Abstract
The COVID‐19 pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has overwhelmed healthcare systems requiring the rapid development of treatments, at least, to reduce COVID‐19 severity. Drug repurposing offers a fast track. Here, we discuss the potential beneficial effects of statins in COVID‐19 patients based on evidence that they may target virus receptors, replication, degradation, and downstream responses in infected cells, addressing both basic research and epidemiological information. Briefly, statins could modulate virus entry, acting on the SARS‐CoV‐2 receptors, ACE2 and CD147, and/or lipid rafts engagement. Statins, by inducing autophagy activation, could regulate virus replication or degradation, exerting protective effects. The well‐known anti‐inflammatory properties of statins, by blocking several molecular mechanisms, including NF‐κB and NLRP3 inflammasomes, could limit the “cytokine storm” in severe COVID‐19 patients which is linked to fatal outcome. Finally, statin moderation of coagulation response activation may also contribute to improving COVID‐19 outcomes.
Linked Articles
This article is part of a themed issue on The Pharmacology of COVID‐19. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v177.21/issuetoc
ABBREVIATIONS
- ACEi
ACE inhibitor
- AMPK
AMP‐activated protein kinase
- Ang II
Angiotensin II
- AP1
activator protein 1
- ARB
angiotensin II receptor blocker
- ATG
autophagy‐related gene
- CEACAM5
carcinoembryonic antigen‐related cell adhesion molecule
- cGAS
cyclic GMP‐AMP synthase
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- CoV
coronavirus
- CRP
C reactive protein
- CYP3A4
Cytochrome P450 3A4
- DAMPs
damage‐associated molecular patterns
- DIC
disseminated intravascular coagulation
- DPP4
dipeptidyl peptidase‐4
- HBV
Hepatitis B virus
- HCV
Hepatitis C virus
- HIV
human immunodeficiency virus
- HMG‐CoA
3‐hydroxy‐3‐methyl‐glutaryl‐CoA reductase
- HR
hazard ratio
- ICU
intensive care unit
- IRFs
IFN regulatory factors
- ISG
IFN stimulated genes
- KLF2
Kruppel‐like factor 2
- KSHV
Kaposi's sarcoma‐associated herpesvirus
- LC3
light chain 3
- MERS
Middle‐East respiratory syndrome
- MHV
murine γ‐herpesvirus
- mTOR
mammalian target of rapamycin
- NLRP‐3
NACHT, LRR and PYD domains‐containing protein 3
- PAMPs
pathogen‐associated molecular patterns
- PfRh5
Plasmodium falciparum reticulocyte binding‐like homologue 5
- PMA
phorbol‐12‐myristate‐13 acetate
- P‐gp
P‐glycoprotein transport system
- PRISM
Platelet Receptor Inhibition in Ischaemic Syndrome Management
- PRR
pattern recognition receptor
- PRRS
porcine reproductive and respiratory syndrome
- RAS
renin angiotensin system
- RBD
receptor‐binding domain
- RIG‐I
retinoic acid‐inducible gene I
- RLR
(RIG‐I)‐like receptor
- ROCK
RhoA/Rho‐kinase
- SARS
severe acute coronary respiratory syndrome
- SKP‐2
S‐phase kinase‐associated protein 2
- SP
spike protein
- SPARCL
Stroke Prevention by Aggressive Reduction in Cholesterol Levels
- ssRNA
single‐stranded RNA
- TF
tissue factor
- TLRs
toll‐like receptors
- TRAF‐3
TNF receptor‐associated factor
- ULK
unc‐51 like autophagy activating kinase
- VSMCs
vascular smooth muscle cells
1. INTRODUCTION
Coronaviruses (CoVs) are enveloped viruses from the order of Nidovirales that have a positive sense, single‐stranded RNA genome (+ssRNA) (Schoeman & Fielding, 2019). Although these viruses primarily affect mammals and birds, the last decades have witnessed outbreaks of human infection, a process known as zoonosis (Schoeman & Fielding, 2019). In 2003, a zoonotic infection causing a severe acute respiratory syndrome (SARS) was reported in Guangdong province (China). Its cause was a novel virus, SARS‐CoV‐1 (Singhal, 2020). Nine years later, another zoonosis called Middle‐East respiratory syndrome (MERS) induced by the MERS‐CoV was first identified in Saudi Arabia (Singhal, 2020), and more recently, by the end of 2019, again, a CoV zoonosis inducing SARS was described for the first time in the city of Wuhan (China) (Singhal, 2020). This new SARS‐CoV‐2 induces a new disease called COVID‐19. The recent events related to COVID‐19 enhance the need of knowing what the virus is, where it comes from, and how it can be defeated. Its high mortality rate and ease of transmission make SARS‐CoV‐2 one of the most important targets of research in recent years, forcing the scientific and medical community to undertake rapid measures to understand the virus behaviour. Currently, there is no effective, approved therapy for CoV infections, only palliative treatment of the symptoms and supportive care. As a result, what we know about SARS‐CoV‐1, MERS‐CoV, or other CoVs becomes important background information. Since the first outbreak of SARS, many studies have addressed the virus structure and the molecular basis of its interaction with the host. Developing a vaccine or an effective new antiviral treatment against an unknown virus needs many experimental studies, clinical trials, and, what is more important in a critical situation, enough time to develop them. For this reason, in recent decades, several authors have pointed out the importance of drug repurposing, identifying already available drugs that may be used to treat future viral infections in order to be prepared for the next worldwide plague (Fedson, 2006; Phadke & Saunik, 2020). Unfortunately, now is “the day,” and it is necessary to identify existing drugs that can be repurposed to help COVID‐19 patients until an effective vaccine can be developed. Currently, there are more than 850 clinical trials using pharmacological interventions to treat COVID‐19 (U.S. National Library of Medicine, 2020).
The HMG‐CoA reductase inhibitors, usually known as statins, are a group of drugs commonly used to lower serum cholesterol by reducing its synthesis in the liver (Liao & Laufs, 2005; Rodrigues Diez et al., 2010). Besides its well‐known lipid‐lowering effects, statins have been postulated to possess pleiotropic beneficial actions by regulating numerous biological pathways implicated in antioxidant, anti‐inflammatory, or anti‐tumour cellular responses (Liao & Laufs, 2005). The pleiotropic effects of statins have been mainly demonstrated in cell cultures and experimental models but in humans is sometimes difficult to dissociate them from the statins hypolipaemic effects (Oesterle, Laufs, & Liao, 2017). However, the anti‐inflammatory non‐lipid effects of statins have been confirmed in a wide range of clinical trials including the AFCAPS/TexCAPS or the JUPITER trials where statins lowered the acute inflammatory marker, C reactive protein (CRP), independently of LDL reduction (Hansson, 2005; Ridker et al., 2001, 2008). Additionally, the JUPITER clinical trial showed that rosuvastatin treatment might modestly reduce the incidence of pneumonia min healthy adults with LDL cholesterol (below 1.3 mg·ml−1) and a high‐sensitivity CRP level ≥20 μg·ml−1 (Novack et al., 2012). These results support the hypothesis that statins can modulate other cellular responses independent of their main lipid lowering action. Since their discovery, statins have been proposed as therapeutic agents in different diseases including infections such as influenza virus or MERS‐CoV (Fedson, 2006; Phadke & Saunik, 2020). Here, we describe different mechanisms through which statins could be potentially helpful in the fight against COVID‐19.
2. SARS‐CoV‐2 INFECTION ENTRY PATHWAYS: THE IMPORTANCE OF ACE2 AND CD147 RECEPTORS
The genome of the CoVs encodes four major structural proteins: the spike protein, the nucleocapsid protein, the membrane protein, and the envelope protein (Schoeman & Fielding, 2019). Recent studies have suggested that some CoVs do not require the full ensemble of the four proteins to form a complete infectious virion (Schoeman & Fielding, 2019) (Figure 1). Among them, the spike glycoprotein (SP) is known to be essential for virus binding to the host cells during the infection process (Shen, Tan, & Tan, 2007). SP is a transmembrane protein that contains protrusions which confers their specificity for some host cell receptors. It is composed of two subunits: S1, which contains the receptor‐binding domain (RBD) responsible for recognizing the cell surface receptors, and S2, which is necessary for membrane fusion (Du et al., 2009). Several host cell receptors bind to S1 and help some CoVs to invade cells, such as dipeptidyl peptidase‐4 (DPP4) (Raj et al., 2013), aminopeptidase N (APN) (Reguera et al., 2012), carcinoembryonic antigen‐related cell adhesion molecule 5 (CEACAM5) (Chan et al., 2016), or ACE2 (Lan et al., 2020).
FIGURE 1.
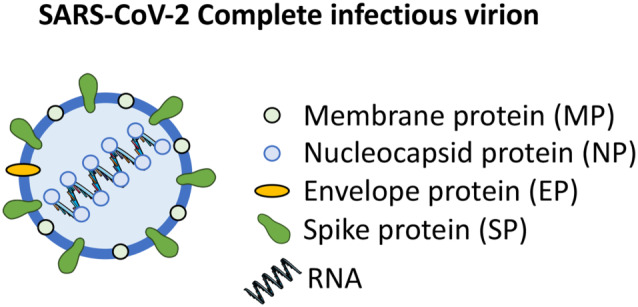
SARS‐CoV‐2 complete infectious virion. The RNA genome encodes a spike protein (SP), an envelope protein (EP), a membrane protein (MP), and a nucleoprotein (NP). The spike protein is the most important surface membrane protein of the SARS‐CoV‐2
ACE2 is one of the best characterized receptors. It binds to the S1 domain, and its relevant role in SARS‐CoV‐1‐induced lung injury has been well established (Kuba et al., 2005). ACE2 is a component of the renin angiotensin system (RAS). This enzyme degrades angiotensin II (Ang II), the effector RAS peptide, to Ang(1–7). Ang II regulates BP and contributes to the pathogenesis of many cardiovascular diseases (Ruiz‐Ortega et al., 2001). Drugs that block Ang II, including ACE inhibitors (ACEi) and/or angiotensin II receptor blockers (ARBs), are currently used to treat many cardiovascular diseases, including hypertension and diabetes, two of the most prevalent COVID‐19 co‐morbidities and clearly associated with the risk of admission to the intensive care unit (ICU), invasive ventilation, or death (Guan et al., 2020). RAS blockers may induce tissue ACE2 overexpression (Ferrario et al., 2005), and therefore, their vascular beneficial effects, besides targeting Ang II actions, could be due to the vasoprotective effects of ACE2/Ang(1–7). Importantly, experimental studies have demonstrated that ACE2 overexpression allowed SARS‐CoV‐2 infection (Zhang, Penninger, et al., 2020). Based on this potential ACE2 activation, an early hypothesis stated that RAS blockers could be detrimental in COVID‐19, and therefore, treatments with ACEi or ARB should be stopped (Fang, Karakiulakis, & Roth, 2020). However, other studies and key scientific societies have argued that there is no empirical basis for this hypothesis and that stopping RAS blockade could be unfavourable (AlGhatrif et al, 2020; European Society of Hypertension, 2020; Zhang, Zhu, et al., 2020). Nevertheless, the discussion on RAS blockers and COVID‐19 is beyond the scope of the present review, and further research is needed to clarify this point.
