Abstract
Background
The recent report of SARS‐CoV‐2 presence in semen samples of six patients, including two subjects who were recovering from the clinical disease, re‐opened the discussion on possible male genital tract infection, virus shedding in semen, sexual transmission and safety of fertility treatments during the pandemic period.
Objectives
To explore current data and hypothesis on the possible sites of SARS‐CoV‐2 infection in the male reproduction system.
Materials and methods
We reviewed the current literature to describe: a) the evidences on angiotensin‐converting enzyme 2 (AC2E) and transmembrane serine protease 2 (TMPRSS2) expression in the testes, accessory glands (including prostate) and the urinary tract; b) other coronaviruses’ (SARS and MERS) ability to infect these sites.
Results
The co‐expression of both ACE2 and TMPRSS2 genes was reported in spermatogonial stem cells, elongated spermatids, in at least a small percentage of prostate hillock cells and in renal tubular cells. Testicular damage was described in autopsies of SARS patients, without evidence of the virus in the specimens. Prostate is a known infection site for MERS‐CoV. SARS‐CoV‐2 was detected in urines.
Discussion
There are still al lot of open questions on the effects of SARS‐CoV‐2 infection on the male reproductive tract. The presence of receptors is not a proof that the testis provides a site for viral infection and it is still unknown if SARS‐CoV‐2 is capable to pass the blood‐testis barrier. The possibility of a prostate involvement has not been investigated yet: we have no data, but theoretically it cannot be excluded. Moreover, the RNA detected in semen could have been just a residual of urinary shedding.
Conclusion
Opening our prospective beyond the testis could be the key to better understand the possibility of a semen‐related viral transmission as well as COVID19 short and long‐term effects on male reproductive function.
Keywords: COVID‐19, SARS‐CoV‐2, SARS‐CoV‐2 in the semen, testis, prostate
A recent report by Li et al described the presence of SARS‐CoV‐2 in semen samples of six patients, including two subjects who were recovering from the clinical disease. This finding re‐opened the discussion on possible male genital tract infection, virus shedding in semen, sexual transmission, and safety of fertility treatments during the pandemic period. 1 As stated by the authors themselves, the small sample size and short follow‐up dictate caution in the interpretation of their results. Moreover, they tested semen samples for SARS‐CoV‐2 by qualitative RT‐PCR, but neither the limits of detection nor the threshold values were described. 2 Perhaps the use of a quantitative PCR assay would be more useful to detect the virus and to test its concentration in semen. Moreover, the semen collection modality is not provided and a possible contamination with RNA fragments from hands or respiratory droplets cannot be excluded. Besides the confirmation of these findings, many questions still remain unanswered such as (a) is the virus located only in seminal fluid, bound to spermatozoa or even internalized? (b) May the virus be removed from spermatozoa by washing procedures? (c) For how long does it remain detectable in semen? (d) And most importantly for clinical practice, is it capable of active replication and potential infection? To answer all these questions, we need the following information: what is the tissue or cell type primarily infected in the male reproductive system? The principal candidates are three: the testes (sperm cells, Sertoli, or Leydig cells), accessory glands (prostate and/or seminal vesicles), and the urinary tract (see Figure 1). Based on early reports, showing expression of ACE2 receptor in Leydig and seminiferous tubules cells, 3 , 4 , 5 recent literature in the field of male reproduction was mostly focused on the possible testis infection by SARS‐CoV‐2. 