Recently, Baumann et al. 1 described the characteristics and outcomes of four patients with chronic lymphocytic leukaemia (CLL) diagnosed with symptomatic COVID‐19. The course of the disease was mild, and no patient required admission in an intensive care unit. The authors speculate that the CLL‐related immunodeficiency might be beneficial in the outcome of COVID‐19 and deserved further investigation. No specific serological testing information was provided. The American Society of Hematology (ASH) has published recommendations on the prevention of COVID‐19 in patients with CLL but again not on serological investigations. 2
However, in the context of COVID‐19, serological testing is a valuable strategy for the diagnosis and the characterization of the course of the disease, for identifying convalescent plasma donors, for epidemiological studies as well as for lockdown exit programmes and COVID‐19 vaccine development. 3 , 4 , 5 Our group recently reported the validation of an electrochemiluminescence immunoassay (ECLIA) for determination of anti‐SARS‐CoV‐2 total antibodies (Elecsys®, Roche Diagnostics®, Bale, Switzerland) and showed excellent analytical and clinical performance of the assay (95·1% of sensitivity and 100% of specificity), if using an optimized cut‐off [i.e. >0·165 cut‐off index (COI)]. 6 In our cohort of COVID‐19 patients, two presented with CLL. Both patients required hospitalization in intensive care units, received hydroxychloroquine for 5 days, and recovered after 18 and 40 days respectively. A control group composed of nine patients nonsuffering from B‐cells abnormalities (median age: 79) was included. Among them, seven required hospitalization, all recovered after a mean time of 13·8 days (min‐max = 6–24) and five received hydroxychloroquine for 5 days. In this group, total antibodies increased rapidly, were all upper the cut‐off by day 14 and remain high overtime; as expected (Fig 1). In comparison, the two patients with CLL showed a different kinetic profile of total antibodies.
Fig 1.
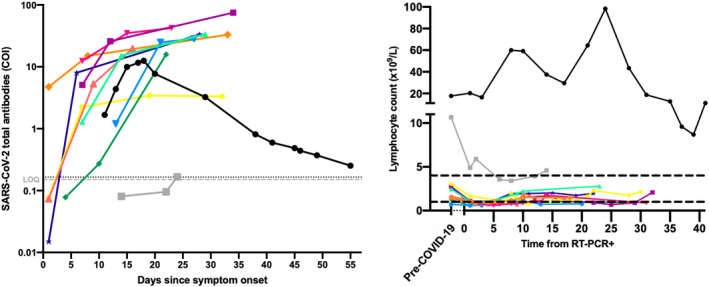
(A) Seroconversion in 11 patients with serial samples (one colour per patient). Optimized cut‐off (i.e. >0·165) and the limit of quantification (LOQ) of the assay (i.e. >0·151) are represented with black‐ and grey‐dotted lines respectively. (B) Lymphocyte kinetics in 11 patients with serial samples (one colour per patient) from reverse transcription polymerase chain reaction (RT‐PCR) positivity. Reference interval is represented with black dotted lines.
