Abstract
Background
Little is known about the incidence and risk of intensive care unit (ICU)‐acquired bloodstream infections (BSI) in critically ill patients with coronavirus disease 2019 (COVID‐19).
Materials and methods
This retrospective, single‐centre study was conducted in Northern Italy. The primary study objectives were as follows: (a) to assess the incidence rate of ICU‐acquired BSI and (b) to assess the cumulative risk of developing ICU‐acquired BSI.
Results
Overall, 78 critically ill patients with COVID‐19 were included in the study. Forty‐five episodes of ICU‐acquired BSI were registered in 31 patients, with an incidence rate of 47 episodes (95% confidence interval [CI] 35‐63) per 1000 patient‐days at risk. The estimated cumulative risk of developing at least one BSI episode was of almost 25% after 15 days at risk and possibly surpassing 50% after 30 days at risk. In multivariable analysis, anti‐inflammatory treatment was independently associated with the development of BSI (cause‐specific hazard ratio [csHR] 1.07 with 95% CI 0.38‐3.04 for tocilizumab, csHR 3.95 with 95% CI 1.20‐13.03 for methylprednisolone and csHR 10.69 with 95% CI 2.71‐42.17 for methylprednisolone plus tocilizumab, with no anti‐inflammatory treatment as the reference group; overall P for the dummy variable = 0.003).
Conclusions
The incidence rate of BSI was high, and the cumulative risk of developing BSI increased with ICU stay. Further study will clarify if the increased risk of BSI we detected in COVID‐19 patients treated with anti‐inflammatory drugs is outweighed by the benefits of reducing any possible pro‐inflammatory dysregulation induced by SARS‐CoV‐2.
Keywords: BSI, coronavirus, COVID‐19, SARS‐CoV‐2, steroid, tocilizumab
1. BACKGROUND
In a very few months, coronavirus disease 2019 (COVID‐19) has become pandemic, and several countries worldwide are currently dealing with unprecedented epidemic foci of severe acute respiratory infection, a possible presentation of COVID‐19 that may require intensive care unit (ICU) admission and carries a high case fatality rate. 1 , 2 , 3 , 4 , 5
While the demographics, clinical characteristics and overall survival of patients with COVID‐19 admitted to ICU have been already extensively characterized by large reports from several parts of the word, little is still known about nonviral infectious complications such as bacterial or fungal bloodstream infections (BSI) that may participate in adversely influencing the outcome of any ICU‐admitted patient. 6 , 7
In the present study, we aimed to retrospectively assess the incidence rate, cumulative risk, predictors and survival of ICU‐acquired BSI in patients with COVID‐19 admitted to two ICUs in a large teaching hospital in Northern Italy, one of the most affected areas in Europe to date. 8
2. METHODS
This retrospective study was conducted in two ICUs (27 and 12 beds, respectively) at Ospedale Policlinico San Martino—IRCCS, a 1200‐bed teaching hospital in Northern Italy. From 20 February to 10 April 2020, all patients with COVID‐19 admitted to the participating ICUs for >48 hours were included in the study. The predefined primary study objectives were as follows: (a) to assess the incidence rate of ICU‐acquired BSI and (b) to assess the cumulative risk of developing ICU‐acquired BSI. Predefined secondary objectives were as follows: (a) to describe the clinical characteristics of ICU‐acquired BSI; (b) to assess predictors of ICU‐acquired BSI; and (c) to describe survival of ICU‐acquired BSI. The collection of anonymized data for the present study was approved by the Ethics Committee of the Liguria Region (registry number 163/2020). Specific informed consent was waived due to the retrospective nature of the study. Reporting of the study conforms to broad EQUATOR guidelines. 9
2.1. Definitions
COVID‐19 was defined in the presence of a positive real‐time polymerase chain reaction (RT‐PCR) for SARS‐CoV‐2 on at least one respiratory specimen (nasopharyngeal swab, sputum and/or lower respiratory tract specimens). ICU‐acquired BSI was defined in the presence of at least one positive blood culture for bacteria or fungi, drawn at >48 hours after ICU admission. For coagulase‐negative staphylococci and other common skin contaminants, at least two consecutive blood cultures positive for the same pathogen were necessary to define BSI. 10 In patients with multiple blood cultures positive for the same organism, novel BSI events were considered as independent if occurring at least 30 days after the last previous positive blood culture. Polymicrobial infections were considered as separate BSI events, one for each causative organism isolated from blood culture.
