incorporate rRT‐PCR into the criterion standard. 9 , 112 Differential verification bias is possible when patients with a positive or concerning index test (e.g., CT findings associated with COVID‐19) are more likely to receive an immediate invasive criterion standard such as repeat rRT‐PCR testing or bronchoalveolar lavage specimens. 82 , 97 Differential verification bias raises specificity in diseases that resolve spontaneously or lowers specificity for diseases that only become detectable during follow‐up. 9 Imperfect criterion standard bias is possible when the standard used to classify the presence or absence of disease misclassifies some patients. Imperfect criterion standard bias raises observed specificity if errors on the index test and “copper standard” are correlated with true disease status and lowers observed specificity if errors on the index test and the copper standard are independent. 9 This is pertinent to COVID‐19 because no well‐accepted criterion standard yet exists. A better criterion standard for COVID‐19 will certainly emerge and we propose some ideas in Table 2. 113 , 114 Temporal bias reflects variation in observed accuracy based on the period of time or stage of disease when index testing occurred. 115 In COVID‐19 viral shedding is highest in the early stages of disease with the highest positive rates noted within the first week. 116 , 117 Spectrum bias is possible when the spectrum of disease severity differs between the study and clinical application (for example, critically ill COVID‐19 patients in the intensive care unit vs. asymptomatic patients evaluated in ambulatory clinics). Spectrum bias skews observed sensitivity upward in sicker populations and skews specificity upward in healthier patients. 9 , 118 Spectrum bias is worth considering when applying diagnostic accuracy results from patients with varying severity of illness to dissimilar populations. For example, among COVID‐19 patients from cruise ships evaluated with CT, those with symptoms more commonly had COVID‐19 CT findings than those without symptoms (80% vs. 40%). 119
Table 2.
Proposed COVID‐19 Criterion Standard
|
Expert consensus months after acute illness, including
|
rRT‐PCR = real‐time reverse transcription–polymerase chain reaction.
Implications for Future Research
The rapidly expanding evidence base around COVID‐19 diagnostic accuracy for clinical examination, routine laboratory tests, imaging, and advanced testing provides important lessons moving forward for clinicians, researchers, and journal reviewers. COVID‐19 researchers need to contemplate myriad biases carefully in reporting observed diagnostic accuracy. If a bias is likely and the anticipated skew in observed sensitivity or specificity is upward and the observed accuracy is already too low, further studies of that diagnostic test may not be warranted. The STARD reporting guidelines provide manuscript protocols to ensure adequate description of methods and results so that diagnostic biases are easier to identify. 7 , 8 Unfortunately, none of the early COVID‐19 diagnostic research cites STARD or adheres to these reporting standards, which is not uncommon in emergency medicine. 120 , 121
Clinical decision aids consist of three or more findings on history, physical examination, routine laboratory tests, or imaging that, in combination, more accurately identify patients at lower or higher risk of a disease or outcome. Diagnostic and prognostic decision aids are commonly developed and employed in emergency medicine to reduce practice variability without compromising patient outcomes. 122 Efforts to develop COVID‐19 decision aids might include something like the Pulmonary Embolism Rule‐Out Criteria (PERC) rule to identify subsets of ED patients at lower risk of COVID‐19 pending definitive testing. 123 Alternatively, a decision aid might serve prognostic purposes to identify COVID‐19 patients more likely to decompensate in response to the viral infection. 124 , 125 , 126 , 127 When decision aid investigators attempt to derive and validate these instruments, higher‐quality emergency medicine research quantifying accuracy and reliability (or the elements of history, physical examination, laboratory tests, and imaging that become predictor variables of the decision aid) will be required as the basis for selecting variables likely to improve model performance.
Most laboratory tests are quantitative rather than qualitative, including COVID‐19 rRT‐PCR and serologic assays. When sensitivity and specificity are reported, the quantitative laboratory tests have been dichotomized at some level. Another approach to evaluating diagnostic accuracy for quantitative data is interval likelihood ratios (iLR). 128 One advantage of iLR’s is that indeterminant results are more readily interpreted. As COVID‐19 diagnosticians identify the rRT‐PCR and serologic tests that best balance availability, accuracy, reliability, and cost, reporting iLR’s could provide added value for clinicians.
Ultimately, ED physicians’ clinical impressions concerning the presence or absence of COVID‐19 are communicated to patients and families—usually without access to definitive testing. Patient communication tools to convey the basics of COVID‐19 personal protection and infection prevention exist, 129 , 130 but shared decision‐making instruments that communicate the uncertainties of clinical examination, imaging, and even rRT‐PCR do not exist. Figure 4 provides one example of a Cates plot that could be used to communicate the accuracy limitations of rRT‐PCR based on current evidence. Actual shared decision‐making instruments will require scientific investigation using accepted methodology before widespread implementation. 131
Figure 4.