The first patient (grey outline) was tested positive for reverse transcription polymerase chain reaction (RT‐PCR) 11 days after symptom onset and had COI values of 0·081, 0·100 and 0·167 at day 14, 22 and 24 since symptom onset respectively. The latter COI value was higher than the optimized cut‐off (i.e. 0·165 COI) meaning that by using the cut‐off of the manufacturer, this patient would not have been considered positive for SARS‐CoV‐2 antibodies. 6 To confirm our classification for positivity and the kinetics observed, these samples were analyzed on another platform (iFlash1800 from YHLO Biotechnology Co., LTD, Shenzhen, China) for specific SARS‐CoV‐2 IgG determination. The negative determination on day 14 was confirmed on the iFlash1800 (i.e. 0·67 AU/ml; manufacturer cut‐off = 10 AU/ml). At days 22 and 24, samples become positive, with increasing antibody titers (22·65 and 110·53 AU/ml) confirming the late antibody kinetics observed. This patient is known with CLL (Binet group A). The patient presented hypogammaglobulinaemia with serum IgG (6·5 g/l) lower than the age‐matched mean [11·5 g/l, reference interval (RI) 7·0–16·0 g/l]. The gamma fraction of the serum electrophoresis also presented low values (values during hospitalization 5·5–7·3 g/l; RI 8·0–13·5 g/l). D‐dimer (up to 2·2 µg/ml; RI < 0·5 µg/ml), C‐reactive protein (CRP) (up to 117·8 mg/dl; RI < 5 mg/dl), creatinine (up to 1·1 mg/dl; RI 0·3–0·9 mg/dl), lactate dehydrogenase (LDH) (up to 389 U/l; RI < 250 U/l) and white blood cells (WBC) (up to 11·3 × 109/l; RI 4·0–10 × 109/l) were increased, and haemoglobin was found to be low (up to 110 g/l; RI 120–160 g/l). We observed a lymphocyte decrease from 10·7 × 109/l (pre‐COVID‐19) to 4·9 × 109/l (at admission) (Fig 1). All these features were associated with COVID‐19 disease. 7 In the nine control patients, a decrease in lymphocyte count following SARS‐CoV‐2 infection was also observed. The patient recovered and was discharged 18 days after admission on May 27, 2020. Unfortunately, no more follow‐up samples were obtained. This case highlights the importance of performing at least two anti‐SARS‐CoV‐2 determinations in case of COVID‐19 symptoms to identify late antibody onset, an observation which strengthens the recommendations made by the Center for Disease Control and Prevention (CDC).
The profile of the second patient (black outline) was also peculiar by presenting a rise of antibody until 18 days after symptom onset followed by a continuous drop of total antibodies until day 55. The patient was tested positive for RT‐PCR 11 days after symptom onset and the RT‐PCR turned out negative at day 41. At days ≥38 and based on the optimized cut‐off (i.e. >0·165 COI), the patient was still considered positive. Four samples of this patient were also analysed on the iFlash1800. Results were consistent with the rise and fall pattern observed on the Elecsys platform with IgG/M values of 4·90/1·10, 10·16/3·69, 21·76/7·00 and 14·68/6·25 AU/ml at days 11, 15, 17 and 28 respectively. Tang et al. 8 also observed a tendency towards a decrease in antibody signals in five patients and speculated about the presence of lower‐affinity IgM antibody binding. However, IgM, as analysed on the iFlash1800 platform, remained negative in our study (manufacturer cut‐off: 10·00 AU/ml). This second patient is also known with CLL (Binet group A). The patient had a serum IgG (4·9 g/l) lower than the age‐matched mean [11·5 g/l, reference interval (RI) 7–16 g/l]. The gamma fraction of the serum electrophoresis also presented low values (values during hospitalization 5·0–7·1 g/l; reference interval 8·0–13·5 g/l). As observed for the first patient, D‐dimer (up to 3·8 µg/ml), CRP (up to 335 mg/dl), creatinine (up to 1·3 mg/dl), LDH (up to 639 U/l) and WBC (up to 76 × 109/l) were increased, and Hb was low (up to 74 g/l). We observed a massive lymphocyte increase up to 98·7 × 109/l at day 24 (Fig 1). Lymphocyte counts then decreased to 9·6, 8·7 and 11·2 × 109/l at day 38, 40 and 42 respectively. Pre‐COVID‐19 lymphocyte count (i.e. before December 2019) was 17·6 × 109/l. No more samples were available for this patient to assess if further COI values will pass below the cut‐off (i.e. becoming negative). The patient recovered and was discharged on May 16, 2020.
Chronic lymphocytic leukaemia is a disease related to a profound immunodeficiency 9 and characterized by hypogammaglobulinaemia in up to 85% of CLL patients. 10 , 11 These particular antibody kinetics in patients suffering from CLL remain unclear. It could be speculated that the related immunodeficiency is part of the explanation. This immunodeficiency is caused by a combination of increased numbers of regulatory T cells and nurse‐like cells, and impaired natural killer cells and T‐cell function. 9 However, the mechanisms by which immunodeficiency develops and evolves in B‐cell abnormalities are still not clear 10 , 11 but it is known that CLL patients are more susceptible to infection. 11 , 12
Several studies aiming at studying the prevalence of COVID‐19 in CLL, its clinical characteristics, optimal therapy and outcome are also needed. These cases also show that interpretation of anti‐SARS‐CoV‐2 antibodies data can be challenging and that further serological investigations are needed in more powered studies as well in patients with heamatological abnormalities. Such information will be of particular interest in the development of COVID‐19 vaccines since vaccination plans may need to consider the seropositivity status in the general population but also in some specific patient groups.