2.2. Data collection
The following data were collected from the patients’ electronic medical records as baseline data at the time of ICU admission: age in years, gender, hypertension, diabetes mellitus, respiratory disease (defined as asthma or chronic obstructive pulmonary disease), end‐stage renal disease (defined as estimated glomerular filtration rate <15 mL/min/1.73 m2), moderate‐to‐severe liver disease (defined as compensated or decompensated liver cirrhosis), solid cancer, haematological malignancy, human immunodeficiency virus infection, sequential organ failure assessment (SOFA) score at ICU admission 11 and antibiotic therapy (yes/no and type of administered antibiotic/s). Since they were constantly continued/started at ICU admission, possible off‐label anti‐inflammatory treatments for COVID‐19 (steroid treatment with intravenous methylprednisolone at 1 mg/kg once daily and/or intravenous tocilizumab at 8 mg/kg single administration or repeated once) were also recorded as dichotomic baseline variables (steroid: yes/no; tocilizumab: yes/no).
The following data were collected related to the onset of BSI episodes (ie they were collected the day when the first positive blood culture was drawn): presence of fever (defined as temperature >37.3°C), requirement of vasoactive agents, presence of acute kidney injury (defined as at least stage 1 of KDIGO [Kidney Disease: Improving Global Outcomes] classification of acute kidney injury 12 ), source of BSI (defined according to CDC/NHSN criteria 13 ), blood neutrophil count in cells × 10−3/mm3, blood platelet count in cells × 10−3/mm3, serum fibrinogen in g/L, serum lactate in mmol/L, serum C‐reactive protein in mg/L, serum procalcitonin in ng/mL, causative agents of BSI and susceptibility test results (the VITEK‐2 automated system, bioMérieux, Marcy l’Etoile, France, was used for isolate identification and antimicrobial susceptibility testing). Finally, the date of the following was collected, whichever came first: death in the ICU and discharge from the ICU.
2.3. Statistical analysis
No sample size calculations were performed a priori for this exploratory, descriptive study. The incidence rate of ICU‐acquired BSI in the study population was calculated as the number of events per 1000 patient‐days at risk (defined as the cumulative days of stay elapsed from 48 hours after ICU admission to death, discharge from ICU or end of the study period, whichever came first). The 95% confidence interval (CI) for the incidence rate estimate was obtained using the mid‐P exact test. 14 The cumulative risk of ICU‐acquired BSI was calculated using the Aalen‐Johansen method, considering the first occurring BSI as the event of interest, death and discharge from the ICU as competing events, and length of ICU stay equal to 30 days or end of the study period (10 Apr 2020) as right‐censoring events. 15 The time of origin was set at 48 hours after ICU admission.
With regard to secondary analyses, demographic and clinical characteristics of BSI episodes were summarized with number and percentages for categorical variables and with median and interquartile range (IQR) for continuous variables. The possible association of demographic and clinical variables with the development of BSI (dependent variable: first occurring ICU‐acquired BSI) was first tested in univariable Cox regression models for estimating the unadjusted cause‐specific hazard ratio (csHR) for the development of BSI. Then, variables potentially associated with the development of BSI in univariable comparisons (P < .10) were included in a multivariable Cox regression model for calculating the adjusted csHR for the development of BSI. Finally, survival of patients with ICU‐acquired BSI was described with the Kaplan‐Meier method, with death as the event of interest and discharge from ICU or the end of the study period (10 Apr 2020) as right‐censoring events. The time of origin was the day when the first positive blood culture of the first occurring BSI episode was drawn.
Statistical analyses were performed using R Statistical Software (version 3.6.0, R Foundation for Statistical Computing, Vienna, Austria) and SPSS Statistics version 21.0 (IBM Corp., Armonk, NY, USA).
3. RESULTS
During the study period, 78 patients with COVID‐19 admitted to ICU for >48 hours were included in the study. Their median age was 66 years (IQR 57‐70), and 77% were males (60/78). Their baseline demographic and clinical characteristics are summarized in Table 1. Of them, 26% (20/78) died in the ICU, 29% (23/78) were discharged from the ICU, and 45% (35/78) were still hospitalized in the ICU at the end of the study period.
TABLE 1.
Characteristics of 78 critically ill patients with COVID‐19
| Variable |
Total 78 (100) |
|---|---|
| Demographics | |
| Age in years, median (IQR) | 66 (57‐70) |
| Male gender | 60 (77) |
| Comorbidities | |
| Diabetes mellitus | 14 (18) |
| Hypertension | 35 (45) |
| Respiratory disease a | 10 (13) |
| End‐stage renal disease b | 0 (0) |
| Moderate/severe liver failure c | 3 (4) |
| Solid cancer | 4 (5) |
| Haematological malignancy | 1 (1) |
| HIV infection | 0 (0) |
| Characteristics at ICU admission | |
| Hospital stay before ICU admission in days, median (IQR) | 2 (0‐5) |
| SOFA score, median (IQR) | 4 (3‐5) |
| Antibiotic therapy d | 75 (96) |
| Anti‐inflammatory treatment | |
| Methylprednisolone | 24 (31) |
| Tocilizumab | 18 (23) |
| Both of them | 14 (18) |
| None of them | 22 (28) |
Results are reported as number of patients (%) unless otherwise indicated.