Although ACE2 is especially abundant in the heart and kidneys (Kuba et al., 2005) where it plays a major role in BP control (Crackower et al., 2002), it is also present in other tissues, including lungs (Kuba et al., 2005). For this reason, modulating tissue ACE2 levels could lead to unwanted and fatal results. For instance, in lung diseases, an impaired ACE2 expression increased vascular permeability and lung oedema and did activate the RAS, contributing to further progression of lung injury (Imai et al., 2005). All these possible negative effects suggest that ACE2 suppression in COVID‐19 should be carefully evaluated. Consequently, alternative solutions based on targeting the ACE2 receptor site have been proposed (Zhang, Penninger, Li, Zhong, & Slutsky, 2020). The most promising approach consists of treatment with a soluble recombinant form of the human ACE2 (APN01), which potentially can bind SARS‐CoV‐2, blocks host cell infection, and protects the lungs from injury (NCT04287686; NCT04324996).
CD147 is another cell surface protein that can act as a CoV receptor. The CD147, also known as basigin, EMMPRIN, or leukocyte activation antigen M6, is a member of the immunoglobulin superfamily expressed in many epithelial, neuronal, lymphoid, and myeloid cell types in its different glycoforms (Grass & Toole, 2015). CD147 is a type I integral membrane receptor that binds to many different ligands including cyclophilin proteins, integrins, or the Plasmodium falciparum reticulocyte binding‐like homologue 5 (PfRh5) (Xiong, Edwards, & Zhou, 2014). CD147 is overexpressed in several cancers, atherosclerosis, inflammation, or microbial diseases (Grass & Toole, 2015). Accordingly, the role of CD147 in infections by pathogens such as human immunodeficiency virus (HIV), hepatitis B (HBV) and C viruses (HCV), or Kaposi's sarcoma‐associated herpesvirus (KSHV) has been reported (Xiong et al., 2014). As described above, CD147 is an essential receptor in erythrocytes for Plasmodium falciparum infection in malaria (Crosnier et al., 2011), and a clinical trial using an anti‐CD147 antibody (Meplazumab) in malaria patients will start this year (NCT04327310). CD147 also facilitates human cytomegalovirus (HCMV) entry to epithelial and endothelial cells (Vanarsdall et al., 2018). More relevant to SARS‐CoV‐2‐induced pulmonary damage, CD147 levels were found up‐regulated in chronic obstructive pulmonary disease (COPD) patients (Jouneau et al., 2011). Additionally, cultured primary bronchial epithelial cells from asthmatic patients showed higher CD147 levels after influenza A virus infection than cells from non‐asthmatic patients (Moheimani et al., 2018). Regarding CoVs, CD147 is a receptor for the S protein in the SARS‐CoV‐1 (Chen et al., 2005) and also for SARS‐CoV‐2 (Wang et al., 2020). In this new study, surface plasmon resonance and co‐immunoprecipitation assays demonstrated a direct interaction between CD147 and the RBD region of the S1. Furthermore, CD147 blockade with Meplazumab inhibited SARS‐CoV‐2 replication in Vero E6 cells. All these data, including an open label clinical trial using the humanized CD147 antibody Meplazumab to treat COVID‐19 pneumonia (NCT04275245), support the concept that CD147 is a potential therapeutic target to fight COVID‐19.
3. STATIN EFFECTS IN THE KEY SARS‐CoV‐2 ENTRY PATHWAYS: ACE2 AND CD147
Statins have been postulated to possess pleiotropic beneficial effects including the inhibition of the untoward effects due to an overactivated RAS such as inflammation and fibrosis (Rodrigues Diez et al., 2010; Ruperez et al., 2007). In this sense, both hypercholesterolaemia and arterial hypertension are often observed in several clinical conditions such as obesity, Type 2 diabetes, atherosclerosis and other cardiovascular diseases. For these reasons, patients are frequently prescribed statins and RAS blockers. As ACE2 is a receptor for SARS‐CoV‐2 entry into host cells, an intense debate about the use of RAS blockers in COVID‐19 patients has recently been generated, based on the fact that both ACEi and ARB are shown to modulate ACE2 tissue levels (South, Diz, & Chappell, 2020; Vaduganathan et al., 2020). Statins have also been included among the drugs that increase ACE2 levels (South et al., 2020). In a model of experimental atherosclerosis in rabbits, atorvastatin increased ACE2 protein levels in heart and kidneys, compared to untreated atherosclerotic animals (Dong et al., 2008; South et al., 2020). Similar results were observed using rosuvastatin or pravastatin in rat vascular balloon injury or diabetes (Li et al., 2013; Min et al., 2018). However, in those studies, ACE2 levels were decreased in injured tissues compared to healthy groups, and therefore, ACE2 up‐regulation induced by statins is only described under disease situations. Thus, the reported up‐regulation of ACE2 by statins in preclinical studies could represent a normalization of ACE2 levels. Therefore, the clinical relevance of these findings is uncertain and could be negligible.
Another pleiotropic effect of statins is the modulation of the CD147 at different levels. Mechanistically, statins alter CD147 expression, structure, and function by inhibiting protein isoprenylation and N‐glycosylation (Sasidhar, Chevooru, Eickelberg, Hartung, & Neuhaus, 2017). In cultured THP‐1 monocytes, pretreatment with atorvastatin, pravastatin, or fluvastatin impaired CD147 translocation to the cell surface, down‐regulating MMP activity and inhibiting THP‐1 differentiation to macrophages after treatment with phorbol‐12‐myristate‐13 acetate (PMA) (Sasidhar et al., 2017). Atorvastatin also down‐regulated CD147 levels and attenuated plaque vulnerability in experimental atherosclerosis in mice (Liang et al., 2017). All these studies suggest that statins, by down‐regulating CD147 in human cells, including pulmonary cells, could impair the ability of the virus to infect cells and could be used as an add‐on or coadjuvant therapy against COVID‐19.
4. COVID‐19 AND LIPID RAFTS
Lipid rafts, defined as small heterogeneous membrane domains enriched in cholesterol and sphingolipids, participate in the compartmentalization of several cellular processes (Lajoie & Nabi, 2007). A relevant role of membrane lipids in the attachment of viruses, including some CoVs, to host cells has been previously reported (Choi, Aizaki, & Lai, 2005; Heaton & Randall, 2011). In the present context, in Vero E6 cells, lipid rafts play an important role in the CoV life cycle during the early stage of SARS (Li, Li, Yamate, Li, & Ikuta, 2007). Closer to COVID‐19, one in vitro study addressed the role of cholesterol‐rich membrane microdomains in the interaction of the S protein of SARS‐CoV‐1 with ACE2 (Glende et al., 2008). ACE2 was present in detergent‐resistant membranes; therefore, cholesterol was required for efficient S‐mediated binding to ACE2‐containing cells. These data suggest that modification of the lipid rafts in the cell membrane could be an option to reduce ACE2‐mediated virus infection.
5. STATINS MODULATE LIPID RAFTS: POTENTIAL ROLE IN SARS‐CoV‐2 INFECTIONS
Statins inhibit the cholesterol biosynthesis pathway by inhibiting HMG‐CoA reductase and modulate cell membrane lipid raft composition. Statins have been proposed to treat disorders associated with changes in lipid rafts. Thus, atorvastatin reversed many of the lipid raft‐associated signalling defects characteristic of autoreactive T cells in systemic lupus erythematosus (Jury, Isenberg, Mauri, & Ehrenstein, 2006). In the viral context, viruses could subvert cholesterol homoeostasis generating a protective membrane environment that facilitates virus assembly and proliferation (Deng, Almsherqi, Ng, & Kohlwein, 2010). Therefore, some authors propose targeting host cell lipid flux as a potential new antibacterial and antiviral strategy (Fernandez‐Oliva, Ortega‐Gonzalez, & Risco, 2019). Accordingly, the use of methyl‐β‐cyclodextrin for cholesterol depletion and lipid raft disruption decreased the infectivity of several viruses, such as HCV or bovine parainfluenza virus, mainly through blocking virus entry into host cells (Fernandez‐Oliva et al., 2019). Similar results were observed using gemfibrozil as a lipid‐lowering drug (Bajimaya et al., 2017). Studies performed in cells infected by several +ssRNA viruses, including from the Coronaviridae family, suggested that viruses induce changes in cell cholesterol metabolism through activation of cellular HMG‐CoA reductase. In 2005, transmission electron microscopy provided evidence that SARS viral infection can result in alterations to the host subcellular membrane inducing a gyroid cubic structure that could modulate viral severity, persistence, and free radical production (Almsherqi, McLachlan, Mossop, Knoops, & Deng, 2005). Thus, structural changes in the plasma membrane of host cells could playing a key role in SARS‐CoV infection (Almsherqi et al., 2005). All these data support the potential use of statins to prevent or reverse host cell lipid raft alterations induced by COVID‐19 infection, which could reduce both cell infection and viral replication.
6. SARS‐CoV‐2 AND AUTOPHAGY
Macroautophagy, thereafter referred to as autophagy, is a highly conserved process in which damaged cellular material is enclosed into a double‐membrane structure called an autophagosome, which finally fuses with lysosomes and forms the autolysosomes for degradation. The main aim of this process is to recycle cellular material, maintain energy levels, and promote cell survival (Yang & Shen, 2020). Canonical autophagy can be divided into three different steps. The first one is the initiation step, where an isolation membrane, also called phagophore, is formed. The second step is the elongation, in which this isolated membrane enlarges and forms the autophagosome. During the third step, maturation, autophagosomes merge with lysosomes forming the autophagolysosomes (Cong, Verlhac, & Reggiori, 2017). Several proteins closely regulate these processes and the signalling network based on the mammalian target of rapamycin (mTOR) is central to autophagy (Munson & Ganley, 2015). Autophagosome formation is mainly controlled by a cluster of genes called autophagy‐related genes (ATGs). Furthermore, the unc‐51 like autophagy activating kinase (ULK) complex and the class III hVPS34 phosphatidylinositol 3‐kinase complex, which includes BECN1 (Beclin 1), are essential in the initiation of the autophagy and autophagosome formation (Wirth, Joachim, & Tooze, 2013). Microtubule‐associated protein light chain 3 (LC3) is involved in elongating and enclosing the phagophore (Yang & Klionsky, 2010). LC3 forms a complex with Atg8 and is cleaved by Atg4, generating LC3‐I which has a glycine residue in the C‐terminal side. Then Atg7 conjugates LC3‐I with a phosphatidylethanolamine resulting in LC3‐II, which is initially attached to both faces of the phagophore membrane, although later on, it will be only present in the inner face, enabling autophagy to continue (Deretic, 2016; Yang & Klionsky, 2010). Apart from its role in cellular homoeostasis, autophagy also participates in the innate immunity response by degrading intracellular pathogens (Maier & Britton, 2012). Regarding viruses, autophagy could act as pro‐viral or anti‐viral process, depending on the virus (Jackson, 2015). Autophagy inhibition increased virulence and replication of some viruses, such as herpes simplex virus 1 (HSV1) (Orvedahl et al., 2007) and the Sindbis virus (Orvedahl et al., 2010). Moreover, some viruses can modulate the autophagy pathway as a mechanism to increase their own replication (Maier & Britton, 2012). This is the case of HSV1, KSHV, and murine γ‐herpesvirus (MHV) which inhibit autophagosome formation by inhibiting Beclin1 (Maier & Britton, 2012). The interaction between the order of Nidovirales and autophagy has been mostly investigated in the CoV and arterovirus families (Cong et al., 2017). One of the most studied arteroviruses causes the porcine reproductive and respiratory syndrome (PRRS). In PRRSV, autophagosome and lysosome fusion are decreased, suggesting that viruses can promote an incomplete autophagy, an abnormal process that may benefit viral replication (Sun et al., 2012). CoVs and other RNA viruses exploit the autophagy for their own replication using the double‐membrane compartments formed during autophagy as a platform for their viral replication machinery which protects viral RNA from the innate immune system of the host cell (Choi, Bowman, & Jung, 2018). A recent study demonstrated that autophagy inhibition favoured MERS‐CoV viral replication. Thus, S‐phase kinase‐associated protein 2 (SKP2) promoted ubiquitination and degradation of Beclin1, while SKP2 inhibition enhanced autophagy and reduced MERS‐CoV replication up to 28,000‐fold (Gassen et al., 2019).