6 , 7 , 8 We know that coronaviruses critically depend on both angiotensin‐converting enzyme 2 (AC2E) and transmembrane serine protease 2 (TMPRSS2) expression for cell entry and spread in the host. 9 Interestingly, the co‐expression of both ACE2 and TMPRSS2 genes was reported only in spermatogonial stem cells and elongated spermatids. 5 , 7 , 8 Of course, the presence of receptors cannot be a proof that testis provides a site for viral infection and it is still unknown if SARS‐CoV‐2 is capable to pass the blood‐testis barrier. However, the mumps virus, a virus with larger dimensions than SARS‐CoV‐2, is able to cause orchitis especially in the presence of an inflammatory status. Moreover, even if we do not have autopsy data on testes of patients infected with SARS‐CoV‐2 yet, we know that autopsies from testes of patients with SARS demonstrated an extensive testicular damage. This condition was reported to be a consequence of generalized severe inflammation with complete subversion of the seminiferous tubules’ architecture, but SARS‐CoV was not detected in the examined tissue. 10 The second possible site of infection is represented by accessory glands, in particular prostate. We know that multiple viruses can survive and actively replicate inside the prostate, relatively sheltered from systemic therapies. 11 Regarding coronaviruses, there is evidence that MERS‐CoV binds to the host cell receptor dipeptidyl peptidase 4 (DPP4), which is broadly expressed on prostate cells. 12 We have no data on the presence of SARS‐CoV‐2 in the prostate, but we know that TMPRSS2 is highly expressed by the epithelium of the human prostate and is androgen‐responsive. 13 Moreover, even if ACE2 expression in the prostate is reported as “very low,” the two receptors are co‐expressed in at least a small percentage of prostate hillock cells 5 , 14 ; therefore, a prostate infection by SARS‐CoV‐2 cannot be excluded. If this hypothesis would be confirmed, it could provide an explanation for the virus presence in the seminal fluid and could justify the persistence of viral RNA in recovering patients. The third site of interest is the urinary tract. Clinical data from patients with SARS‐CoV and MERS‐CoV showed evidence of tubular damage. 15 Moreover, both ACE2 and TMPRSS2 are highly expressed by renal tubular cells 5 , 16 and SARS‐CoV‐2 was detected in urines. 17 Since the distal urinary and reproductive tracts are overlapping in males, the RNA detected in semen could have been just a residual of urinary shedding.
Figure 1.
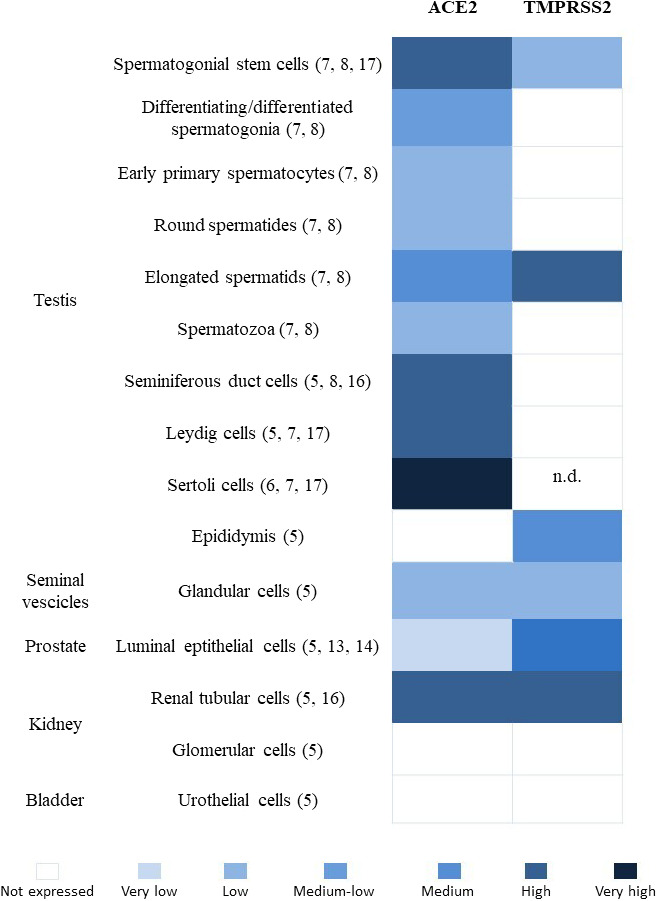
Levels of ACE2 and TMPRSS2 expression in human cells of the male urogenital system. n.d., not determined
In conclusion, opening our prospective beyond the testis could be the key to better implications on possible semen‐related viral transmission as well as COVID‐19 short‐ and long‐term effects on male reproductive function.