Abbreviations: COVID‐19, coronavirus disease 2019; HIV, human immunodeficiency virus; ICU, intensive care unit; IQR, interquartile range; SOFA, sequential organ failure assessment.
Defined as asthma or chronic obstructive pulmonary disease
Defined as estimated glomerular filtration rate <15 mL/min/1.73 m2
Defined as compensated or decompensated liver cirrhosis
Ceftaroline (n = 59), levofloxacin (n = 3), ceftobiprole (n = 2), ceftriaxone plus azithromycin (n = 2), linezolid plus meropenem (n = 2), ceftriaxone (n = 1), ceftolozane/tazobactam (n = 1), ceftaroline plus azithromycin (n = 1), piperacillin/tazobactam plus azithromycin (n = 1), ceftaroline plus meropenem plus tigecycline (n = 1), linezolid plus daptomycin plus ceftolozane/tazobactam (n = 1) and linezolid plus meropenem plus amikacin (n = 1)
Overall, 45 episodes of ICU‐acquired BSI were registered in 31 patients, with an incidence rate of 47 episodes (95% confidence interval [CI] 35‐63) per 1000 patient‐days at risk. The clinical and laboratory characteristics of the 45 episodes of ICU‐acquired BSI are detailed in Table 2. As shown in the table, the lowest prevalence of fever (6/16, 38%) was observed in COVID‐19 patients treated with tocilizumab, whereas the highest was observed in those receiving neither tocilizumab nor steroid (9/10, 90%). Serum C‐reactive protein values were also the lowest among tocilizumab‐treated patients (median 20.6 mg/L, IQR 8.4‐33.6 mg/L) and the highest among those receiving neither tocilizumab nor steroid (median 169 mg/L, IQR 70.4‐194.0 mg/L). The most frequent causative agents of ICU‐acquired BSI were coagulase‐negative staphylococci (11/45, 24%), followed by Enterococcus faecalis (8/45, 18%) and Staphylococcus aureus (6/45, 13%). As shown in Figure 1, the estimated cumulative risk of developing at least one ICU‐acquired BSI episode was of almost 25% after 15 days at risk and possibly surpassing 50% after 30 days a risk.
TABLE 2.
Characteristics of 45 ICU‐acquired BSI episodes in 31 critically ill patients with COVID‐19
| Total no of episodes (n = 45) | Episodes in patients treated with tocilizumab (n = 16) | Episodes in patients treated with steroid (n = 12) | Episodes in patients treated with tocilizumab and steroid (n = 7) | Episodes in patients treated with neither tocilizumab nor steroid (n = 10) | |
|---|---|---|---|---|---|
| Fever (temperature > 37.3°C), n (%) | 24 (53) | 6 (38) | 5 (42) | 4 (57) | 9 (90) |
| Requirement of vasoactive agents, n (%) | 15 (33) | 5 (31) | 3 (25) | 1 (14) | 6 (60) |
| Acute kidney injury a | 9 (2) | 2 (13) | 5 (42) | 0 (0) | 2 (20) |
| Source of BSI b | |||||
| Unknown | 29 (64) | 6 (38) | 11 (92) | 3 (43) | 9 (90) |
| Lower respiratory tract | 10 (22) | 4 (25) | 1 (8) | 4 (57) | 1 (10) |
| Urinary tract | 2 (4) | 2 (13) | 0 (0) | 0 (0) | 0 (0) |
| CVC‐related | 4 (9) | 4 (25) | 0 (0) | 0 (0) | 0 (0) |
| Laboratory results | |||||
| Blood neutrophil count, cell × 10‐3/mm3 | 10.8 (8.1‐14.8) | 8.9 (5.5‐14.7) | 13.4 (8.6‐16.0) | 12.4 (8.7‐16.0) | 10.4 (6.4‐14.5) |