Interestingly, CD147 is also related to autophagy. The low MW compound AC‐73, which targets CD147, elicits autophagy and reduces cell proliferation by inhibiting the ERK/STAT3 pathway (Spinello et al., 2019). Moreover, in human prostate cancer PC‐3 cells, CD147 inhibited autophagy via the PI3K/Akt/mTOR signalling pathway, preventing cell death from unrestrained autophagy (Fang et al., 2015). Taking into account all the available information suggesting a relevant role of autophagy in CoV infection and, potentially, in SARS‐CoV‐2 infection, autophagy should be considered as a potential target in the treatment of COVID‐19.
7. ROLE OF STATINS IN THE AUTOPHAGY RESPONSE
Some of the pleiotropic effects attributed to statins may related to their potential role regulating essential proteins involved in autophagy (Ashrafizadeh, Ahmadi, Farkhondeh, & Samarghandian, 2020). Atorvastatin induced autophagy by enhancing Beclin1 and LC3‐II (Gao et al., 2016) gene and protein expression or via the AMPK/mTOR pathway (Zhang et al., 2013). In cancer cells, lovastatin induced autophagy by up‐regulating LC3‐II (Shi, Felley‐Bosco, Marti, & Stahel, 2012), and atorvastatin through LC3‐I to LC3‐II conversion (Hu et al., 2018). In the same way, pitavastatin stimulated autophagy in melanoma after also increasing LC3‐II levels (Al‐Qatati & Aliwaini, 2017). Interestingly, statins trigger autophagy not only in tumoural tissues. In coronary arterial myocytes, simvastatin increased autophagy by mTOR pathway inhibition (Wei et al., 2013). In relation to lung, the most affected tissue in SARS‐CoV‐2 infection, fluvastatin induced autophagy in two lung adenocarcinoma cell lines (A549 and SPC‐A‐1), by increasing LC3‐II levels (Yang et al., 2017). Additionally, statins could increase autophagy by indirect mechanisms. Thus, in vitro studies showed that lovastatin and simvastatin elicited SKP2 degradation and, therefore, an increase in Beclin1 levels and autophagy (Vosper et al., 2015; Wang, Ho, Lin, Shieh, & Wu, 2017). Altogether, these studies demonstrate that statins can modulate autophagy and therefore add another target supporting their potential beneficial effects in SARS‐CoV‐2 infection.
8. SARS‐CoV‐2 AND NLRP3 INFLAMMASOME ACTIVATION
Viruses infecting host cells need to survive and to replicate. Following infection, host cells activate the innate immune response trying to eliminate the viruses and prevent virus replication (Thompson, Kaminski, Kurt‐Jones, & Fitzgerald, 2011). To this purpose, host cells have developed highly conserved sensors to recognize viral infection and trigger antiviral immune responses. These sensors, known as pattern recognition receptors (PRRs), include toll‐like receptors (TLRs), several DNA sensors such as cyclic GMP‐AMP synthase (cGAS) and retinoic acid‐inducible gene‐I (RIG‐I)‐like receptors (RLRs). The aim of the PRRs is to identify different pathogen‐associated molecular patterns (PAMPs) and damage‐associated molecular patterns (DAMPs) from invading viruses. PRRs binding to their ligands recruit different pathways through activation of the transcription factors NF‐κB, activator protein 1 (AP1), and IFN regulatory factors (IRFs) (Lamkanfi & Dixit, 2014). While IRFs lead to secretion of type I interferons (IFNs), which exert their function by signalling through the JAK‐STAT pathway and the subsequent IFN stimulated genes (ISG) synthesis, NF‐κB activates the production of proinflammatory factors, including IL‐6, and also initiates the first stage of inflammasome activation (Yang, Wang, Kouadir, Song, & Shi, 2019; Zhao & Zhao, 2020). Among the PPRs, the host cell response to an RNA viral infection usually involves the activation of the NACHT, LRR and PYD domains‐containing protein 3 (NLRP3) (Kelley, Jeltema, Duan, & He, 2019). NLRP3 inflammasome activation is a complex process initiated by caspase‐1 activation, followed by the maturation of IL‐1β and IL‐18, leading to inflammation and some mechanisms of cell death, such as pyroptosis (Man & Kanneganti, 2016). In the context of viral infection, the open reading frame 3a from the SARS‐CoV‐1 protein activates the canonical NF‐κB pathway and the NLRP3 inflammasome by promoting TRAF3‐dependent ubiquitination of the p105/NF‐κB subunit (Chen, Moriyama, Chang, & Ichinohe, 2019; Siu et al., 2019). Accordingly, an increased expression of several pro‐inflammatory cytokines, including IL‐1β and IL‐6, has been observed and related to the pathogenesis of acute lung injury in SARS‐CoV‐1 patients (He et al., 2006). Similar results have been observed in SARS‐CoV infection in mice, in which NF‐κB inhibition increased survival (DeDiego et al., 2014). Analogous mechanisms are proposed for the new SARS‐CoV‐2, in which an exacerbated inflammatory response leading to a cytokine storm syndrome is responsible for COVID‐19 severity and mortality (Mehta et al., 2020). In agreement with this hypothesis, clinical trials intended to modulate the inflammatory response have been proposed. Some examples are the use of the anti‐IL drugs anakinra (IL‐1 receptor antagonist), tocilizumab and sarilumab (blocking antibodies against the IL‐6 receptor), and colchicine, which disrupts NLRP3 inflammasome activation and down‐regulates IL‐1β, IL‐18, and IL‐6 levels (Martinez et al., 2015) or even tranilast that targets the NACHT domain of NLRP3 blocking the NLRP3 complex formation (Huang et al., 2018). Additionally, following pilot clinical trials, the antimalarial drugs chloroquine, hydroxychloroquine, or mefloquine are also being used to treat COVID‐19 (Chen et al., 2017; Tang et al., 2018). Several potential mechanisms of action have been proposed for these drugs, including modulation of ACE2 expression and anti‐inflammatory effects including decreased NLRP3 inflammasome activation.
9. STATINS REGULATE NLRP3 INFLAMMASOME‐MEDIATED INFLAMMATION
Probably, one of the best characterized pleiotropic actions of statins is their anti‐inflammatory effects (Blanco‐Colio et al., 2003; Hothersall, McSharry, & Thomson, 2006; Liao & Laufs, 2005). Attenuation of vascular inflammation, added to their lipid‐lowering effect, is thought to contribute to the beneficial effect of statins on cardiovascular outcomes (Albert, Danielson, Rifai, & Ridker, 2001; Blanco‐Colio et al., 2003). At the molecular level, atorvastatin inhibits NF‐κB activation induced by Ang II or TNF‐α in cultured rat vascular smooth muscle cells (VSMCs) and mononuclear cells by a redox‐mediated inhibition of IKK‐1/‐2 (Ortego et al., 2005). Similar results were observed in cultured human endothelial cells, in which cerivastatin prevented activation of the TNFα‐induced NF‐κB pathway by inhibiting PI3K/Akt signalling (Holschermann et al., 2006). Several studies have shown a direct regulation of NLRP3 inflammasome by statins (Parsamanesh et al., 2019). In THP‐1 monocytes, atorvastatin inhibited NLRP3 inflammasome by suppressing the TLR4/MyD88/NF‐κB pathway (Kong et al., 2016). In patients with cardiovascular diseases, treatment with statins down‐regulated the expression of NLRP3 and the downstream cytokines, IL‐18 and IL‐1β (Altaf et al., 2015; Satoh, Tabuchi, Itoh, & Nakamura, 2014). Accordingly, in human peripheral blood mononuclear cells obtained from patients with hyperlipidaemia or healthy controls, stimulation with cholesterol crystals caused NLRP3 inflammasome activation and release of IL‐1β, which was abolished by simvastatin pretreatment (Boland et al., 2018). In terms of the NLRP3 inflammasome, administration of atorvastatin during 8 months in patients with coronary artery disease resulted in a decrease in NLRP3 inflammasome levels (Satoh et al., 2014). There are also some studies suggesting the potential therapeutic role for statins in respiratory diseases such as COPD and asthma (Hothersall et al., 2006; So, Dhungana, Beros, & Criner, 2018). A recent study revealed that statins markedly reduced the risk of subsequent hospitalized exacerbations in patients with a history of frequent COPD exacerbation (Lin et al., 2020). Therefore, taking into account the demonstrated anti‐inflammatory actions of statins, both in NF‐κB‐mediated cytokine induction and in NLRP3 inflammasome activation, these drugs could be considered as a potential way of restraining the uncontrolled inflammation which can be fatal in COVID‐19 patients.
10. COAGULATION COMPLICATIONS IN COVID‐19 PATIENTS
The coagulation system could be regulated by host defences to limit the spread of pathogens during severe infections, as observed with a wide variety of viruses such as HIV, Dengue virus, or Ebola (Antoniak & Mackman, 2014). Nevertheless, in acute viraemia, this situation could lead to disseminated coagulation contributing to multiorgan failure and death (Antoniak & Mackman, 2014). Tissue factor (TF) is an essential cofactor component of the TF‐factor VIIa complex enzyme. TF is transmembrane protein mainly expressed in the vascular adventitia in normal conditions (Butenas, Orfeo, & Mann, 2009). However, during viral infection, TF can be expressed by endothelial cells (and monocytes), and, when exposed to blood, it can activate the coagulation cascade. TF binds to plasma factor VIIa forming the TF‐factor VIIa complex enzyme which triggers blood coagulation by proteolytic activation of the zymogens, factor IX and factor X to the serine proteases, factor IXa and factor Xa (Butenas et al., 2009). Coagulation disorders have been also reported in SARS‐CoV‐1 and MERS‐CoV infections associated with thrombotic complications and haematological manifestations. Different vascular complications, such as vascular endothelial damage (in both small‐ and mid‐sized pulmonary vessels), disseminated intravascular coagulation (DIC), deep venous thrombosis and pulmonary embolism, leading to pulmonary infarction, have been observed in SARS‐Cov‐1 infected patients (Chong et al., 2004; Giannis, Ziogas, & Gianni, 2020; Hwang et al., 2005; Wong et al., 2003). Similarly, DIC was one of the major complications reported in fatal MERS‐CoV cases. Clinical reports include a stable MERS patient who developed MERS‐induced DIC, intracerebral haemorrhage and multiorgan failure (Giannis et al., 2020).
Accordingly, coagulopathy complications are one of the most recently identified features of COVID‐19 infection. Some authors have suggested that clot formation in COVID‐19 patients could be related to the exacerbated inflammatory responses but, as for other viruses, the direct participation of the SARS‐CoV‐2 virus should not be discarded (Connors & Levy, 2020). The first evidence of abnormal coagulation parameters in COVID‐19 patients appeared in China where elevated partial thromboplastin time and prothrombin time (a parameter of how long it takes the blood to clot) were found. In addition, the levels of D‐dimer (a fibrin degradation fragment produced after blood clot lysis) and other inflammation biomarkers, such as IL‐6, erythrocyte sedimentation rate, and CRP, were increased in COVID‐19 patients. (Connors & Levy, 2020). More recent cohort studies from different countries evaluated clotting factors and/or coagulation function in COVID‐19 patients with acute respiratory illness and found increased fibrinogen levels as well as prothrombin time prolongation (Di Micco et al., 2020). However, D‐dimer levels elevation and mild thrombocytopenia are the most consistent haemostatic abnormalities in COVID‐19 patients and are associated with a higher risk of requiring mechanical ventilation, ICU admission, or death (Bikdeli et al., 2020; Klok et al., 2020). Based on these data, it is recommended to consider the preventive and therapeutic use of antithrombotic agents in COVID‐19 patients (Bikdeli et al., 2020). Indeed, autopsies on patients who died of COVID‐19 showed a high incidence of deep venous thrombosis (Wichmann et al., 2020), and anticoagulation treatment was associated with survival in COVID‐19 hospitalized patients (Paranjpe et al., 2020; Tang et al., 2020). At present, a wide range of clinical trials are evaluating the use of low MW heparin in the treatment of COVID‐19 patients, such as NCT04372589 and NCT04345848.
