Dear Editor,
Novel coronavirus disease 2019 (COVID‐19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) was declared a global pandemic on March 2020. 1 Recalcati et al. 2 reported cutaneous manifestations in 20.4% of COVID‐19 patients. We identified six patients (five female and one male) with COVID‐19‐associated cutaneous manifestations in April 2020 on the inpatient dermatology consultation service. The mean age was 51 years (range, 28–71 years). All patients presented with fever and upper respiratory symptoms. The median latency before onset of the rash was 9 days (range, 2–21 days). RNAscope ISH assay targeting the SARS‐CoV‐2 mRNA transcript for the spike protein (V‐nCoV2019‐S, ACD catalog #848569), validated on positive controls from COVID‐19 autopsy lung tissue, was negative in all skin biopsy specimens. Three novel cutaneous patterns were identified: (i) COVID‐19‐associated exfoliative shock syndrome in two patients presenting with fever, hypotension and a diffuse exfoliative rash that spared mucous membranes (Fig. 1a,b). Skin biopsies demonstrated a subcorneal split with intracorneal neutrophils, parakeratosis and scant dermal inflammation (Fig. 1c,d). Pancultures were negative for staphylococcal infection. Given the overlapping features of toxic shock syndrome (TSS) and staphylococcal scalded skin syndrome (SSSS), the patients were diagnosed with COVID‐19‐associated exfoliative shock syndrome. One patient expired, and the second patient improved with linezolid. (ii) COVID‐19‐induced rash and mucositis (CIRM) was diagnosed in one patient who developed a Stevens–Johnson syndrome (SJS)‐like eruption in the absence of prior drug exposure. The SARS‐CoV‐2‐positive patient presented with fever and oral erosions. She then developed widespread dusky targetoid papules and bullae with extensive denudation (Fig. 2a) and worsening mucous membranes involvement. Skin biopsy demonstrated full‐thickness epidermal necrosis (Fig. 2b). (iii) COVID‐19‐associated calciphylaxis with thrombotic vasculopathy was identified in a patient presenting with painful retiform purpura consisting of angulated violaceous plaques with necrotic centres on the bilateral legs (Fig. 2c). Skin biopsy showed epidermal necrosis with vascular thrombi and calcification of small‐ to medium‐sized vessels (Fig. 2d). Laboratory workup was remarkable for elevated D‐dimer and fibrinogen. Creatinine, calcium and phosphorus levels were normal.
Figure 1.
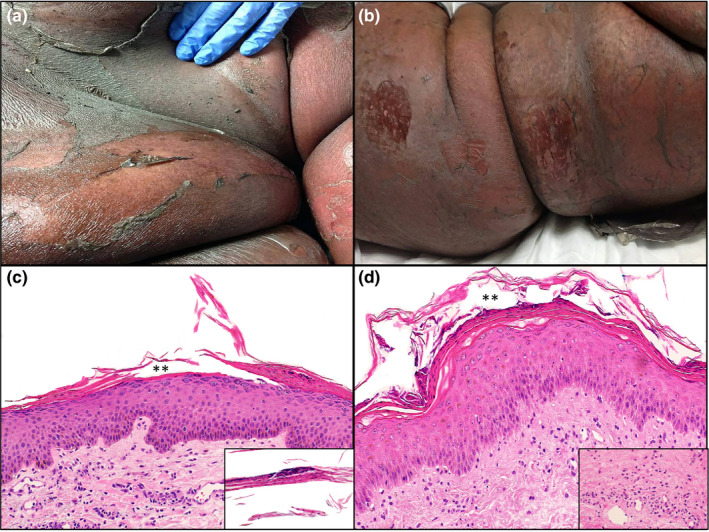
Clinical and histopathologic features of patients with COVID‐19‐associated exfoliative shock syndrome. Patient 1: (a) Erythematous plaques with superficial exfoliation on the abdomen. (b) Patient 2: Erythematous to dusky plaques with superficial exfoliation on the trunk. Patient 1: (c) Haematoxylin and eosin stain of punch biopsy showing subcorneal split (**) with parakeratosis and intracorneal neutrophils (original magnification ×100). Inset showing parakeratotic scale with neutrophilic fragments (original magnification ×200). (d) Patient 2: Haematoxylin and eosin stain of punch biopsy showing subcorneal split (**) with parakeratosis and intracorneal neutrophils (original magnification ×100). Inset showing scant superficial dermal neutrophilic infiltrate (original magnification ×200).
Figure 2.