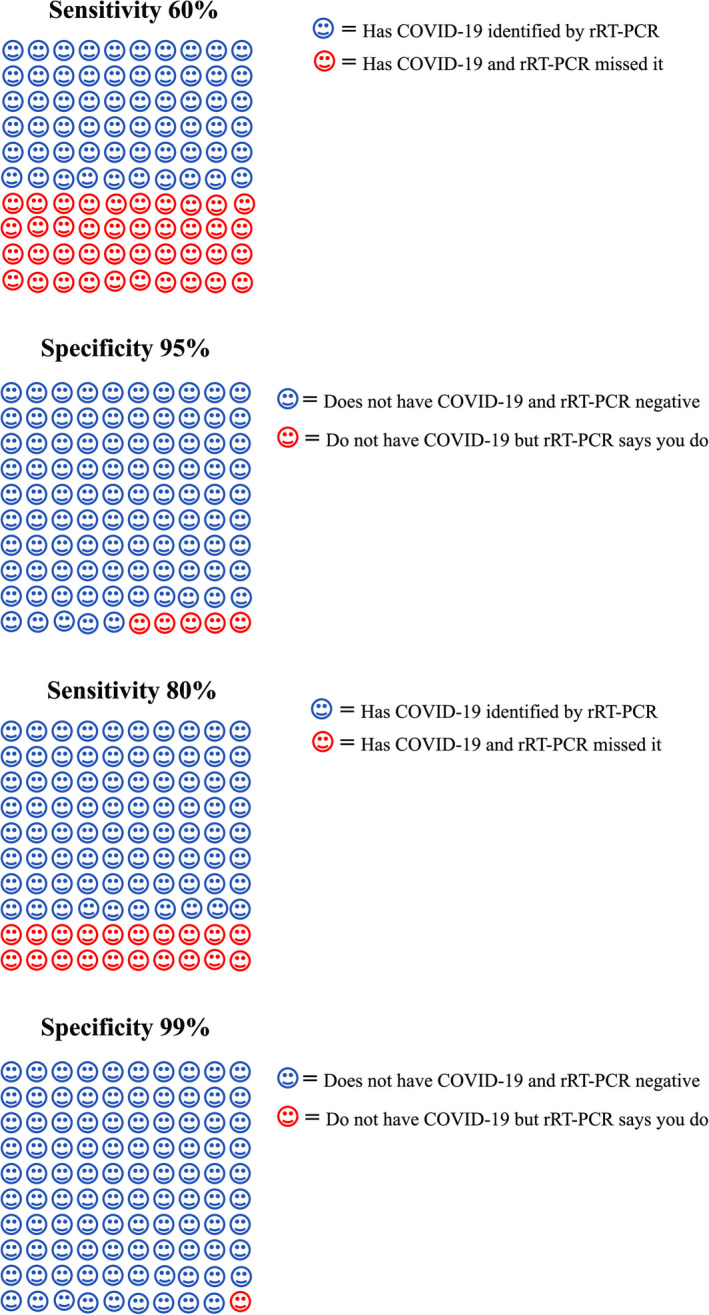
Cates plot for patients. rRT‐PCR = real‐time reverse transcription–polymerase chain reaction.
Limitations
This scoping review has several limitations. The pace of publications around COVID‐19 and diagnostics in the first half of 2020 has been astonishing. At best, this review will serve as a snapshot in time, although hopefully illuminating issues that require higher methodologic standards and peer‐review attention moving forward. Due to time constraints, the search strategy was limited to English language and published research. More research undoubtedly exists in the gray literature. Since earlier systematic reviews exploring aspects of COVID‐19 diagnostic testing did not identify or report additional measures of sensitivity, specificity, or likelihood ratios for hyposmia, hypogeusia, or rRT‐PCR, we are confident that this search presents a complete scoping review of current knowledge. 10 , 131 Others have also noted the absence of diagnostic accuracy reporting amidst the flurry of COVID‐19 publications. 133 , 134 Additionally, this scoping review does not focus on special emergency medicine populations such as pediatrics, geriatrics, or obstetrics because other reviews already exist for these patients. 135 , 136 Most importantly, we report no formal assessment of study quality using accepted instruments such as the QUADAS‐2, 137 although informal assessment of published research to date suggests limited adherence to the full set of recommended methodologic standards for studies of diagnostic test performance.
Conclusions
Clinicians should be aware of the current limited knowledge around history, physical examination, laboratory tests, and imaging for COVID‐19. Fever and acute‐onset disorders of taste and/or smell are the most common findings on history and physical examination associated with COVID‐19. Lymphopenia is associated with COVID‐19 diagnosis, while elevated lactate dehydrogenase and prothrombin time are associated with severe disease. rRT‐PCR has emerged as the primary diagnostic test for suspected COVID‐19, but access has been limited, diagnostic accuracy is underreported, and between‐assay comparative accuracy is rarely evaluated. However, typical testing algorithms and diagnostic accuracy studies rely heavily on rRT‐PCR results with frequent false negatives. Chest computed tomography is indicated for equivocal cases or when considering diagnoses like pulmonary embolism but is not recommended as a general screening protocol. In cases with high clinical suspicions, repeat real‐time reverse transcription–polymerase chain reaction testing with or without computed tomography scanning may be beneficial to reduce community spread. Antigen tests have only recently been approved, and diagnostic accuracy information is similarly limited. Serology may identify past COVID‐19 exposure, but the role of antibody testing and implications for ED decision making remain undefined. Current clinical, imaging, and laboratory studies neglect diagnostic accuracy reporting standards and likely suffer from various biases.