11. ROLE OF STATINS IN THE THROMBOTIC PROCESS
Among the wide range of proposed pleiotropic effects of statins, the interference with the activation of the clotting system and the coagulation cascade is one of the most studied. In 1997, in vitro studies showed that fluvastatin dose‐dependently impaired TF activity and would therefore modulate the coagulation process (Colli et al., 1997). Preclinical studies have addressed the potential mechanisms involved. in vitro data suggest that inhibition of the isoprenylation of small G proteins (Rho, Rac, and Ras) by statins is key to the block of the coagulation cascade (Eto, Kozai, Cosentino, Joch, & Luscher, 2002; Holschermann et al., 2006). Importantly, the RhoA/Rho‐kinase (ROCK) pathway is a key regulator of TF (Ding, Zhao, Li, Liu, & Ma, 2017). Statins can also down‐regulate clot formation by other mechanisms, including the ssthrombomodulin augmentation, via the transcription factor Kruppel‐like factor 2 (KLF2) (Lin et al., 2007; Sen‐Banerjee et al., 2005). Thrombomodulin binds thrombin and promotes protein C activation, lowering factors Va and VIIIa plasma levels and thus having a potent anticoagulant effect (Maruyama, 1999). The anti‐thrombotic effects of statins have also been confirmed in preclinical studies (Undas, Brozek, & Musial, 2002). Interestingly, CD147 inhibition, an effect also attributed to statins, as described above, diminished acute ischaemic stroke in mice by reducing thrombo‐inflammation (Jin, Xiao, Chen, Granger, & Li, 2017).
Importantly, several human studies support the anti‐thrombotic effects of statins. In the Platelet Receptor Inhibition in Ischaemic Syndrome Management (PRISM) study, in patients with acute coronary syndrome, statin administration reduced the risk of negative clinical outcomes, and discontinuation of statin treatment led to the loss of statin‐related beneficial effects (Heeschen et al., 2002). In the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial, statins were efficacious in preventing stroke in patients with acute cerebral ischaemia (Amarenco et al., 2009). In addition to these clinical trials, data from some other human studies are relevant here. Patients with stable atherosclerotic plaque treated with high‐dose (80 mg·day−1) atorvastatin showed a reduction in the ROCK activity, compared with placebo treatment, that could modulate the coagulation process (Nohria et al., 2009). In hypercholesterolaemic patients, rosuvastatin, but not atorvastatin, reduced TF (Panes et al., 2017). Moreover, atorvastatin as well as simvastatin prolonged prothrombin time in patients with hypercholesterolaemia, reducing the tendency to clot generation (Kadikoylu, Yukselen, Yavasoglu, & Bolaman, 2003). Additionally, a meta‐analysis study suggests that statins reduce plasma D‐dimer levels after 3 months suggesting their potential use in some coagulation disorders (Sahebkar et al., 2015). Summarizing, all these proposed anti‐thrombotic effects of statins can be another way of exerting beneficial effects in COVID‐19 patients and their associated clinical complications.
12. STATINS AGAINST COVID‐19: A HYPOTHESIS WORTHY OF CONSIDERATION
Statins are used, or have been proposed to be used, either alone or as adjuvant drugs, in several diseases. These pathologies include hypercholesterolaemia, diabetes, hypertension, cardiovascular diseases, chronic kidney diseases, different types of cancer, rheumatoid arthritis, asthma, or COPD (Fatehi Hassanabad, 2019; McCarey et al., 2004; So et al., 2018; Zhou & Liao, 2009), as well as other infective diseases induced by pathogenic microorganisms such as malaria, Ebola, influenza virus‐related diseases, or MERS (Mehrbod, Omar, Hair‐Bejo, Haghani, & Ideris, 2014; Shrivastava‐Ranjan et al., 2018; Taoufiq et al., 2011; Yuan, 2015). Unfortunately, some of the potential protective effects have not yet been evaluated in some of these diseases, or more thorough studies are needed. Supporting our hypothesis, while writing this review, other authors have also suggested the add‐on therapy of statins in COVID‐19 patients due to their anti‐inflammatory and immuno‐modulatory effects, their ability to curb down cholesterol in lipid rafts, and of course for their extended worldwide use (Bifulco & Gazzerro, 2020; Castiglione, Chiriaco, Emdin, Taddei, & Vergaro, 2020). In addition, several clinical trials in COVID‐19 patients, using simvastatin combined with the JAK‐1/2 inhibitor ruxolitinib to treat COVID‐19 patients (NCT04348695), using atorvastatin alone (NCT04380402) or combined with other drugs (NCT04333407) are currently underway.
Here, we have reviewed some of the pleiotropic effects of statins, such as the down‐regulation of CD147 expression and function, lipid raft disruption, autophagy activation, and attenuation of both the inflammatory response and the coagulation activation (Figure 2). All these processes are thought to be relevant in the infection and replication of SARS‐CoV‐2 in host cells. Although the use of statins would require the assessment of interactions with other experimental therapies for COVID‐19 (The University of Liverpool, 2020), taking into account their effectiveness, safety, low cost, and worldwide distribution, it is worth considering their potential to fight COVID‐19. Additionally, in silico studies to identify FDA‐approved drugs targeting SARS‐CoV‐2 positioned rosuvastatin as the sixth potentially usable drug that may have clinical utility in COVID‐19 (Farag, Wang, Ahmed, Sadek, 2020). As rosuvastatin does not use the cytochrome P4503A4 (CYP3A4) and the P‐glycoprotein transport system, its lower interaction with other drugs employed in these patients, such as remdesivir or chloroquine, would favour its choice as the appropriate statin. For this potential to be realized, first steps would be (a) to analyse COVID‐19 infection databases for potential differential severity or mortality, after adjusting for confounders, of patients already on statins, and (b) providing a biological plausibility basis by studying the impact of statins on viral replication and numbers in cultured cells. This may be followed by a pilot clinical trial that, given the known safety profile of the drugs, may be started without awaiting the results of basic science and epidemiological studies, if these are delayed. So far, only observational evidence on the effects of statins in COVID‐19 patients is available. Although the high mortality among elderly people, who are more likely to be taking statins because of their cardiovascular risk factors, may argue against any benefit of these drugs, it is also true that a high percentage of elderly people with COVID‐19 survive or may even have asymptomatic infection (De Spiegeleer et al., 2020). In this regard, a recent large multinational report of 96,032 hospitalized patients with COVID‐19 supports the potential beneficial effects of statins. Thus, treatment with statins was more frequent among surviving patients than among those patients who died (10.0% vs. 6.9%, P < 0.0001). Indeed, statin use was an independent predictor of low in‐hospital mortality (HR, 95% CI: 0.793, 0.736–0.855), although it was not associated with a differential risk of ventricular arrhythmias during hospitalization (Mehra, Desai, Ruschitzka, & Patel, 2020) These results are in line with a smaller study in 154 nursing home residents in which there was a statistically significant association between statin intake and the absence of symptoms during SARS‐CoV‐2 infection (unadjusted OR 2.91; CI 1.27–6.71; P = 0.011), which remained statistically significant after adjusting for age, sex, functional status, diabetes mellitus, and hypertension (De Spiegeleer et al., 2020).
FIGURE 2.
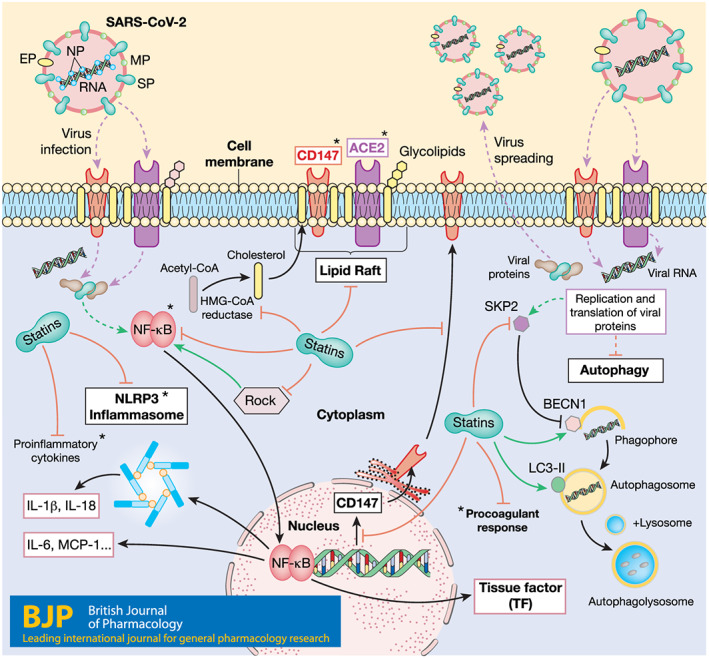
Schematic summary of SARS‐CoV‐2 entry into host cells, replication, effect on host cells, and postulated effects of statins. ACE2 and CD147 are located in the plasma membrane, associated with lipid rafts, and can act as SARS‐CoV‐2 receptors. Statins, by inhibiting cholesterol synthesis, can modify the composition of lipid rafts. Statins can also down‐regulate CD147 expression and its translocation to the cell surface. Autophagy in host cells is altered during SARS‐CoV‐2 infection, by a mechanism that involves SKP2 up‐regulation and subsequent BECN1 degradation. Statins decrease SKP2 levels and induce BECN1 and LC3 II synthesis, which trigger autophagy activation. Another process modulated by SARSCoV‐2 is the activation of the NF‐κB pathway leading to proinflammatory cytokine synthesis, including IL‐6, and NLRP3 inflammasome activation. Statins can down‐regulate NF‐κB pathway activation, proinflammatory cytokine synthesis, and NLRP3 inflammasome activation. Anti‐thrombotic effects of statins by TF modulation could also be beneficial in COVID‐19 patients. Purple, discontinuous lines: viral entry and release. Black lines: cell processes. Green lines: positive regulation of the process. Red lines: negative regulation of the process. Continuous green or red lines: process regulated by statins. Discontinuous green or red lines: process regulated by virus. Viral proteins: EP, envelope protein; NP, nucleocapsid protein; MP, membrane protein; SP: spike protein. *Targets of specific ongoing clinical trials against COVID‐19
2.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander, Christopoulos et al., 2019; Alexander, Fabbro et al., 2019a, 2019b; Alexander, Kelly et al., 2019a, 2019b).