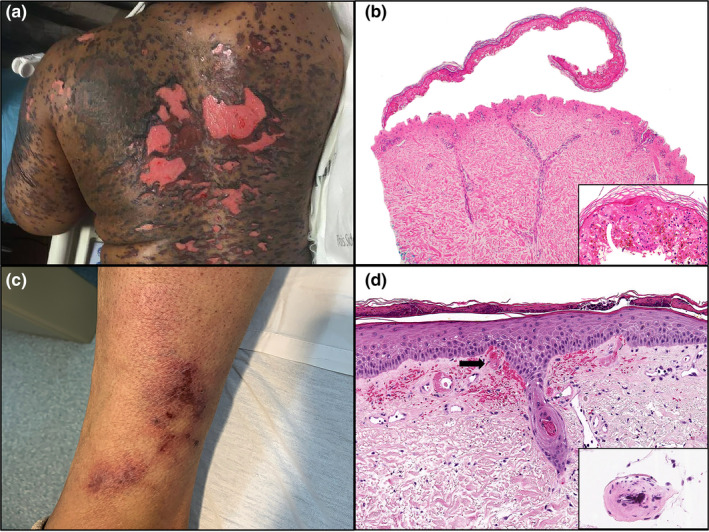
Clinical and histopathologic features of patients 3 and 4. Patient 3: (a) Dusky vesicles and bullae coalescing into plaques with denudation on the back. (b) Haematoxylin and eosin stain of punch biopsy showing full‐thickness epidermal necrosis (original magnification ×100). Inset highlighting confluent epidermal apoptosis (original magnification ×200). Patient 4: (c) Retiform purpura consisting of violaceous angulated plaques with necrotic centres on the lower extremity. (d) Haematoxylin and eosin stain of punch biopsy showing vascular thrombi (arrow) (original magnification ×100). Inset highlighting vessel wall calcium deposition (original magnification ×200).
We present three novel COVID‐19‐associated cutaneous manifestations in hospitalized patients. Two patients presented with a COVID19‐associated exfoliative shock syndrome. Recent reports describe a similar hyperinflammatory shock syndrome in children with overlapping features of Kawasaki disease and TSS. 3 Our patients also presented with fever, hypotension and an exfoliative skin eruption with overlapping features of TSS and SSSS. No gastrointestinal symptoms were observed. Staphylococcus aureus superinfection is a well‐recognized event in the setting of viral diseases due to upregulation of IFN‐α. 4 COVID‐19 induces a cytokine storm, raising the levels of proinflammatory cytokines such as IFN‐α, 5 thus potentially increasing host susceptibility for bacterial superinfection. It is unclear whether this presentation is a direct consequence of COVID‐19‐induced multisystem inflammatory syndrome in predisposed patients or an undetected staphylococcal superinfection. The second pattern identified was CIRM, an eruption that mimics SJS. Exfoliative mucocutaneous eruptions associated with infection are a well‐recognized phenomenon. 6 CD8 cytotoxic T cells, together with the cytokine storm occurring in the setting of COVID‐19, may contribute to this severe cutaneous reaction leading to necrosis of keratinocytes. The histopathologic findings of CIRM are indistinguishable from SJS; however, the time course of events, careful drug history and identification of concurrent infection can allow for distinction. COVID‐19‐associated calciphylaxis with thrombotic vasculopathy is the third pattern observed. The pathogenesis of nonuremic calciphylaxis is primarily related to a hypercoagulable state and thrombotic vasculopathy. 7 COVID‐19‐associated coagulopathy with initial signs of increased D‐dimer and fibrinogen as seen in our patient is well‐documented. 8 The abrupt onset of calciphylaxis with thrombotic vasculopathy and concomitant COVID‐19 infection is suspect and suggests that COVID‐19 infection may increase susceptibility for nonuremic calciphylaxis. Finally, in two patients we observed COVID‐19‐associated morbilliform exanthems that demonstrated a superficial perivascular dermatitis on histopathology.
Viral mRNA was not detected using RNA ISH for SARS‐CoV‐2, suggesting that cutaneous manifestations associated with COVID‐19 are secondary to dysregulation of the immune and coagulation pathways rather than direct viral skin toxicity.
Authors contributions
Drs. Bitar, Lowe and Andea had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1. Cucinotta D, Vanelli M. WHO declares COVID‐19 a pandemic. Acta Biomed 2020; 91: 157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Recalcati S. Cutaneous manifestations in COVID‐ 19: a first perspective. J Eur Acad Dermatol Venereol 2020; 34: e212–e213. [DOI] [PubMed] [Google Scholar]
- 3. Riphagen S, Gomez X, Gonzalez‐Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID‐19 pandemic. Lancet (London, England) 2020; 395: 1607–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shepardson KM, Larson K, Johns LL et al. IFNAR2 is required for anti‐influenza immunity and alters susceptibility to post‐influenza bacterial superinfections. Front Immunol 2018; 9: 2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Astuti I, Ysrafil X. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐ 2): an overview of viral structure and host response. Diabetes Metab Syndr 2020; 14: 407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mazori DR, Nagarajan S, Glick SA. Recurrent reactive infectious mucocutaneous eruption (RIME): insights from a child with three episodes. Pediatr Dermatol 2020. [Epub ahead of print] 10.1111/pde.14142 [DOI] [PubMed] [Google Scholar]
- 7. Jeong HS, Dominguez AR. Calciphylaxis: controversies in pathogenesis, diagnosis and treatment. Am J Med Sci 2016; 351: 217–227. [DOI] [PubMed] [Google Scholar]
- 8. Connors JM, Levy JH. COVID‐19 and its implications for thrombosis and anticoagulation. Blood 2020; 135: 2033–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Acknowledgement
The patients in this manuscript have given verbal informed consent to the publication of their case details.