The authors acknowledge the contributions of Washington University in St. Louis School of Medicine’s medical librarian Michelle Doering for providing electronic medical database search expertise. They also acknowledge illustrator Kai Choummanivong for producing Figure 1.
Supporting information
Data Supplement S1. Appendix.
Academic Emergency Medicine 2020;27:654–670.
The authors have no relevant financial information or potential conflicts to disclose.
References
- 1. Lei P, Fan B, Wang P Differential diagnosis for coronavirus disease (COVID‐ 19): beyond radiologic features. AJR Am J Roentgenol 2020;215:W19. [DOI] [PubMed] [Google Scholar]
- 2. U.S. Food and Drug Administration . Emergency Use Authorization (EUA) information, and list of all current EUAs Food and Drug Administration. 2020. Available at: https://www.fda.gov/emergency‐preparedness‐and‐response/mcm‐legal‐regulatory‐and‐policy‐framework/emergency‐use‐authorization#LDTs. Accessed Jun 3, 2020.
- 3. West CP, Montori VM, Sampathkumar P COVID‐19 testing: the threat of false‐negative results. Mayo Clin Proc 2020;95:1127–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lippi G, Plebani M The critical role of laboratory medicine during coronavirus disease 2019 (COVID‐19) and other viral outbreaks. Clin Chem Lab Med 2020;58:1063–9. [DOI] [PubMed] [Google Scholar]
- 5. Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA‐ScR): checklist and explanation. Ann Intern Med 2018;169:476–3. [DOI] [PubMed] [Google Scholar]
- 6. Cheng MP, Papenburg J, Desjardins M, et al. Diagnostic testing for severe acute respiratory syndrome‐related coronavirus‐2: a narrative review. Ann Intern Med 2020;172:726–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bossuyt PM, Reitsma JB, Bruns DE, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ 2015;351:h5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Simel DL, Rennie D, Bossuyt PM. The STARD statement for reporting diagnostic accuracy studies: application to the history and physical examination. J Gen Intern Med 2008;23:768–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kohn MA, Carpenter CR, Newman TB. Understanding the direction of bias in studies of diagnostic test accuracy. Acad Emerg Med 2013;20:1194–206. [DOI] [PubMed] [Google Scholar]
- 10. Cao Y, Liu X, Xiong L, Cai K. Imaging and clinical features of patients with 2019 novel coronavirus SARS‐CoV‐2: a systematic review and meta‐analysis. J Med Virol 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhu W, Xie K, Lu H, Xu L, Zhou S, Fang S. Initial clinical features of suspected coronavirus disease 2019 in two emergency departments outside of Hubei, China. J Med Virol 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rodriguez‐Moreles AJ, Cardona‐Ospina JA, Gutierrez‐Ocampo E, et al. Clinical, laboratory and imaging features of COVID‐19: a systematic review and meta‐analysis. Travel Med Infect Dis 2020;34:101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peng L, Liu KY, Xue F, Miao YF, Tu PA, Zhou C. Improved early recognition of coronavirus disease‐2019 (COVID‐19): single‐center data from a Shanghai screening hospital. Arch Iran Med 2020;23:272–6. [DOI] [PubMed] [Google Scholar]
- 14. Bwire GM, Paulo LS Paulo LS. Coronavirus disease‐2019: is fever an adequate screening for the returning travelers? Trop Med Health 2020;48:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Benezit F, Le Turnier P, Declerck C, et al. Utility of hyposmia and hypogeusia for the diagnosis of COVID‐19. Lancet Infect Dis 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beltrán‐Corbellini Á, Chico‐García JL, Martínez‐Poles J, et al. Acute‐onset smell and taste disorders in the context of Covid‐19: a pilot multicenter PCR‐based case‐control study. Eur J Neurol 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heidari F, Karimi E, Firouzifar M, Khamushian P, Ansari R, Mohammadi AM. Anosmia as a prominent symptom of COVID‐19 infection. Rhinology 2020;58:302–3. [DOI] [PubMed] [Google Scholar]
- 18. Kaye R, Chang CW, Kazahaya K, Brereton J, Denneny JC 3rd. COVID‐19 anosmia reporting tool: initial findings. Otolaryngol Head Neck Surg 2020;163:132–34. [DOI] [PubMed] [Google Scholar]
- 19. Vaira LA, Deiana G, Fois AG, et al. Objective evaluation of anosmia and ageusia in COVID‐19 patients: single‐center experience on 72 cases. Head Neck 2020;42:1252–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Klopfenstein T, Kadiane‐Oussou NJ, Toko L, et al. Features of anosmia in COVID‐19. Med Mal Infect 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim GU, Kim MJ, Ra SH, et al. Clinical characteristics of asymptomatic and symptomatic patients with mild COVID‐19. Clin Microbiol Infect 2020;26:948.e1–8.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lechien JR, Chiesa‐Estomba CM, Place S, et al. Clinical and epidemiological characteristics of 1,420 European patients with mild‐to‐moderate coronavirus disease 2019. J Intern Med 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ebell MH, Afonso A A systematic review of clinical decision rules for the diagnosis of influenza. Ann Fam Med 2011;2:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Call SA, Vollenweider MA, Hornung CA, Simel DL, McKinney WP. Does this patient have influenza? In: Simel DL, Rennie D, editors. The Rational Clinical Examination: Evidence‐Based Clinical Diagnosis. New York: McGraw‐Hill, 2009. pp. 343–54. [Google Scholar]