ACKNOWLEDGEMENTS
This work and data discussed here were supported by grants from the Instituto de Salud Carlos III (ISCIII) and Fondos FEDER European Union (PI17/00119 and Red de Investigación Renal [REDINREN]: RD16/0009, to M.R.‐O., PI17/01495 to J.E., PI18/01133 to A.M.R., and PI19/00815 to A.O.); Comunidad de Madrid (“NOVELREN” B2017/BMD‐3751 to M.R.‐O., B2017/BMD‐3686 CIFRA2‐CM to A.O.); Spanish Ministry of Economy and Competitiveness MINECO (DTS17/00203 and DTS19/00093) to J.E.; “Convocatoria Dinamización Europa Investigación 2019” MINECO (EIN2019‐103294 to M.R.‐O. and S.R.‐M.); ERA‐PerMed‐JTC2018 (KIDNEY ATTACK AC18/00064 and PERSTIGAN AC18/00071) and DTS18/00032 to A.O.; The “Sara Borrell” postdoctoral training programme of the ISCIII supported the salary of S.R.‐M. (CD19/00021), IMPROVE‐PD project (“Identification and Management of Patients at Risk–Outcome and Vascular Events in Peritoneal Dialysis”) funding from Ministerio de Economía y Competitividad, European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska‐Curie Grant Agreement No. 812699 to M.R.O.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Rodrigues‐Diez RR, Tejera‐Muñoz A, Marquez‐Exposito L, et al. Statins: Could an old friend help in the fight against COVID‐19? Br J Pharmacol. 2020;177:4873–4886. 10.1111/bph.15166
REFERENCES
- Albert, M. A. , Danielson, E. , Rifai, N. , & Ridker, P. M. (2001). Effect of statin therapy on C‐reactive protein levels: The pravastatin inflammation/ CRP evaluation (PRINCE): A randomized trial and cohort study. JAMA, 286, 64–70. 10.1001/jama.286.1.64 [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Peters, J. A. , … CGTP Collaborators . (2019). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: G protein‐coupled receptors. British Journal of Pharmacology, 176, S21–S141. 10.1111/bph.14748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … CGTP Collaborators . (2019a). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Catalytic receptors. British Journal of Pharmacology, 176, S247–S296. 10.1111/bph.14751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … CGTP Collaborators . (2019b). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Enzymes. British Journal of Pharmacology, 176, S297–S396. 10.1111/bph.14752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Faccenda, E. , … CGTP Collaborators . (2019a). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Other Protein Targets. British Journal of Pharmacology, 176, S1–S20. 10.1111/bph.14747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , … CGTP Collaborators . (2019b). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Transporters. British Journal of Pharmacology, 176, S397–S493. 10.1111/bph.14753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- AlGhatrif, M. , Cingolani, O. , & Lakatta, E. G. (2020). The dilemma of coronavirus disease 2019, aging, and cardiovascular disease: Insights from cardiovascular aging science. JAMA Cardiol. 10.1001/jamacardio.2020.1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almsherqi, Z. A. , McLachlan, C. S. , Mossop, P. , Knoops, K. , & Deng, Y. (2005). Direct template matching reveals a host subcellular membrane gyroid cubic structure that is associated with SARS virus. Redox Report, 10, 167–171. 10.1179/135100005X57373 [DOI] [PubMed] [Google Scholar]
- Al‐Qatati, A. , & Aliwaini, S. (2017). Combined pitavastatin and dacarbazine treatment activates apoptosis and autophagy resulting in synergistic cytotoxicity in melanoma cells. Oncology Letters, 14, 7993–7999. 10.3892/ol.2017.7189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altaf, A. , Qu, P. , Zhao, Y. , Wang, H. , Lou, D. , & Niu, N. (2015). NLRP3 inflammasome in peripheral blood monocytes of acute coronary syndrome patients and its relationship with statins. Coronary Artery Disease, 26, 409–421. 10.1097/MCA.0000000000000255 [DOI] [PubMed] [Google Scholar]
- Amarenco, P. , Benavente, O. , Goldstein, L. B. , Callahan, A. 3rd , Sillesen, H. , Hennerici, M. G. , … Welch, K. M. (2009). Results of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial by stroke subtypes. Stroke, 40, 1405–1409. 10.1161/STROKEAHA.108.534107 [DOI] [PubMed] [Google Scholar]
- Antoniak, S. , & Mackman, N. (2014). Multiple roles of the coagulation protease cascade during virus infection. Blood, 123, 2605–2613. 10.1182/blood-2013-09-526277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrafizadeh, M. , Ahmadi, Z. , Farkhondeh, T. , & Samarghandian, S. (2020). Modulatory effects of statins on the autophagy: A therapeutic perspective. Journal of Cellular Physiology, 235, 3157–3168. 10.1002/jcp.29227 [DOI] [PubMed] [Google Scholar]
- Bajimaya, S. , Hayashi, T. , Frankl, T. , Bryk, P. , Ward, B. , & Takimoto, T. (2017). Cholesterol reducing agents inhibit assembly of type I parainfluenza viruses. Virology, 501, 127–135. 10.1016/j.virol.2016.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bifulco, M. , & Gazzerro, P. (2020). Statins in coronavirus outbreak: It's time for experimental and clinical studies. Pharmacological Research, 156, 104803 10.1016/j.phrs.2020.104803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikdeli, B. , Madhavan, M. V. , Jimenez, D. , Chuich, T. , Dreyfus, I. , Driggin, E. , et al. (2020). COVID‐19 and thrombotic or thromboembolic disease: Implications for prevention, antithrombotic therapy, and follow‐up. Journal of the American College of Cardiology, 75, 2950–2973. 10.1016/j.jacc.2020.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco‐Colio, L. M. , Munoz‐Garcia, B. , Martin‐Ventura, J. L. , Lorz, C. , Diaz, C. , Hernandez, G. , & Egido, J. (2003). 3‐hydroxy‐3‐methylglutaryl coenzyme A reductase inhibitors decrease Fas ligand expression and cytotoxicity in activated human T lymphocytes. Circulation, 108, 1506–1513. 10.1161/01.CIR.0000089086.48617.2B [DOI] [PubMed] [Google Scholar]
- Boland, A. J. , Gangadharan, N. , Kavanagh, P. , Hemeryck, L. , Kieran, J. , Barry, M. , … Lucitt, M. (2018). Simvastatin suppresses interleukin Iβ release in human peripheral blood mononuclear cells stimulated with cholesterol crystals. Journal of Cardiovascular Pharmacology and Therapeutics, 23, 509–517. 10.1177/1074248418776261 [DOI] [PubMed] [Google Scholar]
- Butenas, S. , Orfeo, T. , & Mann, K. G. (2009). Tissue factor in coagulation: Which? Where? When? Arteriosclerosis, Thrombosis, and Vascular Biology, 29, 1989–1996. 10.1161/ATVBAHA.108.177402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castiglione, V. , Chiriaco, M. , Emdin, M. , Taddei, S. , & Vergaro, G. (2020). Statin therapy in COVID‐19 infection. Eur. Hear. Journal Cardiovasc. Pharmacother. 10.1093/ehjcvp/pvaa042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, C.‐M. , Chu, H. , Wang, Y. , Wong, B. H.‐Y. , Zhao, X. , Zhou, J. , … Yuen, K. Y. (2016). Carcinoembryonic antigen‐related cell adhesion molecule 5 is an important surface attachment factor that facilitates entry of middle east respiratory syndrome coronavirus. Journal of Virology, 90, 9114–9127. 10.1128/JVI.01133-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, I.‐Y. , Moriyama, M. , Chang, M.‐F. , & Ichinohe, T. (2019). Severe acute respiratory syndrome coronavirus viroporin 3a activates the NLRP3 inflammasome. Frontiers in Microbiology, 10, 50 eCollection 2019. 10.3389/fmicb.2019.00050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X. , Wang, N. , Zhu, Y. , Lu, Y. , Liu, X. , & Zheng, J. (2017). The antimalarial chloroquine suppresses LPS‐induced NLRP3 inflammasome activation and confers protection against murine endotoxic shock. Mediators of Inflammation, 2017, 11 6543237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Z. , Mi, L. , Xu, J. , Yu, J. , Wang, X. , Jiang, J. , … Zhu, P. (2005). Function of HAb18G/CD147 in invasion of host cells by severe acute respiratory syndrome coronavirus. The Journal of Infectious Diseases, 191, 755–760. 10.1086/427811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, K. S. , Aizaki, H. , & Lai, M. M. C. (2005). Murine coronavirus requires lipid rafts for virus entry and cell‐cell fusion but not for virus release. Journal of Virology, 79, 9862–9871. 10.1128/JVI.79.15.9862-9871.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, Y. , Bowman, J. W. , & Jung, J. U. (2018). Autophagy during viral infection—A double‐edged sword. Nature Reviews. Microbiology, 16, 341–354. 10.1038/s41579-018-0003-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong, P. Y. , Chui, P. , Ling, A. E. , Franks, T. J. , Tai, D. Y. H. , Leo, Y. S. , … Travis, W. D. (2004). Analysis of deaths during the severe acute respiratory syndrome (SARS) epidemic in Singapore: Challenges in determining a SARS diagnosis. Archives of Pathology & Laboratory Medicine, 128, 195–204. [DOI] [PubMed] [Google Scholar]
- Colli, S. , Eligini, S. , Lalli, M. , Camera, M. , Paoletti, R. , & Tremoli, E. (1997). Vastatins inhibit tissue factor in cultured human macrophages. A Novel Mechanism of Protection against Atherothrombosis, Arteriosclerosis, Thrombosis, and Vascular Biology, 17, 265–272. 10.1161/01.atv.17.2.265 [DOI] [PubMed] [Google Scholar]
- Cong, Y. , Verlhac, P. , & Reggiori, F. (2017). The interaction between nidovirales and autophagy components. Viruses, 9(7), 182 10.3390/v9070182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors, J. M. , & Levy, J. H. (2020). COVID‐19 and its implications for thrombosis and anticoagulation. Blood, 135, 2033–2040. 10.1182/blood.2020006000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crackower, M. A. , Sarao, R. , Oudit, G. Y. , Yagil, C. , Kozieradzki, I. , Scanga, S. E. , … Penninger, J. M. (2002). Angiotensin‐converting enzyme 2 is an essential regulator of heart function. Nature, 417, 822–828. 10.1038/nature00786 [DOI] [PubMed] [Google Scholar]
- Crosnier, C. , Bustamante, L. Y. , Bartholdson, S. J. , Bei, A. K. , Theron, M. , Uchikawa, M. , … Wright, G. J. (2011). Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum. Nature, 480, 534–537. 10.1038/nature10606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Spiegeleer, A. , Bronselaer, A. , Teo, J. T. , Byttebier, G. , De Tre, G. , Belmans, L. , … De Spiegeleer, B. (2020). The effects of ARBs, ACEIs and statins on clinical outcomes of COVID‐19 infection among nursing home residents. J Am Med Dir Assoc, 2020 10.1016/j.jamda.2020.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeDiego, M. L. , Nieto‐Torres, J. L. , Regla‐Nava, J. A. , Jimenez‐Guardeno, J. M. , Fernandez‐Delgado, R. , Fett, C. , … Enjuanes, L. (2014). Inhibition of NF‐κB‐mediated inflammation in severe acute respiratory syndrome coronavirus‐infected mice increases survival. Journal of Virology, 88, 913–924. 10.1128/JVI.02576-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, Y. , Almsherqi, Z. A. , Ng, M. M. L. , & Kohlwein, S. D. (2010). Do viruses subvert cholesterol homeostasis to induce host cubic membranes? Trends in Cell Biology, 20, 371–379. 10.1016/j.tcb.2010.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic, V. (2016). Autophagy in leukocytes and other cells: mechanisms, subsystem organization, selectivity, and links to innate immunity. Journal of Leukocyte Biology, 100, 969–978. 10.1189/jlb.4MR0216-079R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Micco, P. , Russo, V. , Carannante, N. , Imparato, M. , Rodolfi, S. , Cardillo, G. , & Lodigiani, C. (2020). Clotting factors in COVID‐19: Epidemiological association and prognostic values in different clinical presentations in an Italian cohort. Journal of Clinical Medicine, 9(5), 1371 10.3390/jcm9051371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, R. , Zhao, D. , Li, X. , Liu, B. , & Ma, X. (2017). Rho‐kinase inhibitor treatment prevents pulmonary inflammation and coagulation in lipopolysaccharide‐induced lung injury. Thrombosis Research, 150, 59–64. 10.1016/j.thromres.2016.12.020 [DOI] [PubMed] [Google Scholar]
- Dong, B. , Zhang, C. , Feng, J. B. , Zhao, Y. X. , Li, S. Y. , Yang, Y. P. , … Zhang, Y. (2008). Overexpression of ACE2 enhances plaque stability in a rabbit model of atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology, 28, 1270–1276. 10.1161/ATVBAHA.108.164715 [DOI] [PubMed] [Google Scholar]
- Du, L. , He, Y. , Zhou, Y. , Liu, S. , Zheng, B.‐J. , & Jiang, S. (2009). The spike protein of SARS‐CoV—A target for vaccine and therapeutic development. Nature Reviews. Microbiology, 7, 226–236. 10.1038/nrmicro2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eto, M. , Kozai, T. , Cosentino, F. , Joch, H. , & Luscher, T. F. (2002). Statin prevents tissue factor expression in human endothelial cells: Role of Rho/Rho‐kinase and Akt pathways. Circulation, 105, 1756–1759. 10.1161/01.CIR.0000015465.73933.3B [DOI] [PubMed] [Google Scholar]
- European Society of Hypertension . (2020). ESH recommendations on COVID‐19.