| Blood platelet count, cell × 10‐3/mm3 | 247 (192‐332) | 234 (191‐355) | 260 (188‐445) | 208 (190‐300) | 244 (184‐294) |
| Serum lactate, mmol/L | 1.2 (0.8‐1.6) | 1.3 (1.0‐2.1) | 1.2 (0.8‐1.5) | 1.2 (0.7‐1.4) | 1.1 (0.6‐1.3) |
| Serum fibrinogen, g/L | 4.3 (2.7‐6.5) | 2.8 (2.2‐3.1) | 5.9 (4.3‐8.0) | 4.4 (2.1‐5.4) | 6.5 (4.3‐9.2) |
| Serum C‐reactive protein, mg/L | 44.6 (11.3‐137.0) | 20.6 (8.4‐33.6) | 105.2 (54.0‐164.0) | 43.7 (2.9‐120.0) | 169.0 (70.4‐194.0) |
| Serum procalcitonin | 0.3 (0.1‐1.2) | 0.1 (0.0‐0.3) | 0.9 (0.2‐2.3) | 0.1 (0.1‐0.2) | 1.2 (0.4‐1.9) |
| Causative agent, n (%) | |||||
| Coagulase‐negative staphylococci | 11 (24) | 4 (25) | 4 (33) | 1 (14) | 2 (20) |
| Staphylococcus aureus c | 6 (13) | 4 (25) | 1 (8) | 1 (14) | 0 (0) |
| Enterococcus faecalis d | 8 (18) | 3 (19) | 2 (17) | 1 (14) | 2 (20) |
| Enterococcus faecium e | 4 (9) | 2 (13) | 1 (8) | 1 (14) | 0 (0) |
| Streptococcus pneumoniae f | 1 (2) | 0 (0) | 1 (8) | 0 (0) | 0 (0) |
| Viridans group streptococci | 3 (7) | 0 (0) | 1 (8) | 0 (0) | 2 (20) |
| Pseudomonas aeruginosa g | 2 (4) | 0 (0) | 0 (0) | 1 (14) | 1 (10) |
| Enterobacter aerogenes h | 4 (9) | 0 (0) | 1 (8) | 1 (14) | 2 (20) |
| Escherichia coli h | 1 (2) | 1 (6) | 0 (0) | 0 (0) | 0 (0) |
| Proteus mirabilis h | 1 (2) | 0 (0) | 0 (0) | 1 (14) | 0 (0) |
| Candida spp. i | 3 (7) | 2 (13) | 0 (0) | 0 (0) | 1 (0) |
| Prevotella spp. | 1 (2) | 0 (0) | 1 (8) | 0 (0) | 0 (0) |
Results are reported as median (IQR) unless otherwise indicated.
Abbreviation: IQR, interquartile range.
Defined as at least stage 1 of KDIGO (Kidney Disease: Improving Global Outcomes) stages of acute kidney injury. 12
Defined according to CDC/NHSN criteria. 13
0/6 S aureus were methicillin‐resistant (0%).
0/8 E faecalis were ampicillin‐resistant (0%); none were vancomycin‐resistant (0%).
4/4 E faecium were ampicillin‐resistant (100%); 1/4 were vancomycin‐resistant (25%).
0/1 S pneumoniae were ceftriaxone‐resistant (0%).
0/2 P aeruginosa were piperacillin/tazobactam‐resistant, ceftazidime‐resistant and/or carbapenem‐resistant (0%).
2/6 members of the Enterobacterales were piperacillin/tazobactam‐resistant, ceftazidime‐resistant and/or carbapenem‐resistant (33%)
C albicans (n = 1), C tropicalis (n = 1), C parapsilosis (n = 1).
FIGURE 1.
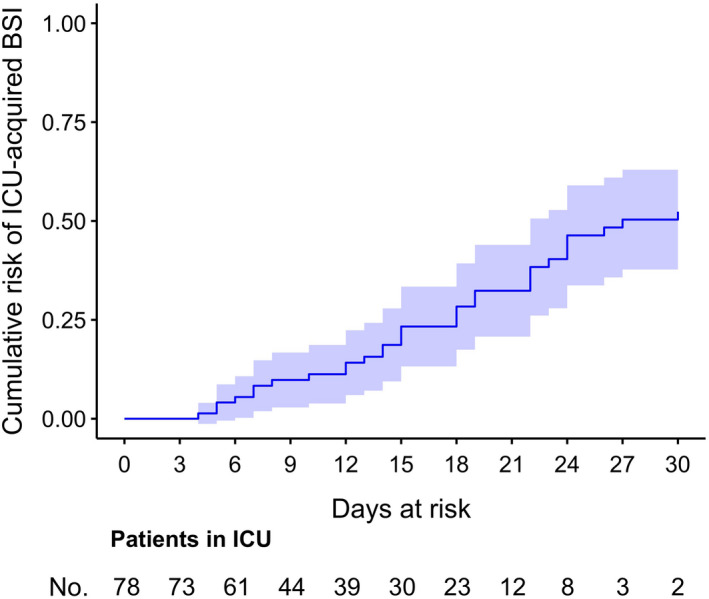
Cumulative risk of ICU‐acquired BSI in critically ill patients with COVID‐19. The cumulative risk of ICU‐acquired BSI in patients with COVID‐19 at different lengths of ICU stay was estimated using the Aalen‐Johansen method, with the first occurring ICU‐acquired BSI as the event of interest and death and discharge from the ICU as competing events. Right censoring was applied in the following cases: (a) persistent ICU stay at the end of the study period and (b) persistent ICU stay at day + 30 after the time of origin (defined as 48 h after ICU admission, see study methods). BSI, bloodstream infection; COVID‐19, coronavirus disease 2019; ICU, intensive care unit