- 25. Villalba NL, Maouche Y, Ortiz MB, et al. Anosmia and dysgeusia in the absence of other respiratory diseases: should COVID‐19 infection be considered? Eur J Case Rep Intern Med 2020;7:001–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roland LT, Gurrola JG 2nd, Loftus PA, Cheung SW, Chang JL. Smell and taste symptom‐based predictive model for COVID‐19 diagnosis. Int Forum Allergy Rhinol 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Amalou A, Turkbey B, Sanford T, et al. Targeted early chest CT in COVID‐19 outbreaks as diagnostic tool for containment of the pandemic ‐ A multinational opinion. Diagn Interv Radiol 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ferrari D, Motta A, Strollo M, Banfi G, Locatelli M. Routine blood tests as a potential diagnostic tool for COVID‐19. Clin Chem Lab Med 2020;58:1095–9. [DOI] [PubMed] [Google Scholar]
- 29. Yang AP, Liu JP, Tao WQ, Li HM. The diagnostic and predictive role of NLR, d‐NLR and PLR in COVID‐19 patients. Int Immunopharmacol 2020;84 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mardani R, Ahmadi Vasmehjani A, Zali F, et al. Laboratory parameters in detection of COVID‐19 patients with positive RT‐PCR; a diagnostic accuracy study. Arch Acad Emerg Med 2020;8:e43. [PMC free article] [PubMed] [Google Scholar]
- 31. Wang F, Hou H, Luo Y, et al. The laboratory tests and host immunity of COVID‐19 patients with different severity of illness. JCI Insight 2020;5:e137799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gao Y, Li T, Han M, et al. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID‐19. J Med Virol 2020;92:791–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li Y, Hu Y, Yu J, Ma T. Retrospective analysis of laboratory testing in 54 patients with severe‐ or critical‐type 2019 novel coronavirus pneumonia. Lab Invest 2020;100:794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Beeching NJ, Fletcher TE. Beadsworth MB. Covid‐19: testing times. BMJ 2020;369:m1403. [DOI] [PubMed] [Google Scholar]
- 35. Pathak M, Patel SK, Jigyasa R, et al. Global threat of SARS‐CoV‐2/COVID‐19 and the need for more and better diagnostic tools. Arch Med Res 2020;51:450–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yelin I, Aharony N, Shaer Tamar E, et al. Evaluation of COVID‐19 RT‐qPCR test in multi‐sample pools. Clin Infect Dis 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Abdalhamid B, Bilder CR, McCutchen EL, Hinrichs SH, Koepsell SA, Iwen PC. Assessment of specimen pooling to conserve SARS CoV‐2 testing resources. Am J Clin Pathol 2020;153:715–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Uhteg K, Jarrett J, Richards M, et al. Comparing the analytical performance of three SARS‐CoV‐2 molecular diagnostic assays. J Clin Virol 2020;127:104384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhen W, Manji R, Smith E, Berry GJ. Comparison of four molecular in vitro diagnostic assays for the detection of SARS‐CoV‐2 in nasopharyngeal specimens. J Clin Microbiol 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhen W, Smith E, Manji R, Schron D, Berry GJ. Clinical evaluation of three sample‐to‐answer platforms for the detection of SARS‐CoV‐2. J Clin Microbiol 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pang J, Wang MX, Ang IY, et al. Potential rapid diagnostics, vaccine and therapeutics for 2019 novel coronavirus (2019‐nCoV): a systematic review. J Clin Med 2020;9:623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rahman H, Carter I, Basile K, et al. Interpret with caution: an evaluation of the commercial AusDiagnostics versus in‐house developed assays for the detection of SARS‐CoV‐2 virus. J Clin Virol 2020;127:104374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lv DF, Ying QM, Weng YS, et al. Dynamic change process of target genes by RT‐PCR testing of SARS‐Cov‐2 during the course of a coronavirus disease 2019 patient. Clin Chim Acta 2020;506:172–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xiao AT, Tong YX, Zhang S False‐negative of RT‐PCR and prolonged nucleic acid conversion in COVID‐19: rather than recurrence. J Med Virol 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yuan J, Kou S, Liang Y, Zeng J, Pan Y, Liu L. PCR Assays turned positive in 25 discharged COVID‐19 patients. Clin Infect Dis 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ren X, Liu Y, Chen H, et al. Application and optimization of RT‐PCR in diagnosis of SARS‐CoV‐2 infection. medRxiv 2020. [preprint]. [Google Scholar]