- Fang, F. , Wang, L. , Zhang, S. , Fang, Q. , Hao, F. , Sun, Y. , … Wang, L. (2015). CD147 modulates autophagy through the PI3K/Akt/mTOR pathway in human prostate cancer PC‐3 cells. Oncology Letters, 9, 1439–1443. 10.3892/ol.2015.2849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang, L. , Karakiulakis, G. , & Roth, M. (2020). Are patients with hypertension and diabetes mellitus at increased risk for COVID‐19 infection? The Lancet Respiratory Medicine, 8(4), e21 10.1016/S2213-2600(20)30116-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farag, A. , Wang, P. , Ahmed, M. , & Sadek, H. (2020). Identification of FDA approved drugs targeting COVID‐19 virus by structure‐ based drug repositioning. ChemRxiv. 10.26434/chemrxiv.12003930.v1 [DOI] [Google Scholar]
- Fatehi Hassanabad, A. (2019). Current perspectives on statins as potential anti‐cancer therapeutics: clinical outcomes and underlying molecular mechanisms. Transl. Lung Cancer Res., 8, 692–699. 10.21037/tlcr.2019.09.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedson, D. S. (2006). Pandemic influenza: A potential role for statins in treatment and prophylaxis. Clinical Infectious Diseases, 43, 199–205. 10.1086/505116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez‐Oliva, A. , Ortega‐Gonzalez, P. , & Risco, C. (2019). Targeting host lipid flows: Exploring new antiviral and antibiotic strategies. Cellular Microbiology, 21, e12996 10.1111/cmi.12996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario, C. M. , Jessup, J. , Chappell, M. C. , Averill, D. B. , Brosnihan, K. B. , Tallant, E. A. , … Gallagher, P. E. (2005). Effect of angiotensin‐converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin‐converting enzyme 2. Circulation, 111, 2605–2610. 10.1161/CIRCULATIONAHA.104.510461 [DOI] [PubMed] [Google Scholar]
- Gao, S. , Zhang, Z.‐M. , Shen, Z.‐L. , Gao, K. , Chang, L. , Guo, Y. , … Wang, A. M. (2016). Atorvastatin activates autophagy and promotes neurological function recovery after spinal cord injury. Neural Regeneration Research, 11, 977–982. 10.4103/1673-5374.184498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassen, N. C. , Niemeyer, D. , Muth, D. , Corman, V. M. , Martinelli, S. , Gassen, A. , … Rein, T. (2019). SKP2 attenuates autophagy through Beclin1‐ubiquitination and its inhibition reduces MERS‐Coronavirus infection. Nature Communications, 10, 5770 10.1038/s41467-019-13659-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannis, D. , Ziogas, I. A. , & Gianni, P. (2020). Coagulation disorders in coronavirus infected patients: COVID‐19, SARS‐CoV‐1, MERS‐CoV and lessons from the past. Journal of Clinical Virology, 127, 104362 10.1016/j.jcv.2020.104362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glende, J. , Schwegmann‐Wessels, C. , Al‐Falah, M. , Pfefferle, S. , Qu, X. , Deng, H. , … Herrler, G. (2008). Importance of cholesterol‐rich membrane microdomains in the interaction of the S protein of SARS‐coronavirus with the cellular receptor angiotensin‐converting enzyme 2. Virology, 381, 215–221. 10.1016/j.virol.2008.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grass, G. D. , & Toole, B. P. (2015). How, with whom and when: An overview of CD147‐mediated regulatory networks influencing matrix metalloproteinase activity. Bioscience Reports, 36, e00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan, W.‐J. , Liang, W.‐H. , Zhao, Y. , Liang, H.‐R. , Chen, Z.‐S. , Li, Y.‐M. , … China Medical Treatment Expert Group for COVID‐19 . (2020). Comorbidity and its impact on 1590 patients with Covid‐19 in China: A nationwide analysis. The European Respiratory Journal, 55, 2000547. 10.1183/13993003.00547-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson, G. K. (2005). Inflammation, atherosclerosis, and coronary artery disease. The New England Journal of Medicine, 352, 1685–1695. 10.1056/NEJMra043430 [DOI] [PubMed] [Google Scholar]
- He, L. , Ding, Y. , Zhang, Q. , Che, X. , He, Y. , Shen, H. , … Jiang, S. (2006). Expression of elevated levels of pro‐inflammatory cytokines in SARS‐CoV‐infected ACE2+ cells in SARS patients: Relation to the acute lung injury and pathogenesis of SARS. The Journal of Pathology, 210, 288–297. 10.1002/path.2067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton, N. S. , & Randall, G. (2011). Multifaceted roles for lipids in viral infection. Trends in Microbiology, 19, 368–375. 10.1016/j.tim.2011.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeschen, C. , Hamm, C. W. , Laufs, U. , Snapinn, S. , Bohm, M. , & White, H. D. (2002). Withdrawal of statins increases event rates in patients with acute coronary syndromes. Circulation, 105, 1446–1452. 10.1161/01.CIR.0000012530.68333.C8 [DOI] [PubMed] [Google Scholar]
- Holschermann, H. , Schuster, D. , Parviz, B. , Haberbosch, W. , Tillmanns, H. , & Muth, H. (2006). Statins prevent NF‐κB transactivation independently of the IKK‐pathway in human endothelial cells. Atherosclerosis, 185, 240–245. 10.1016/j.atherosclerosis.2005.06.019 [DOI] [PubMed] [Google Scholar]
- Hothersall, E. , McSharry, C. , & Thomson, N. C. (2006). Potential therapeutic role for statins in respiratory disease. Thorax, 61, 729–734. 10.1136/thx.2005.057976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, M.‐B. , Zhang, J.‐W. , Gao, J.‐B. , Qi, Y.‐W. , Gao, Y. , Xu, L. , … Wei, Z. Z. (2018). Atorvastatin induces autophagy in MDA‐MB‐231 breast cancer cells. Ultrastructural Pathology, 42, 409–415. 10.1080/01913123.2018.1522406 [DOI] [PubMed] [Google Scholar]
- Huang, Y. , Jiang, H. , Chen, Y. , Wang, X. , Yang, Y. , Tao, J. , … Zhou, R. (2018). Tranilast directly targets NLRP3 to treat inflammasome‐driven diseases. EMBO Molecular Medicine, 10 10.15252/emmm.201708689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang, D. M. , Chamberlain, D. W. , Poutanen, S. M. , Low, D. E. , Asa, S. L. , & Butany, J. (2005). Pulmonary pathology of severe acute respiratory syndrome in Toronto. Mod. Pathol. An off. J. United States Can. Acad. Pathol. Inc, 18, 1–10. 10.1038/modpathol.3800247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai, Y. , Kuba, K. , Rao, S. , Huan, Y. , Guo, F. , Guan, B. , … Penninger, J. M. (2005). Angiotensin‐converting enzyme 2 protects from severe acute lung failure. Nature, 436, 112–116. 10.1038/nature03712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson, W. T. (2015). Viruses and the autophagy pathway. Virology, 479–480, 450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, R. , Xiao, A. Y. , Chen, R. , Granger, D. N. , & Li, G. (2017). Inhibition of CD147 (cluster of differentiation 147) ameliorates acute ischemic stroke in mice by reducing thromboinflammation. Stroke, 48, 3356–3365. 10.1161/STROKEAHA.117.018839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouneau, S. , Khorasani, N. , DE Souza, P. , Macedo, P. , Zhu, J. , Bhavsar, P. K. , & Chung, K. F. (2011). EMMPRIN (CD147) regulation of MMP‐9 in bronchial epithelial cells in COPD. Respirology, 16, 705–712. 10.1111/j.1440-1843.2011.01960.x [DOI] [PubMed] [Google Scholar]
- Jury, E. C. , Isenberg, D. A. , Mauri, C. , & Ehrenstein, M. R. (2006). Atorvastatin restores Lck expression and lipid raft‐associated signaling in T cells from patients with systemic lupus erythematosus. Journal of Immunology, 177, 7416–7422. 10.4049/jimmunol.177.10.7416 [DOI] [PubMed] [Google Scholar]
- Kadikoylu, G. , Yukselen, V. , Yavasoglu, I. , & Bolaman, Z. (2003). Hemostatic effects of atorvastatin versus simvastatin. The Annals of Pharmacotherapy, 37, 478–484. 10.1345/aph.1C189 [DOI] [PubMed] [Google Scholar]
- Kelley, N. , Jeltema, D. , Duan, Y. , & He, Y. (2019). The NLRP3 Inflammasome: An overview of mechanisms of activation and regulation. International Journal of Molecular Sciences, 20 10.3390/ijms20133328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klok, F. A. , Kruip, M. J. H. A. , van der Meer, N. J. M. , Arbous, M. S. , Gommers, D. A. M. P. J. , Kant, K. M. , … Endemane, H. (2020). Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thrombosis Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, F. , Ye, B. , Lin, L. , Cai, X. , Huang, W. , & Huang, Z. (2016). Atorvastatin suppresses NLRP3 inflammasome activation via TLR4/MyD88/NF‐κB signaling in PMA‐stimulated THP‐1 monocytes. Biomedicine & Pharmacotherapy, 82, 167–172. 10.1016/j.biopha.2016.04.043 [DOI] [PubMed] [Google Scholar]
- Kuba, K. , Imai, Y. , Rao, S. , Gao, H. , Guo, F. , Guan, B. , … Penninger, J. M. (2005). A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus‐induced lung injury. Nature Medicine, 11, 875–879. 10.1038/nm1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajoie, P. , & Nabi, I. R. (2007). Regulation of raft‐dependent endocytosis. Journal of Cellular and Molecular Medicine, 11, 644–653. 10.1111/j.1582-4934.2007.00083.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamkanfi, M. , & Dixit, V. M. (2014). Mechanisms and functions of inflammasomes. Cell, 157, 1013–1022. 10.1016/j.cell.2014.04.007 [DOI] [PubMed] [Google Scholar]
- Lan, J. , Ge, J. , Yu, J. , Shan, S. , Zhou, H. , Fan, S. , … Wang, X. (2020). Structure of the SARS‐CoV‐2 spike receptor‐binding domain bound to the ACE2 receptor. Nature, 581, 215–220. 10.1038/s41586-020-2180-5 [DOI] [PubMed] [Google Scholar]