Univariable and multivariable analyses of factors potentially associated with the development of ICU‐acquired BSI are shown in Table 3. In univariable comparisons, the following variables were associated with the development of BSI: diabetes mellitus (csHR 2.76 with 95% CI 1.09‐6.98, P = .032) and anti‐inflammatory treatment (csHR 1.21 with 95% CI 0.44‐3.30 for tocilizumab, csHR 4.48 with 95% CI 1.38‐14.56 for methylprednisolone and csHR 10.84 with 95% CI 2.79‐42.08 for methylprednisolone plus tocilizumab, with no anti‐inflammatory treatment as the reference group; overall p for the dummy variable = 0.002). In multivariable analysis, only anti‐inflammatory treatment (csHR 1.07 with 95% CI 0.38‐3.04 for tocilizumab, csHR 3.95 with 95% CI 1.20‐13.03 for methylprednisolone and csHR 10.69 with 95% CI 2.71‐42.17 for methylprednisolone plus tocilizumab; overall p for the dummy variable = 0.003) retained an independent association with the development of BSI.
TABLE 3.
Univariable and multivariable analyses of risk factors for the development of ICU‐acquired BSI in critically ill patients with COVID‐19 a
| Variable | Unadjusted cause‐specific HR (95% CI) | P | Adjusted cause‐specific HR (95% CI) | P |
|---|---|---|---|---|
| Age in years | 1.00 (0.96‐1.04) | .970 | ||
| Male gender | 1.60 (0.65‐3.94) | .304 | ||
| Diabetes mellitus | 2.76 (1.09‐6.98) | .032 | 2.22 (0.80‐6.20) | .127 |
| Hypertension | 0.89 (0.43‐1.85) | .755 | ||
| Respiratory disease | 1.87 (0.54‐6.43) | .323 | ||
| Moderate/severe liver failure | 6.71 (0.77‐58.29) | .084 | 6.36 (0.59‐68.39) | .127 |
| Solid cancer | 2.71 (0.35‐20.99) | .340 | ||
| Haematological malignancy | b | ‐ | ||
| Hospital stay before ICU admission in days | 1.02 (0.98‐1.08) | .339 | ||
| SOFA score | 1.00 (0.80‐1.25) | .996 | ||
| Antibiotic therapy | b | ‐ | ||
| Anti‐inflammatory treatment | ||||
| Methylprednisolone | 4.48 (1.38‐14.56) | .002 | 3.95 (1.20‐13.03) | .003 |
| Tocilizumab | 1.21 (0.44‐3.30) | 1.07 (0.38‐3.04) | ||
| Both of them | 10.84 (2.79‐42.08) | 10.69 (2.71‐42.17) | ||
| None of them | (ref) | (ref) | ||
Abbreviations: CI, confidence intervals; COVID‐19, coronavirus disease 2019; HR, hazard ratio; ICU, intensive care unit; SOFA, sequential organ failure assessment.
The variables end‐stage renal disease and human immunodeficiency virus (HIV) infection were not included in the model since they were not detected in the study population.
Univariable Cox regression model not converging in the presence of very small samples (n = 1 for patients with haematological malignancies and n = 3 for patients without antibiotic therapy at ICU admission)
As shown in Figure 2, the estimated survival of ICU‐acquired BSI was of almost 75% after 24 days (no longer follow‐up was available) from the first positive blood culture, although with the important limitation that follow‐up after day 12 was available for less than one third of patients at the time of this report.
FIGURE 2.