- 47. Raschke RA, Curry SC, Glenn T, Gutierrez F, Iyengar S. A Bayesian analysis of strategies to rule out COVID19 using reverse transcriptase‐polymerase chain reaction (RT‐PCR). Arch Pathol Lab Med 2020. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 48. Zhang JJ, Cao YY, Dong X, et al. Distinct characteristics of COVID‐19 patients with initial rRT‐PCR positive and negative results for SARS‐CoV‐2. Allergy 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tahamtan A, Ardebili A. Real‐time RT‐PCR in COVID‐19 detection: issues affecting the results. Expert Rev Mol Diagn 2020;20:453–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang X, Yao H, Xu X, et al. Limits of detection of six approved RT‐PCR kits for the novel SARS‐coronavirus‐2 (SARS‐CoV‐2). Clin Chem 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang Y, Kang H, Liu X, Tong Z. Combination of RT‐qPCR testing and clinical features for diagnosis of COVID‐19 facilitates management of SARS‐CoV‐2 outbreak. J Med Virol 2020;92:538–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rhoads DD, Cherian SS, Roman K, Stempak LM, Schmotzer CL, Sadri N. Comparison of Abbott ID Now, Diasorin Simplexa, and CDC FDA EUA methods for the detection of SARS‐CoV‐2 from nasopharyngeal and nasal swabs from individuals diagnosed with COVID‐19. J Clin Microbiol. 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nalla AK, Casto AM, Huang ML, et al. Comparative performance of SARS‐CoV‐2 detection assays using seven different primer/probe sets and one assay kit. J Clin Microbiol 2020;58:e00557–e620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Centers for Disease Control and Prevention . CDC 2019‐Novel Coronavirus (2019‐nCoV) Real‐Time RT‐PCR Diagnostic Panel. 2020. Available at: https://www.fda.gov/media/134922/download. Accessed May 9, 2020
- 55. Ye G, Li Y, Lu M, et al. Experience of different upper respiratory tract sampling strategies for detection of COVID‐19. J Hosp Infect 2020;105:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang X, Tan L, Wang X, et al. Comparison of nasopharyngeal and oropharyngeal swabs for SARS‐CoV‐2 detection in 353 patients received tests with both specimens simultaneously. Int J Infect Dis 2020;94:107–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Santosh KC. AI‐driven tools for coronavirus outbreak: need of active learning and cross‐population train/test models on multitudinal/multimodal data. J Med Syst 2020;44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sullivan PS, Sailey C, Guest JL, et al. Detection of SARS‐CoV‐2 RNA and antibodies in diverse samples: protocol to validate the sufficiency of provider‐observed home‐collected blood, saliva and oropharyngeal samples. JMIR Public Health Surveill. 2020;6:e19054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Azzi L, Carcano G, Gianfagna F, et al. Saliva is a reliable tool to detect SARS‐CoV‐2. J Infect 2020;81:e45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lin C, Xiang J, Yan M, Li H, Huang S, Shen C. Comparison of throat swabs and sputum specimens for viral nucleic acid detection in 52 cases of novel coronavirus (SARS‐Cov‐2)‐infected pneumonia (COVID‐19). Clin Chem Lab Med 2020;58:1089–94. [DOI] [PubMed] [Google Scholar]
- 61. Pan Y, Zhang D, Yang P, Poon LL, Wang Q. Viral load of SARS‐CoV‐2 in clinical samples. Lancet Infect Dis 2020;20:411–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yu F, Yan L, Wang N, et al. Quantitative detection and viral load analysis of SARS‐CoV‐2 in infected patients. Clin Infect Dis 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ghosal S, Sinha B. Rapid sputum testing and not thermal screening alone should be the first‐line screening test at airports: a Bayesian analysis. Diabetes Metab Syndr 2020;14:317–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gallagher EJ. Emergency medicine/editorial. The problem with sensitivity and specificity. Ann Emerg Med 2003;42:298–303. [DOI] [PubMed] [Google Scholar]
- 65. Hayden SR, Brown MD. Likelihood ratio: A powerful tool for incorporating the results of a diagnostic test into clinical decisionmaking. Annals of Emergency Medicine 1999; 33:575–80. [DOI] [PubMed] [Google Scholar]