- Li, G.‐M. , Li, Y.‐G. , Yamate, M. , Li, S.‐M. , & Ikuta, K. (2007). Lipid rafts play an important role in the early stage of severe acute respiratory syndrome‐coronavirus life cycle. Microbes and Infection, 9, 96–102. 10.1016/j.micinf.2006.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y.‐H. , Wang, Q.‐X. , Zhou, J.‐W. , Chu, X.‐M. , Man, Y.‐L. , Liu, P. , … An, Y. (2013). Effects of rosuvastatin on expression of angiotensin‐converting enzyme 2 after vascular balloon injury in rats. Journal of Geriatric Cardiology, 10, 151–158. 10.3969/j.issn.1671-5411.2013.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, X. , Yang, L.‐X. , Guo, R. , Shi, Y. , Hou, X. , Yang, Z. , … Liu, H. (2017). Atorvastatin attenuates plaque vulnerability by downregulation of EMMPRIN expression via COX‐2/PGE2 pathway. Experimental and Therapeutic Medicine, 13, 835–844. 10.3892/etm.2017.4062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao, J. K. , & Laufs, U. (2005). Pleiotropic effects of statins. Annual Review of Pharmacology and Toxicology, 45, 89–118. 10.1146/annurev.pharmtox.45.120403.095748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, C.‐M. , Yang, T.‐M. , Yang, Y.‐H. , Tsai, Y.‐H. , Lee, C.‐P. , Chen, P.‐C. , … Hsieh, M. J. (2020). Statin use and the risk of subsequent hospitalized exacerbations in COPD patients with frequent exacerbations. International Journal of Chronic Obstructive Pulmonary Disease, 15, 289–299. 10.2147/COPD.S229047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, S.‐J. , Chen, Y.‐H. , Lin, F.‐Y. , Hsieh, L.‐Y. , Wang, S.‐H. , Lin, C.‐Y. , … Chen, Y. L. (2007). Pravastatin induces thrombomodulin expression in TNFα‐treated human aortic endothelial cells by inhibiting Rac1 and Cdc42 translocation and activity. Journal of Cellular Biochemistry, 101, 642–653. 10.1002/jcb.21206 [DOI] [PubMed] [Google Scholar]
- Maier, H. J. , & Britton, P. (2012). Involvement of autophagy in coronavirus replication. Viruses, 4, 3440–3451. 10.3390/v4123440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man, S. M. , & Kanneganti, T.‐D. (2016). Converging roles of caspases in inflammasome activation, cell death and innate immunity. Nature Reviews. Immunology, 16, 7–21. 10.1038/nri.2015.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez, G. J. , Robertson, S. , Barraclough, J. , Xia, Q. , Mallat, Z. , Bursill, C. , … Patel, S. (2015). Colchicine acutely suppresses local cardiac production of inflammatory cytokines in patients with an acute coronary syndrome. Journal of the American Heart Association, 4, e002128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama, I. (1999). Recombinant thrombomodulin and activated protein C in the treatment of disseminated intravascular coagulation. Thrombosis and Haemostasis, 82, 718–721. [PubMed] [Google Scholar]
- McCarey, D. W. , McInnes, I. B. , Madhok, R. , Hampson, R. , Scherbakov, O. , Ford, I. , et al. (2004). Trial of atorvastatin in rheumatoid arthritis (TARA): Double‐blind, randomised placebo‐controlled trial. Lancet (London, England), 363, 2015–2021. [DOI] [PubMed] [Google Scholar]
- Mehra, M. R. , Desai, S. S. , Ruschitzka, F. , & Patel, A. N. (2020). Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID‐19: A multinational registry analysis. Lancet (London, England), S0140‐6736(20), 31180–31186. 10.1016/S0140-6736(20)31180-6 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Mehrbod, P. , Omar, A. R. , Hair‐Bejo, M. , Haghani, A. , & Ideris, A. (2014). Mechanisms of action and efficacy of statins against influenza. BioMed Research International, 2014, 872370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta, P. , McAuley, D. F. , Brown, M. , Sanchez, E. , Tattersall, R. S. , & Manson, J. J. (2020). COVID‐19: Consider cytokine storm syndromes and immunosuppression. Lancet (London, England), 395, 1033–1034. 10.1016/S0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min, J. J. , Shin, B.‐S. , Lee, J.‐H. , Jeon, Y. , Ryu, D. K. , Kim, S. , & Shin, Y. H. (2018). Effects of pravastatin on Type 1 diabetic rat heart with or without blood glycemic control. Journal Diabetes Research, 2018. 1067853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moheimani, F. , Koops, J. , Williams, T. , Reid, A. T. , Hansbro, P. M. , Wark, P. A. , & Knight, D. A. (2018). Influenza A virus infection dysregulates the expression of microRNA‐22 and its targets; CD147 and HDAC4, in epithelium of asthmatics. Respiratory Research, 19, 145 10.1186/s12931-018-0851-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson, M. J. , & Ganley, I. G. (2015). MTOR, PIK3C3, and autophagy: Signaling the beginning from the end. Autophagy, 11, 2375–2376. 10.1080/15548627.2015.1106668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohria, A. , Prsic, A. , Liu, P.‐Y. , Okamoto, R. , Creager, M. A. , Selwyn, A. , … Ganz, P. (2009). Statins inhibit Rho kinase activity in patients with atherosclerosis. Atherosclerosis, 205, 517–521. 10.1016/j.atherosclerosis.2008.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novack, V. , MacFadyen, J. , Malhotra, A. , Almog, Y. , Glynn, R. J. , & Ridker, P. M. (2012). The effect of rosuvastatin on incident pneumonia: Results from the JUPITER trial. CMAJ, 184, E367–E372. 10.1503/cmaj.111017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterle, A. , Laufs, U. , & Liao, J. K. (2017). Pleiotropic effects of statins on the cardiovascular system. Circulation Research, 120, 229–243. 10.1161/CIRCRESAHA.116.308537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortego, M. , Gomez‐Hernandez, A. , Vidal, C. , Sanchez‐Galan, E. , Blanco‐Colio, L. M. , Martin‐Ventura, J. L. , … Egido, J. (2005). HMG‐CoA reductase inhibitors reduce IκB kinase activity induced by oxidative stress in monocytes and vascular smooth muscle cells. Journal of Cardiovascular Pharmacology, 45, 468–475. 10.1097/01.fjc.0000159042.50488.e5 [DOI] [PubMed] [Google Scholar]
- Orvedahl, A. , Alexander, D. , Talloczy, Z. , Sun, Q. , Wei, Y. , Zhang, W. , … Levine, B. (2007). HSV‐1 ICP34.5 confers neurovirulence by targeting the Beclin 1 autophagy protein. Cell Host & Microbe, 1, 23–35. 10.1016/j.chom.2006.12.001 [DOI] [PubMed] [Google Scholar]
- Orvedahl, A. , MacPherson, S. , Sumpter, R. J. , Talloczy, Z. , Zou, Z. , & Levine, B. (2010). Autophagy protects against Sindbis virus infection of the central nervous system. Cell Host & Microbe, 7, 115–127. 10.1016/j.chom.2010.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panes, O. , Gonzalez, C. , Hidalgo, P. , Valderas, J. P. , Acevedo, M. , Contreras, S. , … Mezzano, D. (2017). Platelet tissue factor activity and membrane cholesterol are increased in hypercholesterolemia and normalized by rosuvastatin, but not by atorvastatin. Atherosclerosis, 257, 164–171. 10.1016/j.atherosclerosis.2016.12.019 [DOI] [PubMed] [Google Scholar]
- Paranjpe, I. , Fuster, V. , Lala, A. , Russak, A. , Glicksberg, B. S. , Levin, M. A. , … Nadkarni, G. N. (2020). Association of treatment dose anticoagulation with in‐hospital survival among hospitalized patients with COVID‐19. Journal of the American College of Cardiology. 10.1016/j.jacc.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsamanesh, N. , Moossavi, M. , Bahrami, A. , Fereidouni, M. , Barreto, G. , & Sahebkar, A. (2019). NLRP3 inflammasome as a treatment target in atherosclerosis: A focus on statin therapy. International Immunopharmacology, 73, 146–155. 10.1016/j.intimp.2019.05.006 [DOI] [PubMed] [Google Scholar]
- Phadke, M. , & Saunik, S. (2020). COVID‐19 treatment by repurposing drugs until the vaccine is in sight. Drug Development Research. 10.1002/ddr.21666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj, V. S. , Mou, H. , Smits, S. L. , Dekkers, D. H. W. , Muller, M. A. , Dijkman, R. , … Haagmans, B. L. (2013). Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus‐EMC. Nature, 495, 251–254. 10.1038/nature12005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reguera, J. , Santiago, C. , Mudgal, G. , Ordono, D. , Enjuanes, L. , & Casasnovas, J. M. (2012). Structural bases of coronavirus attachment to host aminopeptidase N and its inhibition by neutralizing antibodies. PLoS Pathogens, 8, e1002859 10.1371/journal.ppat.1002859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker, P. M. , Danielson, E. , Fonseca, F. A. H. , Genest, J. , Gotto, A. M. J. , Kastelein, J. J. P. , … JUPITER Study Group . (2008). Rosuvastatin to prevent vascular events in men and women with elevated C‐reactive protein. The New England Journal of Medicine, 359, 2195–2207. 10.1056/NEJMoa0807646 [DOI] [PubMed] [Google Scholar]
- Ridker, P. M. , Rifai, N. , Clearfield, M. , Downs, J. R. , Weis, S. E. , Miles, J. S. , & Gotto, A. M. Jr. (2001). Measurement of C‐reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. The New England Journal of Medicine, 344, 1959–1965. 10.1056/NEJM200106283442601 [DOI] [PubMed] [Google Scholar]
- Rodrigues Diez, R. , Rodrigues‐Diez, R. , Lavoz, C. , Rayego‐Mateos, S. , Civantos, E. , Rodriguez‐Vita, J. , … Ruiz‐Ortega, M. (2010). Statins inhibit angiotensin II/Smad pathway and related vascular fibrosis, by a TGF‐β‐independent process. PLoS ONE, 5, e14145 10.1371/journal.pone.0014145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz‐Ortega, M. , Lorenzo, O. , Ruperez, M. , Esteban, V. , Suzuki, Y. , Mezzano, S. , … Egido, J. (2001). Role of the renin‐angiotensin system in vascular diseases: Expanding the field. Hypertens (Dallas, Tex. 1979), 38, 1382–1387. 10.1161/hy1201.100589 [DOI] [PubMed] [Google Scholar]
- Ruperez, M. , Rodrigues‐Diez, R. , Blanco‐Colio, L. M. , Sanchez‐Lopez, E. , Rodriguez‐Vita, J. , Esteban, V. , … Ruiz‐Ortega, M. (2007). HMG‐CoA reductase inhibitors decrease angiotensin II‐induced vascular fibrosis: Role of RhoA/ROCK and MAPK pathways. Hypertens. (Dallas, Tex. 1979), 50, 377–383. 10.1161/HYPERTENSIONAHA.107.091264 [DOI] [PubMed] [Google Scholar]
- Sahebkar, A. , Serban, C. , Mikhailidis, D. P. , Undas, A. , Lip, G. Y. H. , Muntner, P. , … Lipid and Blood Pressure Meta‐analysis Collaboration (LBPMC) Group . (2015). Association between statin use and plasma D‐dimer levels. A systematic review and meta‐analysis of randomised controlled trials. Thrombosis and Haemostasis, 114, 546–557. 10.1160/TH14-11-0937 [DOI] [PubMed] [Google Scholar]
- Sasidhar, M. V. , Chevooru, S. K. , Eickelberg, O. , Hartung, H.‐P. , & Neuhaus, O. (2017). Downregulation of monocytic differentiation via modulation of CD147 by 3‐hydroxy‐3‐methylglutaryl coenzyme A reductase inhibitors. PLoS ONE, 12, e0189701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh, M. , Tabuchi, T. , Itoh, T. , & Nakamura, M. (2014). NLRP3 inflammasome activation in coronary artery disease: Results from prospective and randomized study of treatment with atorvastatin or rosuvastatin. Clinical Science (London, England), 126, 233–241. 10.1042/CS20130043 [DOI] [PubMed] [Google Scholar]