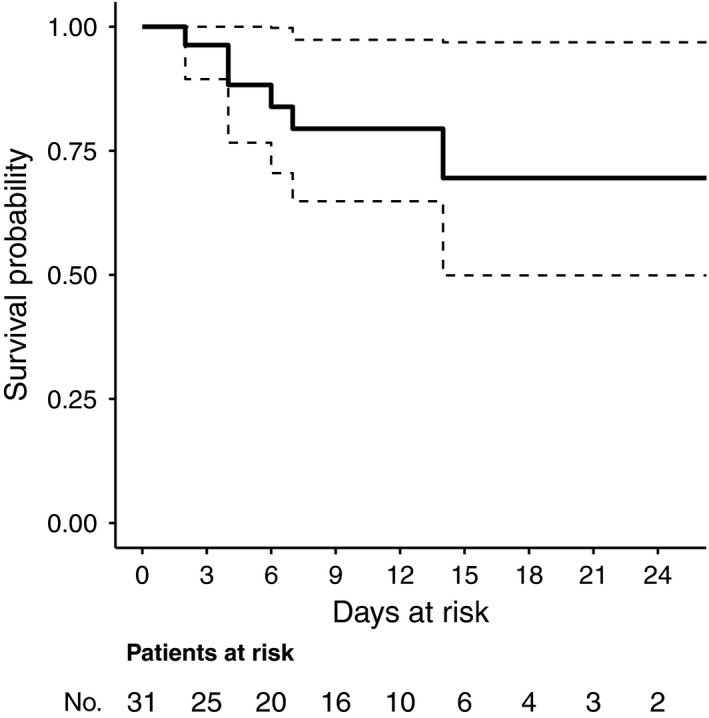
Survival of ICU‐acquired BSI in critically ill patients with COVID‐19. Survival of first occurring ICU‐acquired BSI in patients with COVID‐19 was estimated using the Kaplan‐Meier method. Right censoring was applied in the following cases: (a) discharge from the ICU and (b) persistent ICU stay at the end of the study period. The maximum registered follow‐up was of 24 d after the time of origin. The time of origin was defined as the day when the first positive blood culture of the first occurring BSI episode was drawn. BSI, bloodstream infection; COVID‐19, coronavirus disease 2019; ICU, intensive care unit
4. DISCUSSION
In the present study, we estimated an incidence rate of 47 episodes of ICU‐acquired BSI per 1000 patient‐days at risk in critically ill patients with COVID‐19, which is higher than that of 5‐19 episodes per 1000 patient‐days registered in other heterogeneous, non‐COVID‐19 ICU populations (even if adjusting for the fact that we considered only patient‐days after 48 hours from ICU admission, see Methods). 6 , 16 , 17 , 18
It is also of note that our estimation of a cumulative 30‐day risk of developing BSI of more than 50% is in contrast with the low prevalence of BSI or other bacterial/fungal infections (1%‐12%) reported in other epidemiological reports from China and the United States. 2 , 19 , 20 In our opinion, this could be explained by different, nonmutually exclusive reasons. The first is that the number of patients under follow‐up in the ICU for more than 20 days was limited in our analysis; thus, any generalization of our 30‐day cumulative risk estimate should be made with due caution. On the other hand, the cumulative risk of almost 25% we estimated at day 15 (based on a larger portion of patients still under follow‐up) is already higher than the overall prevalence of BSI registered in critically ill COVID‐19 patients in earlier studies. 2 , 19 , 20 In this regard, a possible explanation may be the difficulty of diagnosing BSI in patients receiving anti‐inflammatory drugs, 21 which prompted us to collect blood cultures in any case of worsening general conditions in COVID‐19 patients, even in the absence of fever and increases in C‐reactive protein serum levels. In support of this approach, in the present study, for example, fever was detected only in 38% of patients with ICU‐acquired BSI previously treated with tocilizumab. Furthermore, serum C‐reactive protein levels were frequently low and other classical inflammatory markers were usually uncharacteristic, making it rather difficult to clinically recognize a BSI event. Therefore, we feel the total number of BSI in COVID‐19 patients may be underestimated wherever anti‐inflammatory drugs are administered, but collection of blood cultures remains based on classical clinical and laboratory indicators of BSI. Finally, and perhaps most importantly, we found an independent association between receipt of anti‐inflammatory agents and development of BSI. This effect seems to be mainly driven by steroids rather than tocilizumab, although it is worth noting that the highest instantaneous risk in Cox models was registered in patients receiving both steroid and tocilizumab. In general, such an unfavourable effect of anti‐inflammatory drugs on the risk of infection is long known. 22 , 23 , 24 What remains unknown in patients with COVID‐19 is as to whether this risk of infectious complications is balanced (or better, outweighed) by possible favourable effects of anti‐inflammatory drugs on relevant clinical outcomes. Indeed, the rational for administering anti‐inflammatory drugs in COVID‐19 patients is mostly based on the intention of attenuating the cytokine storm syndrome possibly prompted by SARS‐CoV‐2, but high‐level evidence supporting the efficacy of this practice is still unavailable, although randomized controlled trials are ongoing. 25
Another different finding from previous reports is that we found a higher prevalence of Gram‐positive than Gram‐negative organisms as causative agents of ICU‐acquired BSI in COVID‐19 patients. 19 Again, at least two different explanations may be considered. The first is that our sample of BSI events was larger than in previous reports, possibly depicting a more reliable estimate of the true distribution of the relative prevalence of the different causative agents. The second is that, borrowing from the observed microbiological epidemiology (higher prevalence of Gram‐positive organisms) in patients with severe influenza, 26 and considering the frequent inability we had in rapidly differentiating between primary viral pneumonia and bacterial pulmonary superinfection in ICU‐admitted COVID‐19 patients, most COVID‐19 patients in our centre were treated with an anti‐methicillin‐resistant S aureus cephalosporin (most frequently ceftaroline, see Table 1) at ICU admission. However, while on the one hand this may be in line with the unusual high relative prevalence of enterococcal BSI (because of the impaired activity of ceftaroline against Enterococcus spp., although possible in vitro activity against E faecalis has been reported 27 ), on the other hand, it is also true that coagulase‐negative staphylococci and S aureus were the other two most prevalent causative agents of BSI in our cohort. From this standpoint, conversely, the true relative prevalence of Gram‐positive organisms could be even higher than that registered in our cohort (because of the anti‐staphylococcal and anti‐pneumococcal activity of ceftaroline that could have reduced the prevalence, absolute and relative, of staphylococci and pneumococci as causative agents of BSI).