- 66. Xie C, Jiang L, Huang G, et al. Comparison of different samples for 2019 novel coronavirus detection by nucleic acid amplification tests. Int J Infect Dis 2020; 93:264–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Baek YH, Um J, Antigua KJ, et al. Development of a reverse transcription‐loop‐mediated isothermal amplification as a rapid early‐detection method for novel SARS‐CoV‐2. Emerg Microbes Infect 2020;9:998–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Huang WE, Lim B, Hsu CC, et al. RT‐LAMP for rapid diagnosis of coronavirus SARS‐CoV‐2. Microb Biotechnol 2020;13:950–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Park GS, Ku K, Baek SH, et al. Development of reverse transcription loop‐mediated isothermal amplification assays targeting severe acute respiratory syndrom coronavirus 2 (SARS‐CoV‐2). J Mol Diagn 2020;22:729–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Guan WD, Chen LP, Ye F, et al. High‐throughput sequencing for confirmation of suspected 2019‐nCoV infection identified by fluorescence quantitative polymerase chain reaction. Chin Med J 2020;133:1385–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hinton DM. Letter to Quidel Corporation. Food and Drug Administration. 2020. Available at: https://www.fda.gov/media/137886/download. Accessed May 11, 2020
- 72. Fact Sheet for Healthcare Providers . Quidel Corporation. 2020. Available at: https://www.fda.gov/media/137884/download. Accessed May 11, 2020
- 73. Hahn SM, Shuren JE.FDA Statement: Coronavirus (COVID‐19) Update: FDA Authorizes First Antigen Test to Help in the Rapid Detection of the Virus that Causes COVID‐19 in Patients. 2020. Available at: https://www.fda.gov/news‐events/press‐announcements/coronavirus‐covid‐19‐update‐fda‐authorizes‐first‐antigen‐test‐help‐rapid‐detection‐virus‐causes. Accessed June 2, 2020
- 74. Guneyli S, Atceken Z, Dogan H, Altinmakas E, Atasoy KC. Radiological approach to COVID‐19 pneumonia with an emphasis on chest CT. Diagn Interv Radiol 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chen SG, Chen JY, Yang YP, Chien CS, Wang ML, Lin LT. Use of radiographic features in COVID‐19 diagnosis: challenges and perspectives. J Chin Med Assoc 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wong HY, Lam HY, Fong AT, et al. Frequency and distribution of chest radiographic findings in COVID‐19 positive patients. Radiology 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Guan CS, Lv ZB, Yan S, et al. Imaging features of coronavirus disease 2019 (COVID‐19): evaluation on thin‐section CT. Acad Radiol 2020;27:609–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kim ES, Chin BS, Kang CK, et al. Clinical course and outcomes of patients with severe acute respiratory syndrome coronavirus 2 infection: a preliminary report of the first 28 patients from the Korean Cohort Study on COVID‐19. J Korean Med Sci 2020;35:e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ng MY, Lee EY, Yang F, et al. Imaging profile of the COVID‐19 infection: radiologic findings and literature review. Radiol Cardiothorac Imaging 2020;2:e200034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Himoto Y, Sakata A, Kirita M, et al. Diagnostic performance of chest CT to differentiate COVID‐19 pneumonia in non‐high‐epidemic area in Japan. Jpn J Radiol 2020;38:400–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Raptis CA, Hammer MM, Short RG, et al. Chest CT and coronavirus disease (COVID‐19): a critical review of the literature to date. AJR Am J Roentgenol 2020;1–4 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 83. Khazaei M, Asgari R, Zarei E, Moharramzad Y, Haghighatkhah H, Sanei TM. Incidentally diagnosed COVID‐19 Infection in trauma patients; a clinical experience. Arch Acad Emerg Med 2020;8:e31. [PMC free article] [PubMed] [Google Scholar]
- 84. Hao W, Li M. Clinical features of atypical 2019 novel coronavirus pneumonia with an initially negative RT‐PCR assay. J Infect 2020;80:671–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Huang P, Liu T, Huang L, et al. Use of chest CT in combination with negative RT‐PCR assay for the 2019 novel coronavirus but high clinical suspicion. Radiology 2020;295:22–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Xie X, Zhong Z, Zhao W, Zheng C, Wang F, Liu J. Chest CT for typical 2019‐nCoV pneumonia: relationship to negative RT‐PCR testing. Radiology 2020;200343 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Xu J, Wu R, Huang H, et al. Computed tomographic imaging of 3 patients with coronavirus disease 2019 pneumonia with negative virus real‐time reverse‐transcription polymerase chain reaction test. Clin Infect Dis 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Shi H, Han X, Cao Y, Alwalid O, Zheng C. CT screening for early diagnosis of SARS‐CoV‐2 infection ‐ authors' reply. Lancet Infect Dis 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Chen X, Tang Y, Mo Y, et al. A diagnostic model for coronavirus disease 2019 (COVID‐19) based on radiological semantic and clinical features: a multi‐center study. Eur Radiol 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Feng H, Liu Y, Lv M, Zhong J. A case report of COVID‐19 with false negative RT‐PCR test: necessity of chest CT. Jpn