- Schoeman, D. , & Fielding, B. C. (2019). Coronavirus envelope protein: current knowledge. Virology Journal, 16, 69 10.1186/s12985-019-1182-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen‐Banerjee, S. , Mir, S. , Lin, Z. , Hamik, A. , Atkins, G. B. , Das, H. , … Jain, M. K. (2005). Kruppel‐like factor 2 as a novel mediator of statin effects in endothelial cells. Circulation, 112, 720–726. 10.1161/CIRCULATIONAHA.104.525774 [DOI] [PubMed] [Google Scholar]
- Shen, S. , Tan, T. H. P. , & Tan, Y.‐J. (2007). Expression, glycosylation, and modification of the spike (S) glycoprotein of SARS CoV. Methods in Molecular Biology, 379, 127–135. 10.1007/978-1-59745-393-6_9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, Y. , Felley‐Bosco, E. , Marti, T. M. , & Stahel, R. A. (2012). Differential effects of lovastatin on cisplatin responses in normal human mesothelial cells versus cancer cells: Implication for therapy. PLoS ONE, 7, e45354 10.1371/journal.pone.0045354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrivastava‐Ranjan, P. , Flint, M. , Bergeron, E. , McElroy, A. K. , Chatterjee, P. , Albarino, C. G. , … Spiropoulou, C. F. (2018). Statins suppress Ebola virus infectivity by interfering with glycoprotein processing. MBio, 9 10.1128/mBio.00660-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal, T. (2020). A review of coronavirus disease‐2019 (COVID‐19). Indian Journal of Pediatrics, 87, 281–286. 10.1007/s12098-020-03263-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu, K.‐L. , Yuen, K.‐S. , Castano‐Rodriguez, C. , Ye, Z.‐W. , Yeung, M.‐L. , Fung, S.‐Y. , … Jin, D.‐Y. (2019). Severe acute respiratory syndrome coronavirus ORF3a protein activates the NLRP3 inflammasome by promoting TRAF3‐dependent ubiquitination of ASC. FASEB J. off. Publ. Fed. Am. Soc. Exp. Biol., 33, 8865–8877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So, J. Y. , Dhungana, S. , Beros, J. J. , & Criner, G. J. (2018). Statins in the treatment of COPD and asthma—Where do we stand? Current Opinion in Pharmacology, 40, 26–33. 10.1016/j.coph.2018.01.001 [DOI] [PubMed] [Google Scholar]
- South, A. M. , Diz, D. , & Chappell, M. C. (2020). COVID‐19, ACE2 and the Cardiovascular Consequences. American Journal of Physiology. Heart and Circulatory Physiology, 318, H1084–H1090. 10.1152/ajpheart.00217.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinello, I. , Saulle, E. , Quaranta, M. T. , Pasquini, L. , Pelosi, E. , Castelli, G. , … Labbaye, C. (2019). The small‐molecule compound AC‐73 targeting CD147 inhibits leukemic cell proliferation, induces autophagy and increases the chemotherapeutic sensitivity of acute myeloid leukemia cells. Haematologica, 104, 973–985. 10.3324/haematol.2018.199661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, M.‐X. , Huang, L. , Wang, R. , Yu, Y.‐L. , Li, C. , Li, P.‐P. , … Mao, X. (2012). Porcine reproductive and respiratory syndrome virus induces autophagy to promote virus replication. Autophagy, 8, 1434–1447. 10.4161/auto.21159 [DOI] [PubMed] [Google Scholar]
- Tang, N. , Bai, H. , Chen, X. , Gong, J. , Li, D. , & Sun, Z. (2020). Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. Journal of Thrombosis and Haemostasis, 18, 1094–1099. 10.1111/jth.14817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, T.‐T. , Lv, L.‐L. , Pan, M.‐M. , Wen, Y. , Wang, B. , Li, Z.‐L. , … Liu, B. C. (2018). Hydroxychloroquine attenuates renal ischemia/reperfusion injury by inhibiting cathepsin mediated NLRP3 inflammasome activation. Cell Death & Disease, 9, 351 10.1038/s41419-018-0378-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taoufiq, Z. , Pino, P. , N'dilimabaka, N. , Arrouss, I. , Assi, S. , Soubrier, F. , … Mazier, D. (2011). Atorvastatin prevents Plasmodium falciparum cytoadherence and endothelial damage. Malaria Journal, 10, 52 10.1186/1475-2875-10-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The University of Liverpool . (2020). Covid‐19 drug interactions.
- Thompson, M. R. , Kaminski, J. J. , Kurt‐Jones, E. A. , & Fitzgerald, K. A. (2011). Pattern recognition receptors and the innate immune response to viral infection. Viruses, 3, 920–940. 10.3390/v3060920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. National Library of Medicine . (2020). Covid‐19 clinical trials.
- Undas, A. , Brozek, J. , & Musial, J. (2002). Anti‐inflammatory and antithrombotic effects of statins in the management of coronary artery disease. Clinical Laboratory, 48, 287–296. [PubMed] [Google Scholar]
- Vaduganathan, M. , Vardeny, O. , Michel, T. , McMurray, J. J. V. , Pfeffer, M. A. , & Solomon, S. D. (2020). Renin‐angiotensin‐aldosterone system inhibitors in patients with Covid‐19. The New England Journal of Medicine, 382, 1653–1659. 10.1056/NEJMsr2005760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanarsdall, A. L. , Pritchard, S. R. , Wisner, T. W. , Liu, J. , Jardetzky, T. S. , & Johnson, D. C. (2018). CD147 promotes entry of pentamer‐expressing human cytomegalovirus into epithelial and endothelial cells. MBio, 9 10.1128/mBio.00781-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosper, J. , Masuccio, A. , Kullmann, M. , Ploner, C. , Geley, S. , & Hengst, L. (2015). Statin‐induced depletion of geranylgeranyl pyrophosphate inhibits cell proliferation by a novel pathway of Skp2 degradation. Oncotarget, 6, 2889–2902. 10.18632/oncotarget.3068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, K. , Chen, W. , Zhou, Y.‐S. , Lian, J.‐Q. , Zheng, Z. , Peng, D. , … Yu‐Meng Zhu, Z.‐N. C. (2020). SARS‐CoV‐2 invades host cells via a novel route: CD147‐spike protein. BioRxiv. 10.1101/2020.03.14.988345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S.‐T. , Ho, H. J. , Lin, J.‐T. , Shieh, J.‐J. , & Wu, C.‐Y. (2017). Simvastatin‐induced cell cycle arrest through inhibition of STAT3/SKP2 axis and activation of AMPK to promote p27 and p21 accumulation in hepatocellular carcinoma cells. Cell Death & Disease, 8, e2626 10.1038/cddis.2016.472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, Y.‐M. , Li, X. , Xu, M. , Abais, J. M. , Chen, Y. , Riebling, C. R. , … Zhang, Y. (2013). Enhancement of autophagy by simvastatin through inhibition of Rac1‐mTOR signaling pathway in coronary arterial myocytes. Cellular Physiology and Biochemistry, 31, 925–937. 10.1159/000350111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann, D. , Sperhake, J.‐P. , Lutgehetmann, M. , Steurer, S. , Edler, C. , Heinemann, A. , … Kluge, S. (2020). Autopsy findings and venous thromboembolism in patients with COVID‐19: A prospective cohort study. Annals of Internal Medicine. 10.7326/M20-2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth, M. , Joachim, J. , & Tooze, S. A. (2013). Autophagosome formation—The role of ULK1 and Beclin1‐PI3KC3 complexes in setting the stage. Seminars in Cancer Biology, 23, 301–309. 10.1016/j.semcancer.2013.05.007 [DOI] [PubMed] [Google Scholar]
- Wong, R. S. M. , Wu, A. , To, K. F. , Lee, N. , Lam, C. W. K. , Wong, C. K. , … Sung, J. J. Y. (2003). Haematological manifestations in patients with severe acute respiratory syndrome: Retrospective analysis. BMJ, 326, 1358–1362. 10.1136/bmj.326.7403.1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong, L. , Edwards, C. K. 3rd , & Zhou, L. (2014). The biological function and clinical utilization of CD147 in human diseases: A review of the current scientific literature. International Journal of Molecular Sciences, 15, 17411–17441. 10.3390/ijms151017411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, N. , & Shen, H.‐M. (2020). Targeting the endocytic pathway and autophagy process as a novel therapeutic strategy in COVID‐19. International Journal of Biological Sciences, 16, 1724–1731. 10.7150/ijbs.45498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y. , Wang, H. , Kouadir, M. , Song, H. , & Shi, F. (2019). Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death & Disease, 10, 128 10.1038/s41419-019-1413-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Z. , & Klionsky, D. J. (2010). Mammalian autophagy: core molecular machinery and signaling regulation. Current Opinion in Cell Biology, 22, 124–131. 10.1016/j.ceb.2009.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Z. , Su, Z. , DeWitt, J. P. , Xie, L. , Chen, Y. , Li, X. , … Yuan, Z. (2017). Fluvastatin prevents lung adenocarcinoma bone metastasis by triggering autophagy. eBioMedicine, 19, 49–59. 10.1016/j.ebiom.2017.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, S. (2015). Statins may decrease the fatality rate of middle east respiratory syndrome infection. MBio, 6, e01120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. , Penninger, J. M. , Li, Y. , Zhong, N. , & Slutsky, A. S. (2020). Angiotensin‐converting enzyme 2 (ACE2) as a SARS‐CoV‐2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Medicine, 46, 586–590. 10.1007/s00134-020-05985-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. , Yang, Z. , Xie, L. , Xu, L. , Xu, D. , & Liu, X. (2013). Statins, autophagy and cancer metastasis. The International Journal of Biochemistry & Cell Biology, 45, 745–752. 10.1016/j.biocel.2012.11.001 [DOI] [PubMed] [Google Scholar]
- Zhang, P. , Zhu, L. , Cai, J. , Lei, F. , Qin, J.‐J. , Xie, J. , … Li, H. (2020). Association of inpatient use of angiotensin converting enzyme inhibitors and Angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID‐19. Circulation Research, 126(12), 1671–1681. 10.1161/CIRCRESAHA.120.317134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, C. , & Zhao, W. (2020). NLRP3 inflammasome—A key player in antiviral responses. Frontiers in Immunology, 11, 211 10.3389/fimmu.2020.00211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Q. , & Liao, J. K. (2009). Statins and cardiovascular diseases: From cholesterol lowering to pleiotropy. Current Pharmaceutical Design, 15, 467–478. 10.2174/138161209787315684 [DOI] [PMC free article] [PubMed] [Google Scholar]