Author contributions
FJ, CE, ME, JD, JMD performed the research; FJ, CE, ME, JD, JMD designed the research study, Roche Diagnostics contributed essential reagents or tools; FJ, CE, ME, CG, KL, PG, FJ, JD and JMD analysed the data; FJ, JD and JMD wrote the paper.
Conflict of interest
Among the authors, Jonathan Douxfils is chief executive officer and founder of QUALIblood sa and reports personal fees from Diagnostica Stago, Roche, Roche Diagnostics, Daiichi‐Sankyo and Portola, outside the submitted work. Roche Diagnostics generously provided the kits for the validation.
Acknowledgements
We wish to thank the personnel of the Saint‐Luc Bouge laboratory for its technical assistance.
References
- 1. Baumann T, Delgado J, Montserrat E. CLL and COVID‐19 at the Hospital Clinic of Barcelona: an interim report. Leukemia. 2020. 10.1038/s41375-020-0870-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. COVID‐19 and CLL: Frequently Asked Questions. https://www.hematology.org/covid‐19/covid‐19‐and‐cll Accessed June 4, 2020.
- 3. Winter AK, Hegde ST. The important role of serology for COVID‐19 control. Lancet Infect Dis. 2020. 10.1016/S1473-3099(20)30322-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Long QX, Liu BZ, Deng HJ, Wu GC, Deng K, Chen YK, et al. Antibody responses to SARS‐CoV‐2 in patients with COVID‐19. Nat Med. 2020;26(6):845–8. [DOI] [PubMed] [Google Scholar]
- 5. Farnsworth CW, Anderson NW. SARS‐CoV‐2 serology: much hype, little data. Clin Chem. 2020. 10.1093/clinchem/hvaa107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Favresse J, Eucher C, Elsen M, Marie TH, Dogne JM, Douxfils J. Clinical performance of the Elecsys electrochemiluminescent immunoassay for the detection of SARS‐CoV‐2 total antibodies. Clin Chem. 2020. 10.1093/clinchem/hvaa131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lippi G, Plebani M. Laboratory abnormalities in patients with COVID‐2019 infection. Clin Chem Lab Med. 2020;58(7):1131–4. [DOI] [PubMed] [Google Scholar]
- 8. Tang MS, Hock KG, Logsdon NM, Hayes JE, Gronowski AM, Anderson NW, et al. Clinical performance of the Roche SARS‐CoV‐2 serologic assay. Clin Chem. 2020. 10.1093/clinchem/hvaa132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Riches JC, Gribben JG. Understanding the immunodeficiency in chronic lymphocytic leukemia: potential clinical implications. Hematol Oncol Clin North Am. 2013;27:207–35. [DOI] [PubMed] [Google Scholar]
- 10. Dhalla F, Lucas M, Schuh A, Bhole M, Jain R, Patel SY, et al. Antibody deficiency secondary to chronic lymphocytic leukemia: should patients be treated with prophylactic replacement immunoglobulin? J Clin Immunol. 2014;34:277–82. [DOI] [PubMed] [Google Scholar]
- 11. Levinson AI. The impact of malignancy on adaptive immunity. Stiehm's Immune Deficiencies. Cambridge, MA: Academic Press. 2014; p. 875–87. [Google Scholar]
- 12. Kalpadakis C, Pangalis GA, Sachanas S, Vassilakopoulos TP, Kyriakaki S, Korkolopoulou P, et al. New insights into monoclonal B‐cell lymphocytosis. Biomed Res Int. 2014;2014:258917. [DOI] [PMC free article] [PubMed] [Google Scholar]