J Radiol 2020;38:409–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Al‐Tawfiq JA, Memish ZA. Diagnosis of SARS‐CoV‐2 Infection based on CT scan vs. RT‐PCR: reflecting on experience from MERS‐CoV. J Hosp Infect 2020;105:154–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Majidi H, Niksolat F. Chest CT in patients suspected of COVID‐19 infection: a reliable alternative for RT‐PCR. Am J Emerg Med 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Huang Y, Cheng W, Zhao N, Qu H, Tian J. CT screening for early diagnosis of SARS‐CoV‐2 infection. Lancet Infect Dis 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Bai HX, Hsieh B, Xiong Z, et al. Performance of radiologists in differentiating COVID‐19 from viral pneumonia on chest CT. Radiology 2020;200823 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Bai HX, Wang R, Xiong Z, et al. AI augmentation of radiologist performance in distinguishing COVID‐19 from pneumonia of other etiology on chest CT. Radiology 2020;201491 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Laghi A. Cautions about radiologic diagnosis of COVID‐19 infection driven by artificial intelligence. Lancet Digital Health 2020;2:e225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Fang Y, Zhang H, Xie J, et al. Sensitivity of chest CT for COVID‐19: comparison to RT‐PCR. Radiology 2020;200432 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Long C, Xu H, Shen Q, et al. Diagnosis of the coronavirus disease (COVID‐19): rRT‐PCR or CT? Eur J Radiol 2020;126:108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Nair A, Rodrigues JC, Hare S, et al. A British Society of Thoracic Imaging statement: considerations in designing local imaging diagnostic algorithms for the COVID‐19 pandemic. Clin Radiol 2020;75:329–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Tavare AN, Braddy A, Brill S, et al. Managing high clinical suspicion COVID‐19 inpatients with negative RT‐PCR: a pragmatic and limited role for thoracic CT. Thorax 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Whitman JD, Hiatt J, Mowery CT, et al. Test performance evaluation of SARS‐CoV‐2 serological assays. medRxiv 2020. [preprint]. [Google Scholar]
- 102. Bendavid E, Mulaney B, Sood N, et al. COVID‐19 antibody seroprevalence in Santa Clara County, California. medRxIV 2020. [preprint]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Abbasi J. The promise and perils of antibody testing for COVID‐19. JAMA 2020. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 104. World Health Organization . “Immunity passports" in the Context of COVID‐19. World Health Organization. 2020. Available at: https://www.who.int/news‐room/commentaries/detail/immunity‐passports‐in‐the‐context‐of‐covid‐19. Accessed May 6, 2020
- 105. U.S. Food and Drug Administration . Policy for Coronavirus Disease‐2019 Tests During the Public Health Emergency (Revised). 2020. Available at: https://www.fda.gov/media/135659/download. Accessed May 7, 2020.
- 106. Rong XM, Yang L, Chu HD, Fan M. Effect of delay in diagnosis on transmission of COVID‐19. Math Biosci Eng 2020;17:2725–40. [DOI] [PubMed] [Google Scholar]
- 107. Koenig KL, Beÿ CK, McDonald EC. 2019‐nCoV: the identify‐isolate‐inform (3I) tool applied to a novel emerging coronavirus. West J Emerg Med 2020;21:184–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Orsi MA, Oliva AG, Cellina M. Radiology department preparedness for COVID‐19: facing an unexpected outbreak of the disease. Radiology 2020;295:E8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Chen H, Ai L, Lu H, Li H. Clinical and imaging features of COVID‐19. Radiol Infect Dis 2020. [in press]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Rubin GD, Ryerson CJ, Haramati LB, et al. The role of chest imaging in patient management during the COVID‐19 pandemic: a multinational consensus statement from the Fleischner Society. Chest 2020;296:172–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Centers for Disease Control and Prevention . FACT SHEET FOR HEALTHCARE PROVIDERS CDC ‐ 2019‐nCoV Real‐Time RT‐PCR Diagnostic Centers for Disease Control. 2020. Available at: https://www.cdc.gov/coronavirus/2019‐ncov/downloads/Factsheet‐for‐Healthcare‐Providers‐2019‐nCoV.pdf. Accessed May 6, 2020
- 112. Karch A, Koch A, Zapf A, Zerr I, Karch A. Partial verification bias and incorporation bias affected accuracy estimates of diagnostic studies for biomarkers that were part of an existing composite gold standard. J Clin Epidemiol 2016;78:73–82. [DOI] [PubMed] [Google Scholar]
- 113. Lei P, Fan B, Mao J, Wang P. Multiple parameters required for diagnosis of COVID‐19 in clinical practice. J Infect 2020;80:e27–e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Glasziou P, Irwig L, Deeks JJ. When should a new test become the current reference standard? Ann Intern Med 2008;149:816–21. [DOI] [PubMed] [Google Scholar]