Finally, although we apparently observed an improved survival of BSI in comparison with previous large studies conducted in non‐COVID‐19 ICU patients, 7 it should be reminded that our sample size of BSI patients was limited (n = 31) and that follow‐up was very short. Consequently, any related conclusion should be drawn cautiously. Other important limitations of our study are its retrospective nature (mainly because of possible information and selection biases), its single‐centre nature that may impact generalization of results, and the lack of adequate power for exploring possible predictors of improved survival in COVID‐19 patients with ICU‐acquired BSI.
In conclusion, in critically ill patients with COVID‐19, the incidence rate of ICU‐acquired BSI was high and the cumulative risk of developing BSI increased with ICU stay. Diagnosis of BSI in tocilizumab‐treated COVID‐19 patients may prove difficult because of the frequently absent fever and reduced serum concentrations of classical inflammatory markers. Further study will clarify if the increased risk of BSI we detected in COVID‐19 patients treated with anti‐inflammatory drugs is outweighed by the benefits of reducing any possible pro‐inflammatory dysregulation of the host response induced by SARS‐CoV‐2.
CONFLICT OF INTEREST
Outside the submitted work, DR Giacobbe reports honoraria from Stepstone Pharma GmbH and unconditional grants from MSD Italia and Correvio Italia. Outside the submitted work, M. Bassetti has received funding for scientific advisory boards, travel and speaker honoraria from Angelini, Astellas, AstraZeneca, Basilea, Bayer, BioMèrieux, Cidara, Correvio, Cubist, Menarini, Molteni, MSD, Nabriva, Paratek, Pfizer, Roche, Shionogi, Tetraphase, Thermo Fisher and The Medicine Company.
AUTHORS’ CONTRIBUTION
DRG made substantial contributions to the study concept and design, acquisition of data, analysis and interpretation of data, first drafting of the manuscript and critical revision of the manuscript for important intellectual content. MB and PP made substantial contributions to the study concept and design and critical revision of the manuscript for important intellectual content. DB, LT, LM, AS and AV made substantial contributions to the study concept and design, and analysis and interpretation of data. LM, BB, FC, GC, CD, LM, CR, AV and LT made substantial contributions to the acquisition of data and critical revision of the manuscript for important intellectual content. LB, IB, GI, ADM, ADB, AM, MM, NP and AO made substantial contributions to the interpretation of data and critical revision of the manuscript for important intellectual content. All authors read and approved the final manuscript.
ACKNOWLEDGEMENTS
We would like to thank the GECOVID study group.