Massarotti C, Garolla A, Maccarini E, et al. SARS-CoV-2 in the semen: Where does it come from? Andrology.2021;9:39–41. 10.1111/andr.12839
REFERENCES
- 1. Li D, Jin M, Bao P, Zhao W, Zhang S. Clinical characteristics and results of semen tests among men with coronavirus disease 2019. JAMA Netw Open. 2020;3(5):e208292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Paoli D, Pallotti F, Turriziani O, et al. SARS‐CoV‐2 presence in seminal fluid: Myth or reality. Andrology. 2020. [Epub ahead of print]. 10.1111/andr.12825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Douglas GC, O'Bryan MK, Hedger MP, et al. The novel angiotensin‐converting enzyme (ACE) homolog, ACE2, is selectively expressed by adult Leydig cells of the testis. Endocrinology. 2004;145(10):4703‐4711. [DOI] [PubMed] [Google Scholar]
- 4. Chen Y, Guo Y, Pan Y, Zhao ZJ. Structure analysis of the receptor binding of 2019‐nCoV. Biochem Biophys Res Commun. 2020;525(1):135‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Proteinatlas https://www.proteinatlas.org/. Accessed on May 10th, 2020.
- 6. Abobaker A, Raba AA. Does COVID‐19 affect male fertility? World J Urol. 2020. [Epub ahead of print]. 10.1007/s00345-020-03208-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shen Q, Xiao X, Aierken A, Liao M, Hua J. The ACE2 Expression in Sertoli cells and Germ cells may cause male reproductive disorder after SARS‐CoV‐2 Infection. OSF preprint. 2020;February:24. 10.31219/osf.io/fs5hd [DOI] [PMC free article] [PubMed]
- 8. Stanley KE, Thomas E, Leave M, Wells D. Coronavirus disease (COVID‐19) and fertility: viral host entry protein expression in male and female reproductive tissues. Fert Ster. 2020;114(1):33‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stopsack KH, Mucci LA, Antonarakis ES, Nelson PS, Kantoff PW. TMPRSS2 and COVID‐19: serendipity or opportunity for intervention? Cancer Discov. 2020;10(6):779‐782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xu J, Qi L, Chi X, et al. Orchitis: a complication of severe acute respiratory syndrome (SARS). Biol Reprod. 2006;74(2):410‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Spencer JL, Lahon A, Tran LL, et al. Replication of zika virus in human prostate cells: a potential source of sexually transmitted virus. J Infect Dis. 2018;217(4):538‐547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Magrone T, Magrone M, Jirillo E. Focus on receptors for coronaviruses with special reference to angiotensin‐converting enzyme 2 as a potential drug target – a perspective. Endocr Metab Immune Disord Drug Targets. 2020;20(6):807‐811. 10.2174/1871530320666200427112902 [DOI] [PubMed] [Google Scholar]
- 13. Chen Y‐W, Lee M‐S, Lucht A, et al. TMPRSS2, a serine protease expressed in the prostate on the apical surface of luminal epithelial cells and released into semen in prostasomes, is misregulated in prostate cancer cells. Am J Pathol. 2010;176(6):2986‐2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Song H, Seddighzadeh B, Cooperberg MR, Huang FW. Expression of ACE2, the SARS‐CoV‐2 receptor, and TMPRSS2 in prostate epithelial cells. Eur Urol. 2020;78(2):296‐298. 10.1016/j.eururo.2020.04.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alsaad KO, Hajeer AH, Al Balwi M, et al. Histopathology of Middle East respiratory syndrome coronovirus (MERS‐ CoV) infection — clinicopathological and ultrastructural study. Histopathology. 2018;72:516‐524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li MY, Li L, Zhang Y, Wang XS. Expression of the SARS‐CoV‐2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang W, Xu Y, Gao R, et al. Detection of SARS‐CoV‐2 in different types of clinical specimens. JAMA. 2020;323(18):1843‐1844. [DOI] [PMC free article] [PubMed] [Google Scholar]