- 115. Carpenter CR. Understanding bias in diagnostic research. In: Pines JM, Carpenter CR, Raja AS, Schuur JD, editors. Evidence‐Based Emergency Care: Diagnostic Testing and Clinical Decision Rules. 2nd ed. London: John Wiley & Sons, 2013. pp. 54–64. [Google Scholar]
- 116. Xiao AT, Tong YX, Zhang S. Profile of RT‐PCR for SARS‐CoV‐2: a preliminary study from 56 COVID‐19 patients. Clin Infect Dis 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. To KK, Tsang OT, Leung WS, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS‐CoV‐2: an observational cohort study. Lancet Infect Dis 2020;20:565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Leeflang MM, Rutjes AW, Reitsma JB, Hooft L, Bossuyt PM. Variation of a test's sensitivity and specificity with disease prevalence. CMAJ 2013;185:E537–E544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Inui S, Fujikawa A, Jitsu M, et al. Chest CT findings in cases from the cruise ship “Diamond Princess” with coronavirus disease 2019 (COVID‐19). Radiol Cardiothorac Imaging 2020;2:e200110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Gallo L, Hua N, Mercuri M, Silveira A, Worster A. Adherence to standards for reporting diagnostic accuracy in emergency medicine research. Acad Emerg Med 2017;24:914–9. [DOI] [PubMed] [Google Scholar]
- 121. Carpenter CR, Meizel Z. Overcoming the Tower of Babel in medical science by finding the "EQUATOR": research reporting guidelines. Acad Emerg Med 2017;24:1030–3. [DOI] [PubMed] [Google Scholar]
- 122. Hunter BR, Carpenter CR. The development of clinical prediction rules. In: Wilson MP, Guluma K, Hayden SR, editors. Doing Research in Emergency and Acute Care: Making Order Out of Chaos. Oxford: Wiley‐Blackwell, 2016. pp. 139–47. [Google Scholar]
- 123. Kline JA, Courtney DM, Kabrhel C, et al. Prospective multicenter evaluation of the pulmonary embolism rule‐out criteria. J Thromb Haemost 2008;6:772–80. [DOI] [PubMed] [Google Scholar]
- 124. Wynants L, Van Calster B, Bonten MM, et al. Prediction models for diagnosis and prognosis of covid‐19 infection: systematic review and critical appraisal. BMJ 2020;369:m1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Ji D, Zhang D, Xu J, et al. Prediction for progression risk in patients with COVID‐19 pneumonia: the CALL score. Clin Infect Dis 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Gong J, Ou J, Qiu X, et al. A tool to early predict severe corona virus disease 2019 (COVID‐19): a multicenter study using the risk nomogram in Wuhan and Guangdong, China. Clin Infect Dis 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Hu H, Yao N, Qiu Y. Comparing rapid scoring systems in mortality prediction of critically ill patients with novel coronavirus disease. Acad Emerg Med 2020;27:461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Brown MD, Reeves MJ. Interval likelihood ratios: Another advantage for the evidence‐based diagnostician. Annals of Emergency Medicine 2003; 42:292–7. [DOI] [PubMed] [Google Scholar]
- 129. Desai AN, Aronoff DM. Masks and coronavirus disease 2019 (COVID‐19). JAMA 2020;323:2103. [DOI] [PubMed] [Google Scholar]
- 130. Desai AN, Patel P. Stopping the spread of COVID‐19. JAMA 2020;323:1516. [DOI] [PubMed] [Google Scholar]
- 131. Sepucha KR, Breslin M, Graffeo C, Carpenter CR, Hess EP. State of the science: tools and measurement for shared decision making. Acad Emerg Med 2016;23:1325–31. [DOI] [PubMed] [Google Scholar]
- 132. Pascarella G, Strumia A, Piliego C, et al. COVID‐19 diagnosis and management: a comprehensive review. J Intern Med. 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Marcotte LM, Liao JM. Incorporating Test Characteristics Into SARS‐CoV‐2 Testing Policy—Sense and Sensitivity. JAMA Health Forum Web site. 2020. Available at: https://jamanetwork.com/channels/health‐forum/fullarticle/2764750. Accessed Jun 2, 2020 [DOI] [PubMed]
- 134. Bachelet VC. Do we know the diagnostic properties of the tests used in COVID‐19? A rapid review of recently published literature. Medwave 2020;20:e7890. [DOI] [PubMed] [Google Scholar]
- 135. Elshafeey F, Magdi R, Hindi N, et al. A systematic scoping review of COVID‐19 during pregnancy and childbirth. Int J Gynaecol Obstet 2020;150:47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Malone ML, Hogan TM, Perry A, et al.COVID‐19 in Older Adults: Key Points for Emergency Department Providers. Geriatric Emergency Department Collaborative. Journal of Geriatric Emergency Medicine Web Site. 2020. Available at: https://gedcollaborative.com/article/covid‐19‐in‐older‐adults‐key‐points‐for‐emergency‐department‐providers/. Accessed May 9, 2020
- 137. Whiting PF, Rutjes AW, Westwood MD, et al. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529–36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Supplement S1. Appendix.