Giacobbe DR, Battaglini D, Ball L, et al. Bloodstream infections in critically ill patients with COVID‐19. Eur J Clin Invest. 2020;50:e13319. 10.1111/eci.13319
DATA AVAILABILITY STATEMENT
The data sets used and/or analysed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Bassetti M, Vena A, Giacobbe DR. The novel Chinese coronavirus (2019‐nCoV) infections: challenges for fighting the storm. Eur J Clin Invest. 2020;50(3):e13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of Covid‐19 in New York City. N Engl J Med. 2020;382(24):2372‐2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected With SARS‐CoV‐2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239. [DOI] [PubMed] [Google Scholar]
- 5. Smyk W, Janik MK, Portincasa P, Milkiewicz P, Lammert F, Krawczyk M. COVID‐ 19: focus on the lungs but do not forget the gastrointestinal tract. Eur J Clin Invest. 2020;e13276. 10.1111/eci.13276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bassetti M, Giacobbe DR, Vena A, et al. Incidence and outcome of invasive candidiasis in intensive care units (ICUs) in Europe: results of the EUCANDICU project. Crit Care. 2019;23(1):219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tabah A, Koulenti D, Laupland K, et al. Characteristics and determinants of outcome of hospital‐acquired bloodstream infections in intensive care units: the EUROBACT International Cohort Study. Intensive Care Med. 2012;38(12):1930‐1945. [DOI] [PubMed] [Google Scholar]
- 8. European Centre for Disease Prevention and Control (ECDC) . COVID‐19 situation update worldwide as of 3 June 2020 [Accessed 3 Jun 2020]. Available at: https://www.ecdc.europa.eu/en/geographical‐distribution‐2019‐ncov‐cases
- 9. Simera I, Moher D, Hoey J, Schulz KF, Altman DG. A catalogue of reporting guidelines for health research. Eur J Clin Invest. 2010;40(1):35‐53. [DOI] [PubMed] [Google Scholar]
- 10. Elzi L, Babouee B, Vögeli N, et al. How to discriminate contamination from bloodstream infection due to coagulase‐negative staphylococci: a prospective study with 654 patients. Clin Microbiol Infect. 2012;18(9):E355‐E361. [DOI] [PubMed] [Google Scholar]
- 11. Vincent J‐L, Moreno R, Takala J, et al. The SOFA (Sepsis‐related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis‐Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707‐710. [DOI] [PubMed] [Google Scholar]
- 12. Kellum JA, Lameire N. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care. 2013;17(1):204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care‐associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309‐332. [DOI] [PubMed] [Google Scholar]
- 14. Rothman KJ, Boice JD Jr. Epidemiologic analysis with a programmable calculator. Bethesda: MD. NIH Publication; 1979. [Google Scholar]
- 15. Aalen OO, Johansen S. An empirical transition matrix for non‐homogeneous Markov chains based on censored observations. Scand J Stat. 1978;5(3):141‐150. [Google Scholar]
- 16. Hugonnet S, Sax H, Eggimann P, Chevrolet JC, Pittet D. Nosocomial bloodstream infection and clinical sepsis. Emerg Infect Dis. 2004;10(1):76‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Laupland KB, Zygun DA, Davies HD, Church DL, Louie TJ, Doig CJ. Population‐based assessment of intensive care unit‐acquired bloodstream infections in adults: Incidence, risk factors, and associated mortality rate. Crit Care Med. 2002;30(11):2462‐2467. [DOI] [PubMed] [Google Scholar]
- 18. Rello J, Ricart M, Mirelis B, et al. Nosocomial bacteremia in a medical‐surgical intensive care unit: epidemiologic characteristics and factors influencing mortality in 111 episodes. Intensive Care Med. 1994;20(2):94‐98. [DOI] [PubMed] [Google Scholar]
- 19. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bari SF, Khan A, Lawson T. C reactive protein may not be reliable as a marker of severe bacterial infection in patients receiving tocilizumab. BMJ Case Rep. 2013;2013):bcr2013010423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fardet L, Petersen I, Nazareth I. Common infections in patients prescribed systemic glucocorticoids in primary care: a population‐based cohort study. PLoS Med. 2016;13(5):e1002024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lang VR, Englbrecht M, Rech J, et al. Risk of infections in rheumatoid arthritis patients treated with tocilizumab. Rheumatology (Oxford). 2012;51(5):852‐857. [DOI] [PubMed] [Google Scholar]
- 24. Stuck AE, Minder CE, Frey FJ. Risk of infectious complications in patients taking glucocorticosteroids. Rev Infect Dis. 1989;11(6):954‐963. [DOI] [PubMed] [Google Scholar]
- 25. Bassetti M, Giacobbe DR, Aliberti S, et al. Balancing evidence and frontline experience in the early phases of the COVID‐19 pandemic: current position of the Italian Society of Anti‐Infective Therapy (SITA) and the Italian Society of Pulmonology (SIP). Clin Microbiol Infect. 2020;26(7):880‐894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Metersky ML, Masterton RG, Lode H, File TM Jr, Babinchak T. Epidemiology, microbiology, and treatment considerations for bacterial pneumonia complicating influenza. Int J Infect Dis. 2012;16(5):e321‐e331. [DOI] [PubMed] [Google Scholar]
- 27. Saravolatz LD, Stein GE, Johnson LB. Ceftaroline: a novel cephalosporin with activity against methicillin‐resistant Staphylococcus aureus . Clin Infect Dis. 2011;52(9):1156‐1163. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets used and/or analysed during the current study are available from the corresponding author on reasonable request.


